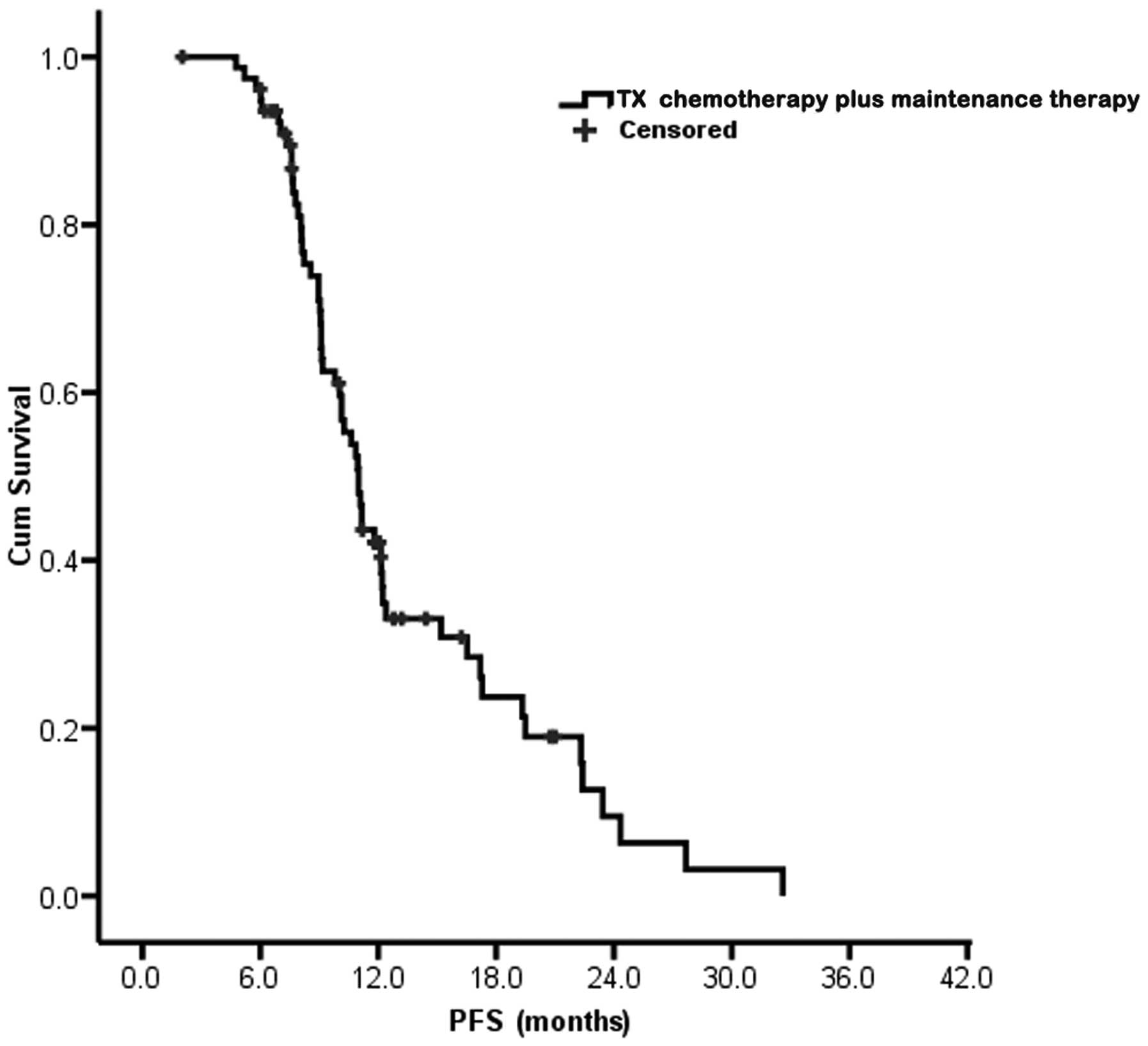
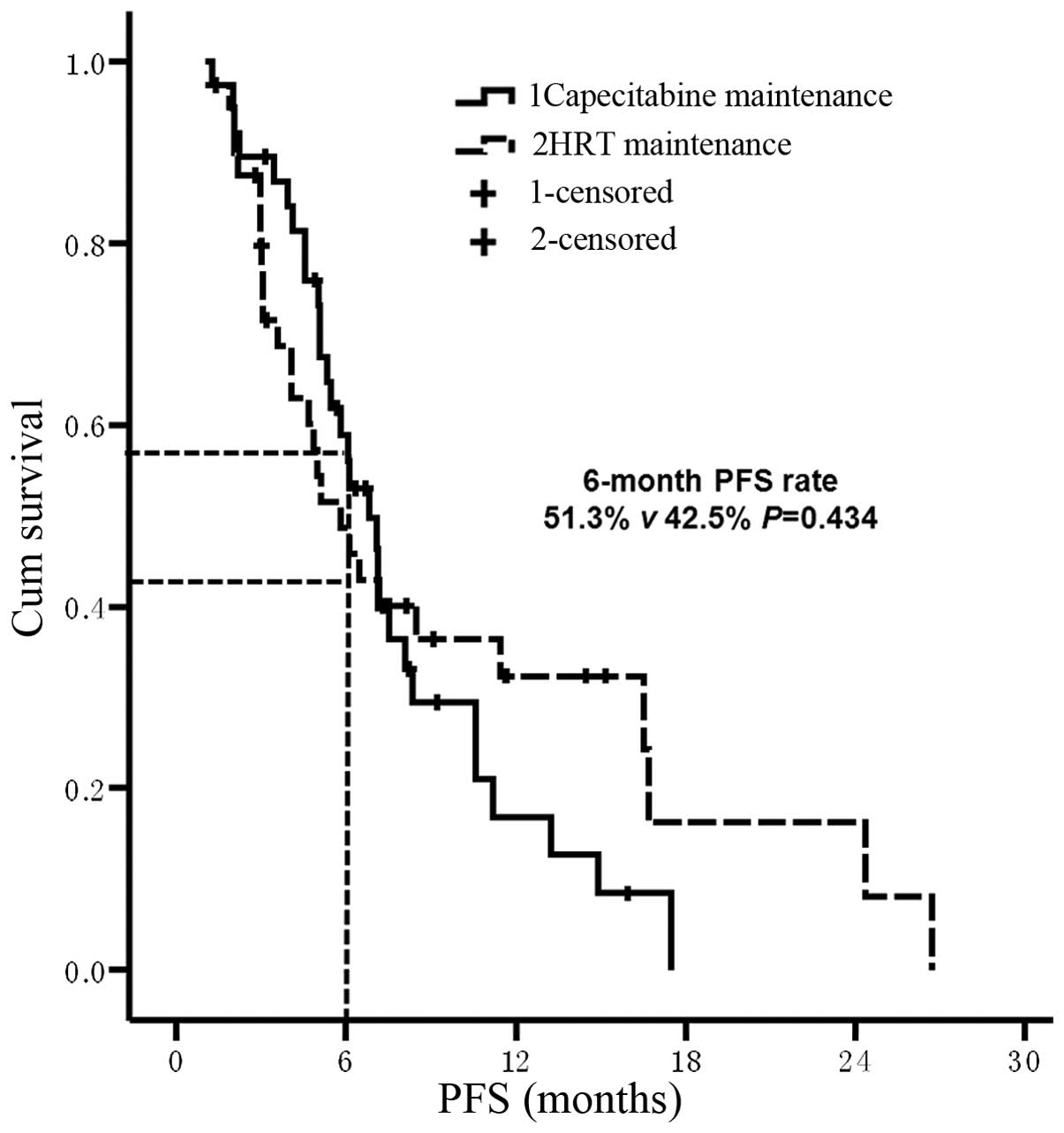
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Thun MJ, Jemal A and Ward E: Global cancer
incidence and mortality. Cancer: Principles and Practice of
Oncology. DeVita VT Jr: 9th edition. Lippincott Williams &
Wilkins; Philadelphia, PA: pp. 241–266. 2011
|
|
3
|
Gennari A, Amadori D, De Lena M, et al:
Lack of benefit of maintenance paclitaxel in first-line
chemotherapy in metastatic breast cancer. J Clin Oncol.
24:3912–3918. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Alba E, Ruiz-Borrego M, Margelí M, et al:
Maintenance treatment with pegylated liposomal doxorubicin versus
observation following induction chemotherapy for metastatic breast
cancer: GEICAM 2001–01 study. Breast Cancer Res Treat. 122:169–176.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gennari A, Stockler M, Puntoni M, et al:
Duration of chemotherapy for metastatic breast cancer: a systematic
review and meta-analysis of randomized clinical trials. J Clin
Oncol. 29:2144–2149. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dufresne A, Pivot X, Tournigand C, et al:
Maintenance hormonal treatment improves progression free survival
after a first line chemotherapy in patients with metastatic breast
cancer. Int J Med Sci. 5:100–105. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bertelli G, Garrone O, Bertolotti L, et
al: Maintenance hormone therapy with letrozole after first-line
chemotherapy for advanced breast cancer. Oncology. 68:364–370.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mayer IA: Treatment of HER2-positive
metastatic breast cancer following initial progression. Clin Breast
Cancer. 9(Suppl 2): S50–S57. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Leonard R, Hennessy BT, Blum JL and
O’Shaughnessy J: Dose-adjusting capecitabine minimizes side effects
while maintaining efficacy: a retrospective review of capecitabine
for metastatic breast cancer. Clin Breast Cancer. 11:349–356. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Górnaś M and Szczylik C: Oral treatment of
metastatic breast cancer with capecitabine: what influences the
decision-making process? Eur J Cancer Care (Engl). 19:131–136.
2010. View Article : Google Scholar
|
|
11
|
Reichardt P, Von Minckwitz G,
Thuss-Patience PC, et al: Multicenter phase II study of oral
capecitabine (Xeloda®) in patients with metastatic
breast cancer relapsing after treatment with a taxane-containing
therapy. Ann Oncol. 14:1227–1233. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kusama M, Nomizu T, Aogi K, et al: Phase
II study of 4-weekly capecitabine monotherapy in
advanced/metastatic breast cancer. Breast Cancer. 17:233–240. 2010.
View Article : Google Scholar
|
|
13
|
Hortobagyi GN, Gomez HL, Li RK, et al:
Analysis of overall survival from a phase III study of ixabepilone
plus capecitabine versus capecitabine in patients with MBC
resistant to anthracyclines and taxanes. Breast Cancer Res Treat.
122:409–418. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
O’Shaughnessy J, Miles D, Vukelja S, et
al: Superior survival with capecitabine plus docetaxel combination
therapy in anthracycline-pretreated patients with advanced breast
cancer: phase III trial results. J Clin Oncol. 20:2812–2823. 2002.
View Article : Google Scholar
|
|
15
|
Blum JL, Dees EC, Vukelja SJ, et al: Phase
II trial of capecitabine and weekly paclitaxel in patients with
metastatic breast cancer previously treated with every-3-week
taxane therapy. Clin Breast Cancer. 7:465–470. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chan A and Verrill M: Capecitabine and
vinorelbine in metastatic breast cancer. Eur J Cancer.
45:2253–2265. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Eisenhauer EA, Therasse P, Bogaerts J, et
al: New response evaluation criteria in solid tumours: revised
RECIST guideline (version 1.1). Eur J Cancer. 45:228–247. 2009.
View Article : Google Scholar
|
|
18
|
Oken MM, Creech RH, Tormey DC, et al:
Toxicity and response criteria of the Eastern Cooperative Oncology
Group. Am J Clin Oncol. 5:649–655. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Trotti A, Colevas AD, Setser A, et al:
CTCAE v3.0: development of a comprehensive grading system for the
adverse effects of cancer treatment. Semin Radiat Oncol.
13:176–181. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nicolini A, Giardino R, Carpi A, et al:
Metastatic breast cancer: an updating. Biomed Pharmacother.
60:548–556. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cardoso F, Bedard PL, Winer EP, et al;
ESO-MBC Task Force. International guidelines for management of
metastatic breast cancer: combination vs. sequential single-agent
chemotherapy. J Natl Cancer Inst. 101:1174–1181. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Park YH, Jung KH, Im SA, et al: Phase III,
multicenter, randomized trial of maintenance chemotherapy versus
observation in patients with metastatic breast cancer after
achieving disease control with six cycles of gemcitabine plus
paclitaxel as first-line chemotherapy: KCSG-BR07-02. J Clin Oncol.
31:1732–1739. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Martín M and López-Tarruella S:
Chemotherapy: Maintenance therapy in breast cancer - many questions
remain. Nat Rev Clin Oncol. 10:370–372. 2013. View Article : Google Scholar
|
|
24
|
Sledge GW Jr, Hu P, Falkson G, Tormey D
and Abeloff M: Comparison of chemotherapy with chemohormonal
therapy as first-line therapy for metastatic, hormone-sensitive
breast cancer: an Eastern Cooperative Oncology Group Study. J Clin
Oncol. 18:262–266. 2000.PubMed/NCBI
|
|
25
|
Beaver JA and Park BH: The BOLERO-2 trial:
the addition of everolimus to exemestane in the treatment of
postmenopausal hormone receptor-positive advanced breast cancer.
Future Oncol. 8:651–657. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ali S and Coombes RC: Endocrine-responsive
breast cancer and strategies for combating resistance. Nat Rev
Cancer. 2:101–112. 2002. View
Article : Google Scholar
|
|
27
|
Burstein HJ: Novel agents and future
directions for refractory breast cancer. Semin Oncol. 38(Suppl 2):
S17–S24. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mouridsen H, Sun Y, Gershanovich M, et al:
Superiority of letrozole to tamoxifen in the first-line treatment
of advanced breast cancer: evidence from metastatic subgroups and a
test of functional ability. Oncologist. 9:489–496. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nabholtz JM, Buzdar A, Pollak M, et al:
Anastrozole is superior to tamoxifen as first-line therapy for
advanced breast cancer in postmenopausal women: results of a north
american multicenter randomized trial. Arimidex Study Group. J Clin
Oncol. 18:3758–3767. 2000.PubMed/NCBI
|
|
30
|
Mauri D, Pavlidis N, Polyzos NP and
Ioannidis JP: Survival with aromatase inhibitors and inactivators
versus standard hormonal therapy in advanced breast cancer:
meta-analysis. J Natl Cancer Inst. 98:1285–1291. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Falkson G, Gelman RS, Pandya KJ, et al:
Eastern Cooperative Oncology Group randomized trials of observation
versus maintenance therapy for patients with metastatic breast
cancer in complete remission following induction treatment. J Clin
Oncol. 16:1669–1676. 1998.PubMed/NCBI
|
|
32
|
Nooij MA, de Haes JC, Beex LV, et al;
EORTC Breast Cancer Group. Continuing chemotherapy or not after the
induction treatment in advanced breast cancer patients: Clinical
outcomes and oncologists’ preferences. Eur J Cancer. 39:614–621.
2003. View Article : Google Scholar : PubMed/NCBI
|