Introduction
Oncocytic carcinomas (OCs) are very rare neoplasms
that have been reported to occur in the nasal and thoracic
cavities, ovary, kidney, thyroid gland, salivary gland, breast and
parathyroid (1,2). These tumors represent 11% of all
oncocytic salivary gland neoplasms, 0.5% of all epithelial salivary
gland malignancies and 0.18% of all epithelial salivary gland
tumors (1). Bauer and Bauer
(3) reported the first case in
1953, and, in total, only 31 cases have been reported in
English-language literature (4–22).
Oncocytes are typically large epithelial cells with
a low nuclear-to-cytoplasmic ratio, a centrally situated round
nucleus with a prominent nucleolus and an abundant bright
eosinophilic granular cytoplasm that is ultrastructurally
characterized by numerous mitochondria (14). Thymic epithelial tumors include
thymomas and thymic carcinomas. Although occurence is rare
(accounting for 0.2–1.5% of all malignancies) (23), they present the most common tumor of
the anterior mediastinum (24).
Thymomas are neoplasms arising from or exhibiting differentiation
towards thymic epithelial cells. Thymomas are classified into two
major types depending on whether the neoplastic epithelial cells
have an oval shape and are uniformly bland (type A thymoma) or
whether the cells have a predominantly round or polygonal
appearance (type B thymoma). Thymomas which exhibit type A and
B-like features are classified as type AB (25). In this case report, the patient not
only had OC but also thymoma. To the best of our knowledge, this is
the first reported case of an OC patient exhibiting type AB thyoma.
Written informed consent was obtained from the patient.
Case report
Case presentation
A 66-year-old male was admitted to the General
Hospital of Jinan Military Command (Jinan, China) with a 1-year
history of a painless left parotid mass that was gradually
increasing in size. Physical examination revealed a fixed, hard,
3×2-cm mass with a smooth surface in the left parotid region. There
was no palpable lymph node in the parotid gland or on the left side
of the neck. Systemic physical and laboratory examinations revealed
no abnormalities. Echography of the neck revealed an area of mixed
echoes in the left parotid gland. Computed tomography (CT)
demonstrated a 3×2-cm solid lesion in the left parotid gland and a
4.5×4.5-cm mass in the region of thymus. Radical resection of the
parotid tumor and thoracotomy resection of the thymic tumor were
performed.
Tissue staining
The specimen was fixed in neutral buffered formalin
and routinely processed with tissue sections embedded in paraffin.
The sections were cut into 4-μm slices and stained with hematoxylin
and eosin (H&E) for conventional evaluation. In addition to
H&E, the following immunostains and special tissue stains were
used: Cytokeratin (CK, AE1/AE3; Dako, Carpinteria, CA, USA),
carcinoembryonic antigen (CEA; Zymed, San Francisco, CA, USA), p53
(Dako), S-100 (4c4.9; Zymed), Ki-67 (Dako) and phosphotungstic
acid-hematoxylin (PTAH; Shanghai Lanji Science and Technology Co.,
Ltd., Shanghai, China).
Macroscopic findings
The parotid tumor consisted of unencapsulated,
irregular, cord-like, tan to gray masses. The cut surface was light
brown, solid, and non-homogeneous with cystic degeneration,
necrosis or hemorrhage (Fig. 1).
The tumor of the thymus was encapsulated and its cut surface was
solid and light brown (Fig. 2).
Microscopic findings and
immunohistochemistry
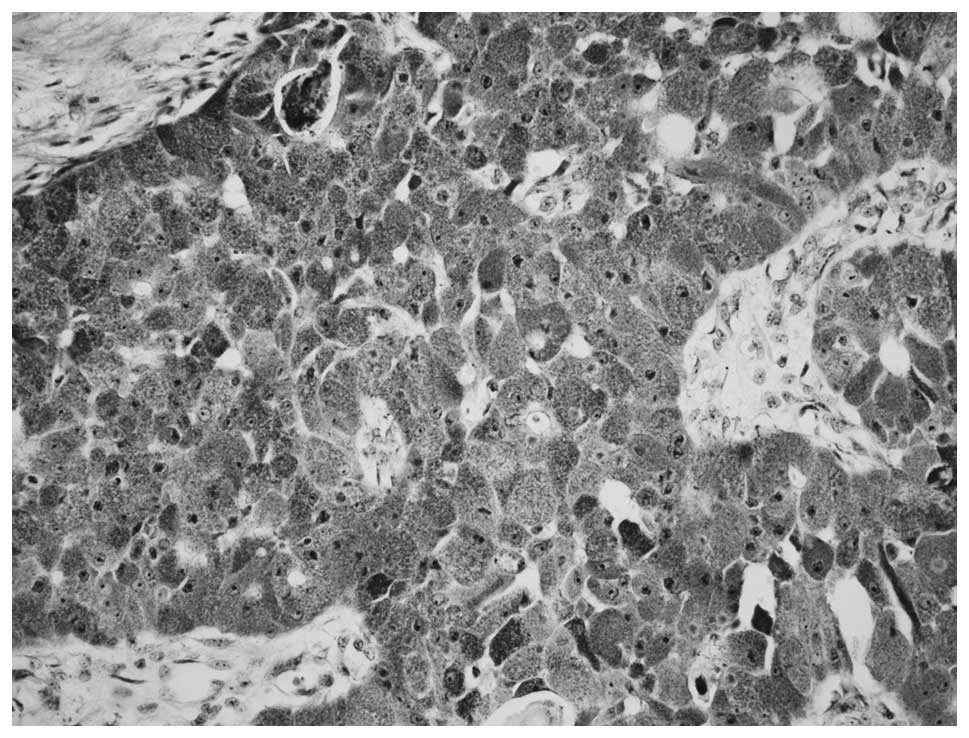
The parotid tumor had replaced a large area of the
parotid gland, but perineural invasion and vascular invasion were
not found. Neoplastic elements were large, round or polyhedral
cells and were arranged in solid sheets, islands and cords. The
cytoplasm was abundant, eosinophilic and finely granular. The
nuclei were large and centrally or peripherally located, and the
nucleoli were distinct and large (Fig.
3). PTAH staining distinctly illustrated positive, small,
dark-blue cytoplasmic granules, which represented mitochondria
(Fig. 4). Tumor cells were positive
for CK, CEA, S-100 and p53 by immunohistochemistry. Additionally,
PTAH staining illustrated positive dark-blue cytoplasmic granules.
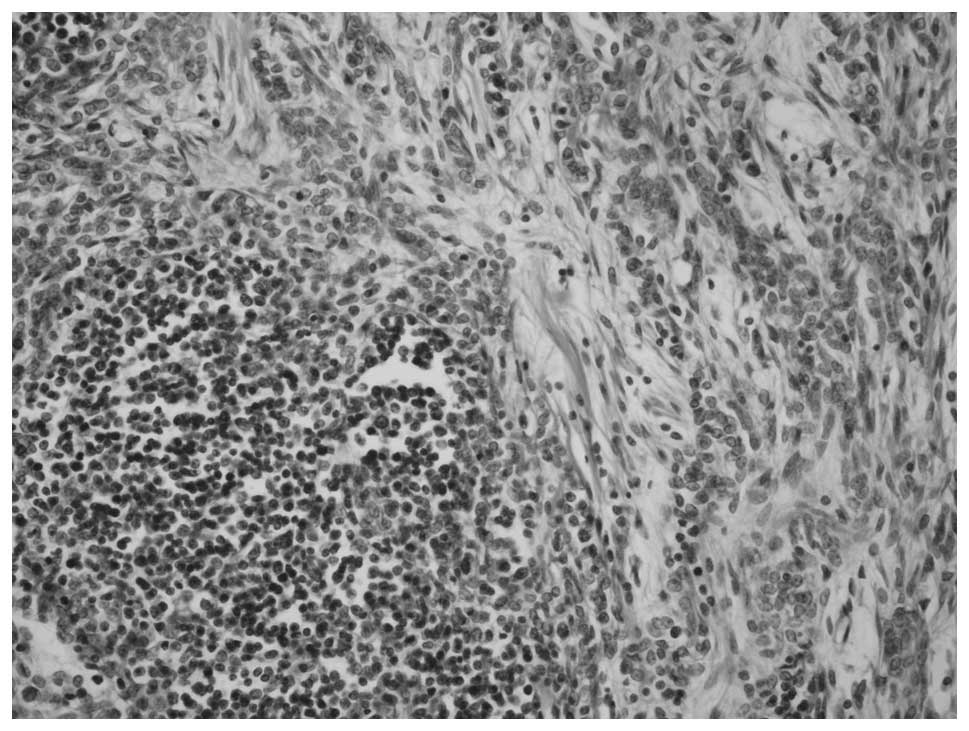
The tumor of the thymus consisted of a homogeneous population of
neoplastic epithelial cells that were spindle- or oval-shaped and
lacked nuclear atypia, admixed with foci rich in lymphocytes. The
segregation of the two patterns was sharp and distinct (Fig. 5).
Discussion
Oncocytes are large, granular, eosinophilic
epithelial cells that are primarily found in glandular tissue,
including that of the salivary glands and thyroid. In 1931, the
pathologist Hamperl (26) used the
term ‘oncocyte’ for this distinctive and typical cell type, which
was taken from the Greek word ‘onkousthai’ (27). Normal oncocytes are observed in the
salivary glands of aged patients and are considered to represent an
age-related metaplasia or degenerative process (28). In salivary gland ductal epithelium,
the appearance of oncocytes (oncocytic metaplasia) is rare prior to
the age of 50; however, it is nearly universal beyond age 70. In
1989, Linnane et al (29)
hypothesized that aging is the accumulation of mtDNA errors that
lead to mitochondrial ‘respiratory failure’ and multisystem
degeneration.
According to the World Health Organization
histologic classification of salivary gland tumors (30), parotid oncocytic neoplasms are
divided into three categories, including oncocytosis, oncocytoma
and OC. OC has been given several names in the past, including
oncocytic adenocarcinoma, malignant oncycytoma and malignant
oxyphilic adenoma. Sugimoto et al (31) reported that OC commonly presents as
a parotid mass with pain and facial nerve paralysis, and that such
symptoms were observed in one of three patients with OC. However,
the primary symptom in the patient reported in the current study
was a slowly progressive, painless mass. Oncocytic carcinomas
appear to arise from benign oncocytomas; however, they may arise
de novo (30). In the
current case, the malignant nature of the neoplasm was evidenced by
the regional and distant lymph node metastases. No perineural
invasion or infiltration of subcutaneous tissue was observed.
Criteria for the diagnosis of oncocytic carcinoma of the salivary
gland include: i) distant metastasis; ii) local lymph node
metastasis; iii) perineural, vascular, or lymphatic invasion; and
iv) frequent mitoses and cellular pleomorphism with extensive
invasion and destruction of adjacent structures (32).
It has been reported that OC occurs predominately in
the parotid gland of older adults with a mean age of occurrence of
62.5 years, and two-thirds of all cases occurring in males
(30). We reviewed previous
literature from the past 15 years (Table I) and found only 32 cases of parotid
OC. In the current case, the patient age (historically ranging from
41 to 86 years with a median age of 62.5 years) and tumor location
(historically 62.5% in the parotid gland) were in agreement with
those of the previous reports.
 | Table IReports of oncocytic carcinomas in the
salivary gland. |
Table I
Reports of oncocytic carcinomas in the
salivary gland.
| Author (ref) | Age | Sex | Site | Size (cm) | Rec. | LM |
|---|
| Guclu (4) | 65 | F | P | 3 | Y | N |
| Mizutari (5) | 55 | M | Sm | 3 | N | N |
| Kimura (6) | 61 | M | P | 4 | N | Y |
| Wischerath (7) | 59 | M | Sm | 2 | N | Y |
| Lombardi (8) | 45 | M | Oth | N | N | N |
| Sugiyama (9) | 84 | M | Oth | 4 | N | N |
| Ardekian (10) | 64 | M | P | 8 | N | N |
| Cinar (11) | 48 | F | P | 6 | N | Y |
| Muramatsu (12) | 82 | M | Sm | 4.5 | N | Y |
| Ozawa (13) | 58 | M | P | 3 | N | Y |
| Nakada (14) | 68 | M | Sm | 3 | N | Y |
| Corbridge (15) | 57 | M | P | 4 | N | Y |
| Yang (16) | 64 | M | Sm | 3.8 | N | Y |
| Wang (17) | 73 | M | P | 3 | N | Y |
| Tian (18) | 66 | M | P | 3 | Y | N |
| Dong (19) | 57 | M | Sm | 3 | N | N/A |
| Zhou (20) | 60 | M | Oth | 3.5 | Y | Y |
| 57 | M | P | 7 | N | Y |
| 48 | M | P | 3 | N | N |
| 59 | M | P | 8 | N | Y |
| 75 | M | P | 3 | Y | Y |
| 68 | M | P | 4 | Y | N |
| 41 | M | P | 3 | N | N |
| 55 | M | P | 2.5 | N | Y |
| 67 | F | P | 3.5 | Y | N |
| 86 | M | P | 1 | Y | N |
| 51 | F | Oth | 4 | Y | Y |
| 68 | M | P | 3 | Y | N |
| Lee (21) | 51 | M | Sm | 3 | N | Y |
| Gallego (22) | 65 | M | P | 2.5 | N | Y |
| Present case | 66 | M | P | 2.5 | N | Y |
Oncocytic carcinoma can be differentiated from
benign oncocytoma, since the former includes distant metastases;
local lymph node metastases; perineural, intravascular, or
lymphatic invasion; and mitoses and cellular polymorphisms with
destructive invasion of adjacent structures. Ki-67 immunostaining
has been proposed as a tool for distinguishing OC from oncocytomas
(33). In a previous study, the
frequency of Ki-67 positive cells with nuclear staining was higher
in OC compared to oncocytomas (34).
In contrast to oncocytic carcinoma, salivary duct
carcinoma forms duct-like spaces with papillary and cribriform
growth, and displays comedonecrosis (2). In addition, the presence of numerous
mitochondria in the cytoplasm of the oncocytes, as confirmed by
ultrastructural examination, is not found in the neoplastic cells
of the other malignancies mentioned above, which can also be used
for adjuvant diagnosis. However, the processes of fixing or
embedding the specimens for light microscopy often destroys the
fine structure of organelles in the cytoplasm, making it difficult
to observe mitochondria clearly.
Acinic cell adenocarcinoma may be differentiated
from oncocytic carcinoma by its amphophilic or basophilic
cytoplasmic granules, negative staining for mithochondrial antigens
and the presence of a connective tissue capsule. Cytologic
examination of Warthin’s tumor shows oncocytes together with
lymphocytes, amorphous material and cystic fluid. However, the
possibility of oncocytoma should be considered when the smear
contains only oncocytes (35).
PTAH staining has been successfully used to identify
oncocytes; Brandwein and Huvos (36) particularly recommended the use of
prolonged (48 h) PTAH staining, which results in positive,
dark-blue cytoplasmic granules. It has also been reported that
immunohistochemistry using an anti-mitochondrial antibody is a
highly sensitive and specific method for identifying mitochondria
using light microscopy (37).
Surgical excision is the most widely accepted method
of treatment for OC (15), and the
majority of the cases described in the literature have included
neck dissection. Goode and Corio (38) reported that patients with tumors
<2 cm in diameter appeared to have a better prognosis than those
that were larger. Adjuvant radiotherapy has been used for the
treatment of oncocytic carcinoma, but its true contribution has not
yet been elucidated. OC has a potential risk of distant metastasis,
and lung, liver and brain metastases have been reported (32). The long-term survival of patients
with OC is poor due to distant disease, and long-term follow-up is
necessary after therapy (2). In the
current study, the patient not only had OC but also thymoma, which
is exceedingly rare and may represent the first documented case in
the literature.
Acknowledgements
The authors thank Medjaden Bioscience Limited for
assisting in the preparation of this manuscript.
References
|
1
|
Ellis GL, Auclair PL, Gnepp DR and Goode
PK: Other malignant epithelial neoplasms. Surgical Pathology of the
Salivary Glands. Ellis GL, Auclair PL and Gnepp DR: Philadelphia:
W. Saunders; pp. 455–488. 1991
|
|
2
|
Ellis GL and Auclair PL: Tumors of the
salivary glands. Atlas of Tumor Pathology: Third Series, Fascicle
17. Armed Forces Institute of Pathology; Washington, DC: pp.
318–324. 1996
|
|
3
|
Bauer WH and Bauer JD: Classification of
glandular tumors of salivary glands; study of one-hundred
forty-three cases. AMA Arch Pathol. 55:328–346. 1953.PubMed/NCBI
|
|
4
|
Guclu E, Oghan F, Ozturk O, Alper M and
Egeli E: A rare malignancy of the parotid gland: oncocytic
carcinoma. Eur Arch Otorhinolaryngol. 262:567–569. 2005. View Article : Google Scholar
|
|
5
|
Mizutari K, Naganishi H and Tanaka Y:
Oncocytic carcinoma in the submandibular gland: report of a case
based on anti-mitochondrial immunohistochemical observations. Auris
Nasus Larynx. 32:305–308. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kimura M, Furuta T, Hashimoto S, Takano T
and Nagao K: Oncocytic carcinoma of the parotid gland. A case
report. Acta Cytol. 47:1099–1102. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wischerath H, Brehmer D, Hesse G and
Laubert A: Oncocytic adenocarcinoma of the submandibular gland.
HNO. 50:565–569. 2002.(In German). View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lombardi D, Piccioni M, Farina D, Morassi
ML and Nicolai P: Oncocytic carcinoma of the maxillary sinus: a
rare neoplasm. Eur Arch Otorhinolaryngol. 263:528–531. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sugiyama T, Nakagawa T, Narita M, Nakamura
S, Inui M and Tagawa T: Pedunculated oncocytic carcinoma in buccal
mucosa: immunohistochemical and ultrastructural studies. Oral Dis.
12:324–328. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ardekian L, Manor R, Peled M and Laufer D:
Malignant oncocytoma of the parotid gland: case report and analysis
of the literature. Oral Maxillofac Surg. 57:325–328. 1999.
View Article : Google Scholar
|
|
11
|
Cinar U, Vural C, Basak T and Turgut S:
Oncocytic carcinoma of the parotid gland: report of a new case. Ear
Nose Throat J. 82:699–701. 2003.PubMed/NCBI
|
|
12
|
Muramatsu T, Hashimoto S, Lee MW, Chung
CK, Matsuzaki K, Inoue T, Noma H and Shimono M: Oncocytic carcinoma
arising in submandibular gland with immunohistochemical
observations and review of the literature. Oral Oncol. 39:199–203.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ozawa H, Fujii M, Matsunaga T, Masuda K,
Hirose S and Taiji H: Oncocytic carcinoma of the parotid gland. J
Otolaryngol. 35:189–192. 2006.PubMed/NCBI
|
|
14
|
Nakada M, Nishizaki K, Akagi H, Masuda Y
and Yoshino T: Oncocytic carcinoma of the submandibular gland: a
case report and literature review. J Oral Pathol Med. 27:225–228.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Corbridge RJ, Gallimore AP, Dalton CG and
O’Flynn PE: Oncocytomas of the upper jaw. Head Neck. 18:374–380.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang GZ, Gao LX and Ding HY: Salivary
gland carcinoma with eosinophilia neuroendocrine differentiation.
Chin J Pathol. 33:582–583. 2004.
|
|
17
|
Wang SY, Lou JL and DU XH: Oncocytic
Carcinoma of Parotid Gland: A Case Report. J Oncol. 14:689–691.
2008.
|
|
18
|
Tian XQ and Wang WZ: Oncocytic carcinoma
of Salivary gland, a case report. Zhong liu yan jiu yu lin chuang.
14:252–253. 2002.
|
|
19
|
Dong DX, Shi PX and Li YL: Salivary Gland
Cancer: A case report. J Diagn Pathol. 8:183–184. 2001.
|
|
20
|
Zhou CX, Shi DY, Ma DQ, Zhang JG, Yu GY
and Gao Y: Primary oncocytic carcinoma of the salivary glands: A
clinicopathologic and immunohistochemical study of 12 cases. Oral
Oncol. 46:773–778. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lee WY and Chang SL: Fine needle
aspiration cytology of oncocytic carcinoma of the submandibular
gland with pre-existing oncocytoma: a case report. J Cytopathology.
21:339–341. 2010. View Article : Google Scholar
|
|
22
|
Gallego L, García-Consuegra L, Fuente E,
Calvo N and Junquera L: Oncocytic carcinoma of the parotid gland
with late cervical lymph node metastases: a case report. J Med Case
Rep. 5:112011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fornasiero A, Daniele O, Ghiotto C, et al:
Chemotherapy of invasive thymoma. J Clin Oncol. 8:1419–1423.
1990.PubMed/NCBI
|
|
24
|
Detterbeck FC and Parsons AM: Thymic
tumors. Ann Thorac Surg. 77:1860–1869. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Marx A, Harris NL, Strobel Ph, et al:
Thymomas. World Health Organization Classification of Tumours.
Pathology and Genetics of Tumours of the Lung, Pleura, Thymus and
Heart. Travis DW, Brambilla E, Muller-Hermelink HK and Harris CC:
IARC Press; Lyon: pp. 152–157. 2004
|
|
26
|
Hamperl H: Beitrage zur normalen und
pathologischen histology menschlicher speicheldrusen. Z Microanat
Forsch. 27:1–55. 1931.(In German).
|
|
27
|
Alberty J, August C and Stoll W: Oncocytic
neoplasms of the parotid gland. Differential diagnosis, clinical
course and review of the literature. HNO. 49:109–117. 2001.(In
German). View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Dardick I, Birek C, Lingen MW and Rowe PE:
Differentiation and the cytomorphology of salivary gland tumors
with specific reference to oncocytic metaplasia. Oral Surg Oral Med
Oral Pathol Oral Radiol Endod. 88:691–701. 1999. View Article : Google Scholar
|
|
29
|
Linnane AW1, Marzuki S, Ozawa T and Tanaka
M: Mitochondrial DNA mutations as an important contributor to
ageing and degenerative diseases. Lancet. 25:642–645. 1989.
View Article : Google Scholar
|
|
30
|
Sciubba JJ and Shimono M: Oncocytic
carcinoma. World Health Organization Classification of Tumors:
Pathology and Genetics: Head and Neck Tumours. Barnes L, Eveson JW,
Reichart D and Sidransky D: IARC Press; Lyon: pp. 2352005
|
|
31
|
Sugimoto T, Wakizono S, Uemura T,
Tsuneyoshi M and Enjoji M: Malignant oncocytoma of the parotid
gland: a case report with an immunohistochemical and
ultrastructural study. J Laryngol Otol. 107:69–74. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gray SR, Cornog JL Jr and Seo IS:
Oncocytic neoplasms of salivary glands: a report of fifteen cases
including two malignant oncocytomas. Cancer. 38:1306–1317. 1976.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ito K, Tsukuda M, Kawabe R, et al: Benign
and malignant oncocytoma of the salivary glands with an
immunohistochemical evaluation of Ki-67. ORL J Otorhinolaryngol
Relat Spec. 62:338–341. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhou CX and Gao Y: Oncocytoma of the
salivary glands: a clinicopathologic and immunohistochemical study.
Oral Oncol. 45:e232–e238. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yang T and Guo LN: Oncocytic carcinoma.
Diagnostic Pathology: First Series. Liu TH: People’s Medical
Publishing Press; Beijing: pp. 617–618. 2013
|
|
36
|
Brandwein MS and Huvos AG: Oncocytic
tumors of major salivary glands: a study of 68 cases with follow-up
of 44 patients. Am J Surg Pathol. 15:514–528. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Shintaku M and Honda T: Identification of
oncocytic lesions of salivary glands by anti-mitochondrial
immunohistochemistry. Histopathology. 31:408–411. 1997. View Article : Google Scholar
|
|
38
|
Goode RK and Corio RL: Oncocytic
adenocarcinoma of salivary glands. Oral Surg Oral Med Oral Pathol.
65:61–66. 1988. View Article : Google Scholar : PubMed/NCBI
|