Introduction
Neoadjuvant chemotherapy for the treatment of breast
cancer has been reported to be equivalent to adjuvant chemotherapy
in terms of survival and overall disease progression (1). In addition, neoadjuvant chemotherapy
offers certain attractive benefits, since it can downstage the
primary tumor in the majority of patients, increasing
breast-conserving surgery (BCS) rates or improving resectability
(2). Neoadjuvant chemotherapy also
provides an in vivo assessment of tumor response to
chemotherapy, as the patients that attain pathological complete
response (pCR) during neoadjuvant therapy exhibit a significantly
improved disease-free survival rate (3,4).
Trastuzumab is a humanized monoclonal antibody that
blocks the activity of human epidermal growth factor receptor (HER)
2. Combined with neoadjuvant chemotherapy for the treatment of
HER2-positive breast cancer patients, trastuzumab offers a
substantial benefit in terms of pCR, with no additional toxicity
(5). Lapatinib is an orally-active
small molecule that reversibly inhibits HER1 and HER2. As
demonstrated by cell line and xenograft models (6,7),
lapatinib blocks the activating signaling cascades in the MAPK and
PI3K pathways, resulting in cell growth arrest or apoptosis.
Clinical trials have demonstrated that lapatinib is efficacious in
HER2-positive metastatic breast cancer (8).
A potential therapeutic option to improve HER2
inhibition is the combination of lapatinib and trastuzumab. The
theoretical advantage of this combination is that the
non-overlapping mechanism of action between the two agents and the
ability to overcome the primary and acquired resistance to the
agents by dual blockade. Certain randomized controlled trials
(RCTs) that have explored the potential advantages by using
lapatinib in the neoadjuvant setting have been previously reported
(9–12).
To evaluate the efficacy and safety of lapatinib
combined with neoadjuvant therapy for the treatment of
HER2-positive breast cancer, a meta-analysis of all relevant
published RCTs was performed.
Patients and methods
Eligibility criteria
The eligibility and exclusion criteria were
pre-specified. Studies were considered eligible for the present
meta-analysis if they were RCTs that evaluated the administration
of trastuzumab-based chemotherapy compared with neoadjuvant
chemotherapy using a combination of agents including lapatinib. All
cytotoxic chemotherapy regimens were considered eligible for the
present meta-analysis if the same chemotherapy agents were
administered at the same dose in all treatment arms and that the
arms differed systematically only in the anti-HER2 therapy
administered. If multiple publications of the same trial or a case
mix between publications was found, only the most recent or most
informative publication was included. The study was approved by the
ethics committee of Renmin Hospital of Wuhan University (Wuhan,
China).
Search strategy
MEDLINE (National Library of Medicine, Bethesda, MD,
USA), Embase (Elsevier, Amsterdam, Netherlands) and the Cochrane
Central Register of Controlled Trials (Cochrane Library, Hoboken,
NJ, USA) were searched between inception and 15 December 2013,
using the following searching algorithm: (neoadjuvant OR
preoperative OR induction OR primary systemic OR primary
chemotherapy) AND (lapatinib OR tykerb OR tyverb). The proceedings
of the major international congresses, comprising the San Antonio
Breast Cancer Symposium (Symposia Cancer Therapy & Research
Center at UT Health Science Center San Antonio, San Antonio, TX,
USA) and the American Society of Clinical Oncology Annual Meeting
(American Society of Clinical Oncology, Alexandria, VA, USA), were
also electronically searched to avoid the exclusion of unpublished
recent trials using lapatinib in neoadjuvant chemotherapy. Finally,
the reference lists of the key articles were reviewed to search for
further studies.
Data extraction
The data was independently extracted from all
included studies. Disagreements were discussed to achieve
consensus, or underwent third-party adjudication. From each
eligible trial, the following items were recorded: authors’ name;
journal name and year of publication; years of patient enrollment;
country of origin; number of medical centers involved; number of
patients randomized and analyzed per arm; patient age and gender;
hormone receptor (HR) status; tumor size; node status; median
follow-up time; technique used for HER2 identification; type and
dose of chemotherapy; dose and duration of trastuzumab therapy; and
dose and duration of lapatinib therapy. Primary and secondary
outcome measures, consisting of pCR, BCS and all adverse events,
were also recorded.
Outcome definition
The primary outcome assessed was the rate of pCR
achieved. If the primary study reported a separate pCR rate for
breast tissue and breast tissue plus axilla, only the pCR rate for
breast tissue plus axilla was included. The secondary outcomes
assessed were the rate of BCS and all adverse events. The primary
adverse events included grade 3–4 diarrhea, hepatic toxicity,
dermatological toxicity and neutropenia. HR-positive was defined as
immunohistochemical estrogen receptor level ≥10% or progesterone
receptor level ≥10%.
Assessment of risk of bias
Cochrane’s risk of bias tool was utilized to assess
the individual risk of bias in each study (13). The criteria used for quality
assessment were sequence generation of allocation, allocation
concealment, masking of participants, personnel and outcome
assessors, incomplete outcome data, selective outcome reporting and
other sources of bias. The risk of bias in each eligible trial was
independently assessed. Potential publication bias was assessed
visually using a funnel plot and was statistically analyzed using
Egger’s and Begg’s tests (14,15).
Statistical analysis
Two by two tables were constructed, using the
intention to treat assignment when applicable, and the risk ratio
(RR) was calculated for each primary study to estimate the relative
risk of each outcome in patients with HER2-positive breast cancer
receiving trastuzumab-based chemotherapy versus combination of
lapatinib as neoadjuvant therapy. For each eligible study group,
the RR for the outcome measures was estimated and compared between
the groups, and the 95 % confidence interval (CI) was also
estimated. The data were then synthesized for all studies using
fixed effects (Mantel-Haenszel) or random effects (Der Simonian and
Laird) modeling when heterogeneity was present between studies.
The Q statistic was used to test heterogeneity
between trials. The presence of statistical heterogeneity was
assessed using Cochran’s Q test and quantified using I2
and respective 95% CIs. P<0.10 was considered to indicate a
statistically significant difference. For the I2 values,
≥40% indicated a large heterogeneity and >75% indicated an
extremely large heterogeneity. When substantial heterogeneity,
classified as I2≥40%, was identified, subgroup analyses
were performed. The subgroup analyses performed were defined a
priori to investigate the effects of HR in pCR.
The meta-analysis was conducted using Review Manager
software version 5.1 (The Cochrane Collaboration, Copenhagen,
Denmark). Begg’s and Egger’s tests were performed using the Stata
software package version 12.0 (StataCorp LP, College Station, TX,
USA).
Results
Literature selection and study
characteristics
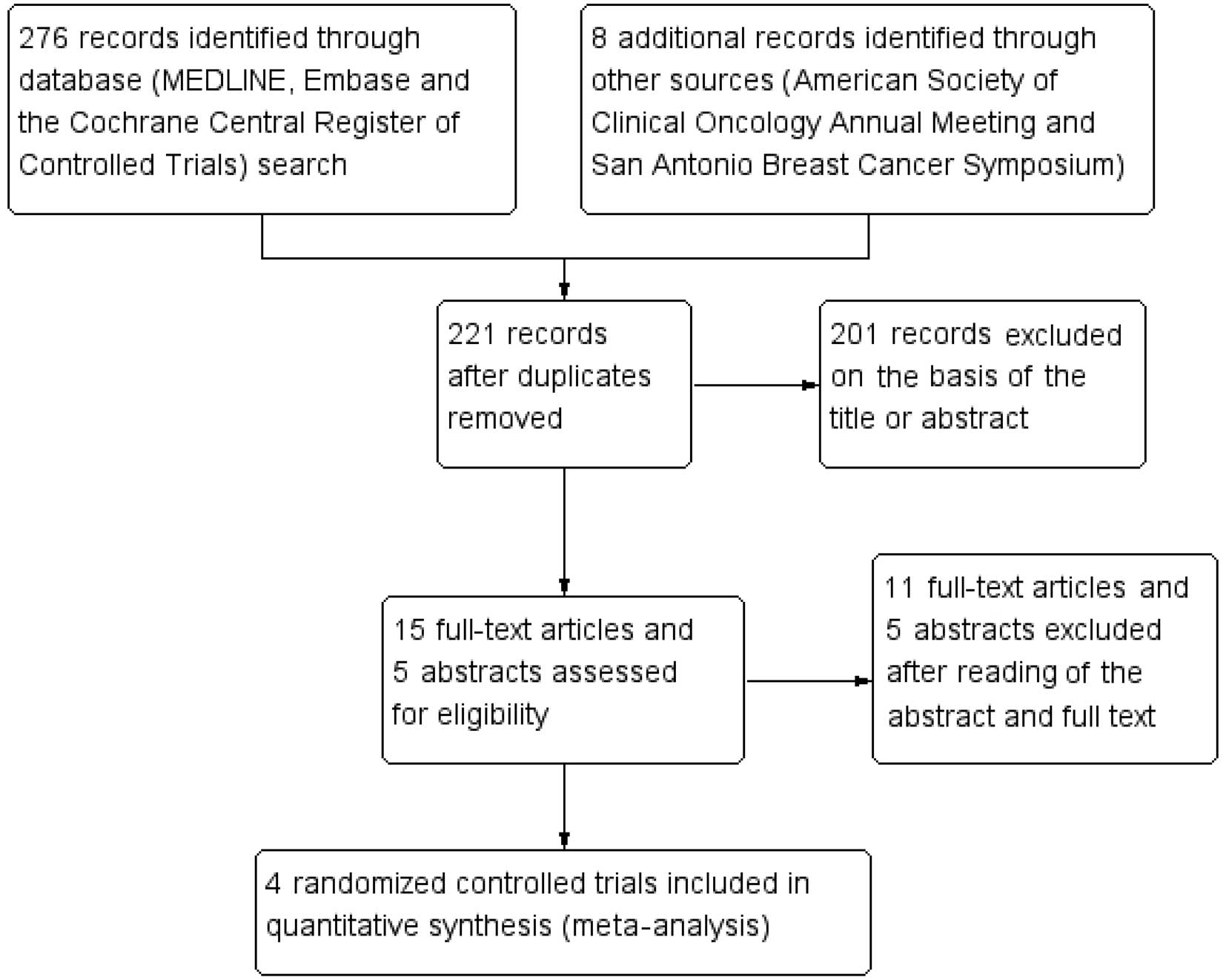
The process of identifying eligible trials is
presented in Fig. 1. The titles and
abstracts of 221 unique records identified through the literature
search were screened. Four eligible full-text articles were
retrieved, all of which were randomized controlled trials and from
peer-reviewed studies (9–12). These trials were included in the
meta-analysis.
Table I reports the
characteristics of the four trials that met the eligibility
criteria for the present study. The four trials were three-armed
and compared the administration of trastuzumab with the
administration of lapatinib or a combination of the two for
neoadjuvant chemotherapy, with all regimens including paclitaxel in
the regimen. All trials reported the pCR rate and primary adverse
events. To investigate the potential role of lapatinib in
neoadjuvant therapy, only the data from the trastuzumab-based
chemotherapy arm, termed the no lapatinib group, and the lapatinib
plus trastuzumab-based chemotherapy arm, termed the lapatinib
group, were extracted. In total, 779 patients were included in the
present meta-analysis. Of those, 388 patients had been randomly
allocated to the no lapatinib group and 391 to the lapatinib
group.
 | Table ICharacteristics of eligible
trials. |
Table I
Characteristics of eligible
trials.
| Clinical trial
(reference) | Total number of
patients, n | HER2 status
assessment | Number of patients
analyzed, n | Treatment arm | Number of patients
per arm, n | HR-positive tumors, n
(%) | Neoadjuvant
chemotherapy | Neoadjuvant anti-HER2
therapy | Duration of anti-HER2
therapy, weeks |
|---|
| CHER-LOB (9) | 121 | IHC3+ or
FISH amplification | 82 | No Lap | 36 | 21 (58) | P weekly → 4 ×
FEC | T 4→2 mg/kg
weekly | 26 |
| Lap | 46 | 28 (62) | P weekly → 4 ×
FEC | Lap 1000 mg daily + T
2 mg/kg weekly | 26 |
| Holmes et al
(10) | 100 | IHC3+ or
FISH ratio >2.2 | 66 | No Lap | 33 | 15 (45) | 4 × FEC → P
weekly | T 4→2 mg/kg
weekly | 26 |
| Lap | 33 | 20 (61) | 4 × FEC → P
weekly | Lap 750 mg daily + T
2 mg/kg weekly | 26 |
| NeoALTTO (11) | 455 | IHC3+ or
FISH amplification | 301 | No Lap | 149 | 75 (50) | P weekly | T 4→2 mg/kg
weekly | 18 |
| Lap | 152 | 77 (51) | P weekly | Lap 1000 mg daily +
T 2 mg/kg weekly | 18 |
| NSABP B-41
(12) | 529 | IHC3+,
FISH or CISH amplification | 355 | No Lap | 181 | 122 (67) | 4 × AC → P
weekly | T 4→2 mg/kg
weekly | 16 |
| Lap | 174 | 108 (62) | 4 × AC → P
weekly | Lap 750 mg daily +
T 2 mg/kg weekly | 16 |
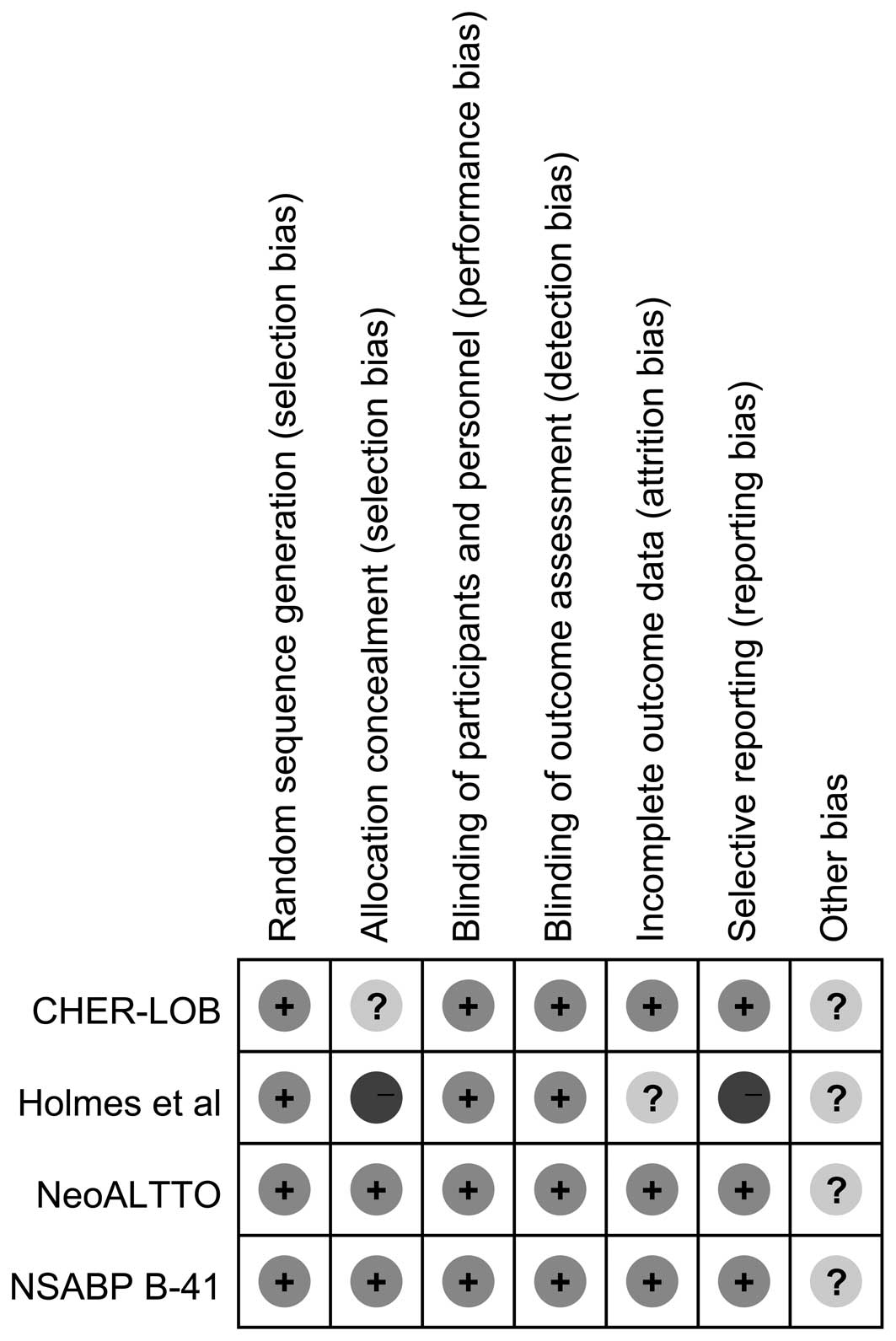
Risk of bias
Two studies (9,10) were
phase II clinical trials and two studies (11,12)
were phase III clinical trials. The pCR rate and the primary
adverse events were reported for all studies. According to the
Cochrane risk of bias tool, each risk of bias item for each RCT
included in the present study was assessed, and the results are
summarized in Fig. 2 and presented
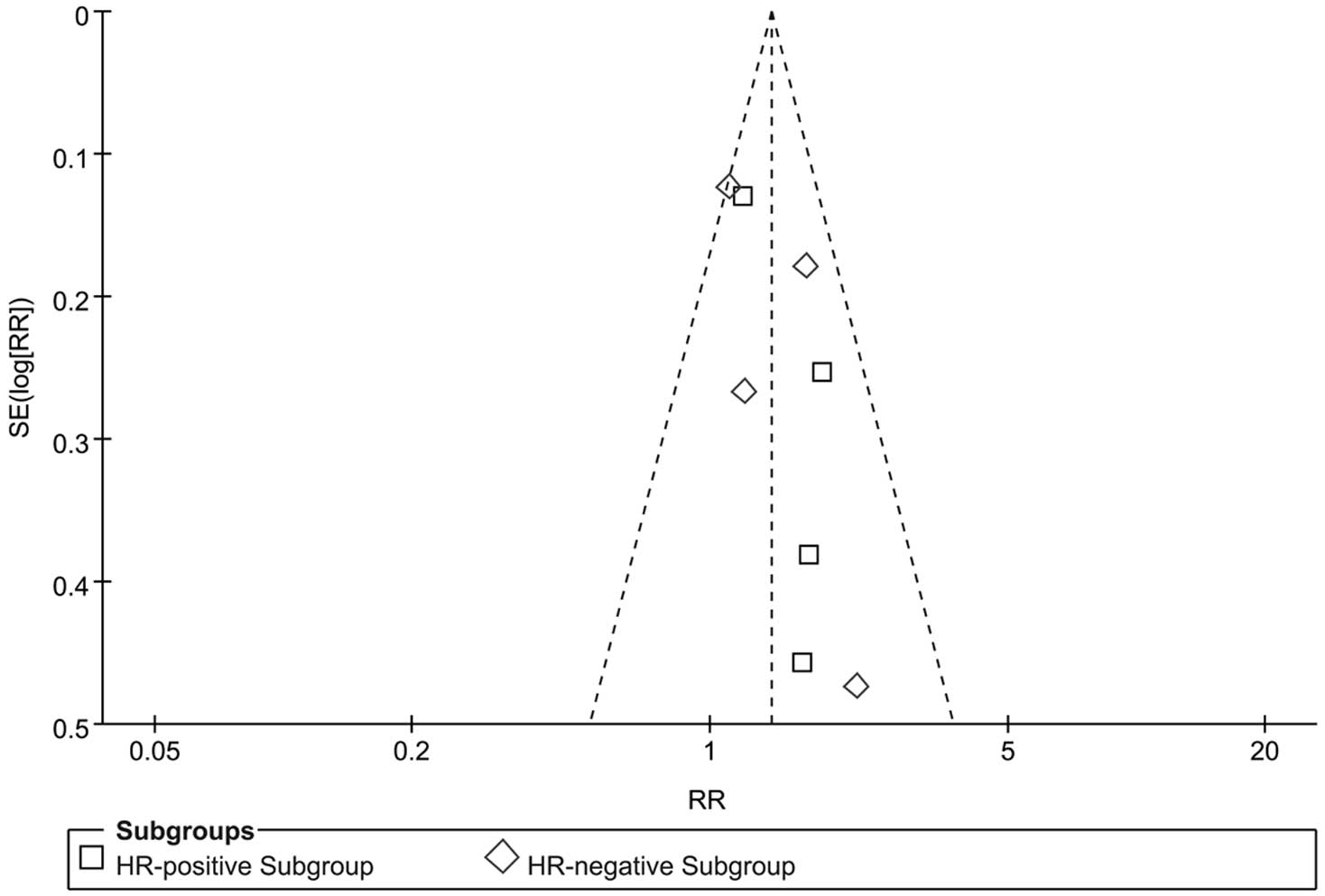
as percentages across all included studies in Fig. 3. In summary, the total risk of bias
was low. A funnel plot was drawn to assess the publication bias in
terms of pCR. A high risk of publication bias was not visually
identified (Fig. 4). This result
was confirmed by Begg’s (P=0.216) and Egger’s (P=0.122) tests.
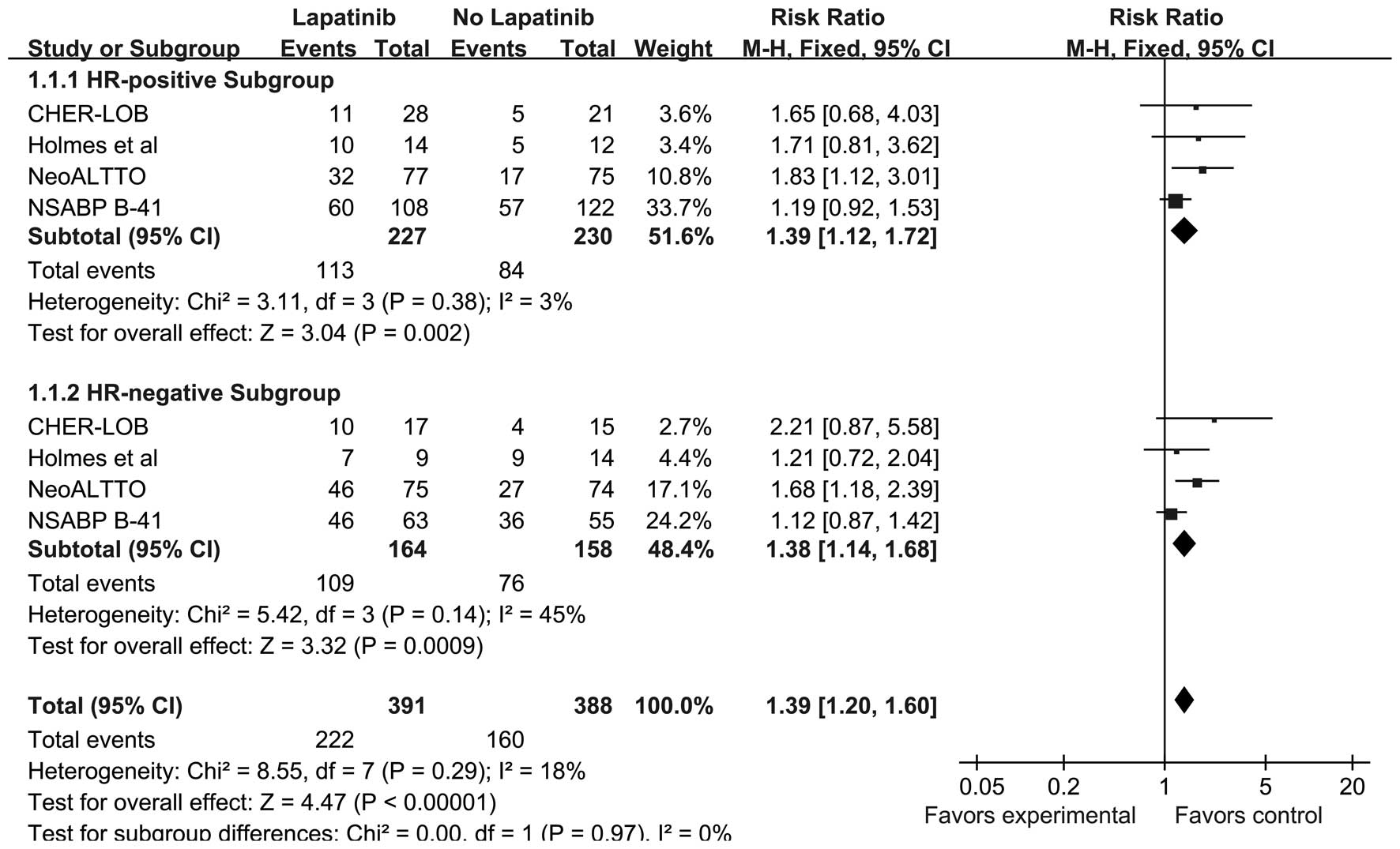
Overall effect of lapatinib on pCR
All four trials, including 779 patients, included
data for pCR. The absolute pCR rate was 56.78% (222 out of 391
patients) in the lapatinib group and 41.24% (160 out of 388
patients) in the no lapatinib group (RR, 1.39; 95% CI, 1.20–1.60;
P<0.0001). A subgroup meta-analysis was conducted using the HR
status. The probability of pCR was significantly higher in the
lapatinib group compared with the no lapatinib group for
HR-positive (RR, 1.39; 95% CI, 1.12–1.72; P=0.002) and HR-negative
(RR, 1.38; 95% CI, 1.14–1.68; P=0.0009) patients (Fig. 5). There was no significant
heterogeneity between the studies according to the subgroup
analysis (HR-positive, P=0.38 and I2=3%; HR-negative,
P=0.14 and I2=45%). No recurrence and survival analysis
was performed due to the short-term follow-up performed in the
assessed trials and a lack of the necessary data.
Overall effect of lapatinib on the BCS
rate and adverse events
Data on the number of patients that underwent BCS
was available in two trials, totaling 382 patients (9,11). No
difference was identified in terms of BCS between the two treatment
arms (RR, 1.14; 95% CI, 0.89–1.47; P=0.31).
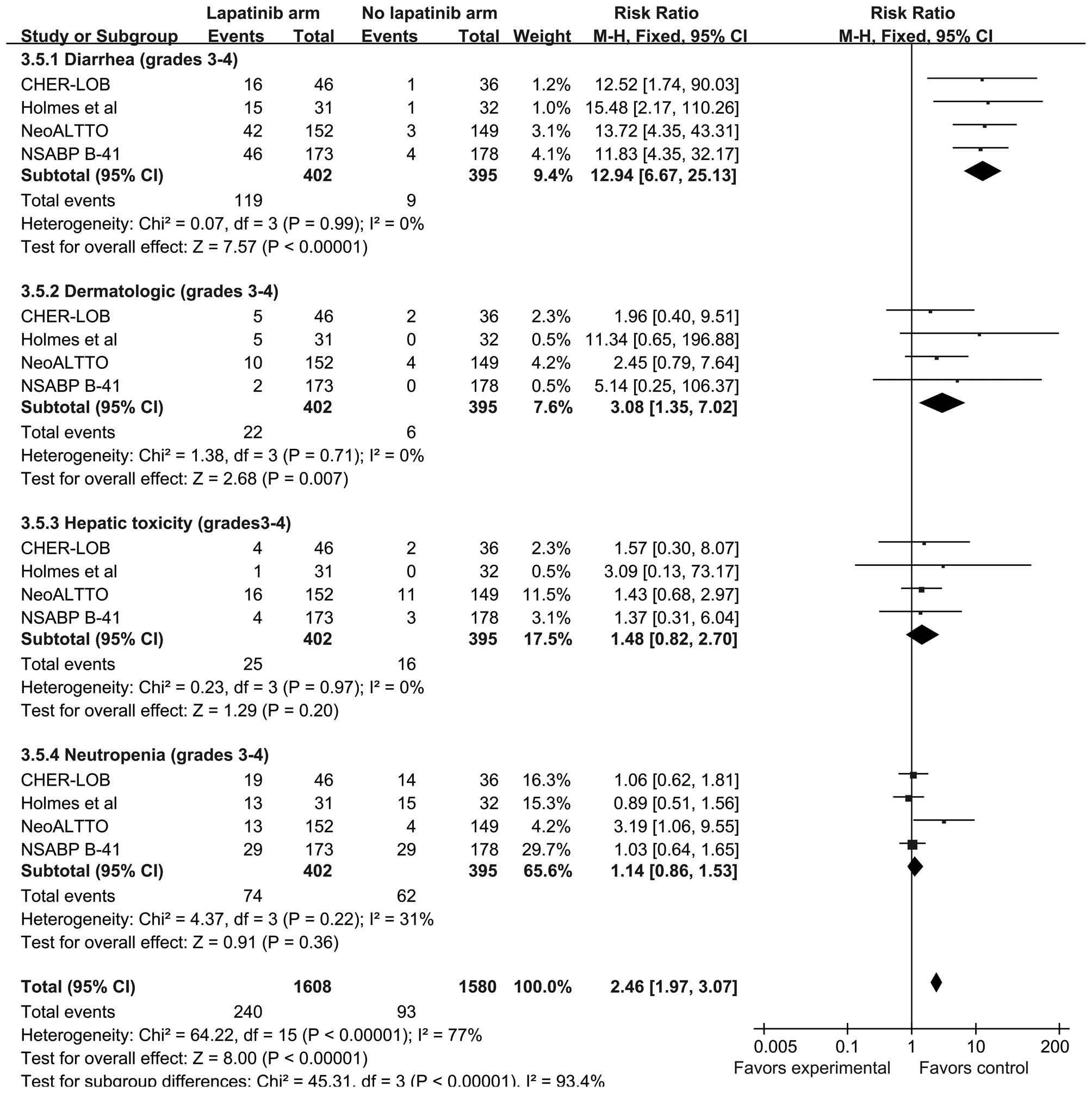
The primary adverse events, consisting of grade 3–4
diarrhea, dermatological toxicity, hepatic toxicity and
neutropenia, were reported in all four trials. The proportion of
patients that experienced primary adverse events was higher in the
lapatinib group compared with the no lapatinib group (RR 2.46; 95%
CI, 1.97–3.07; P<0.0001). The heterogeneity of each subgroup was
moderate, with all subgroups demonstrating
I2<40%.
The incidence of grade 3–4 diarrhea (RR, 12.94; 95%
CI, 6.67–25.13; P<0.0001) and grade 3–4 dermatological toxicity
(RR, 3.08; 95% CI, 1.35–7.02; P=0.007) was significantly higher in
the lapatinib group compared with the no lapatinib group. Despite
there being no significant difference between the groups, grade 3–4
hepatic toxicity and neutropenia appeared to occur more frequently
in the lapatinib group (Fig.
6).
The pooled RRs for additional adverse events and the
95% CI for the use of lapatinib in neoadjuvant chemotherapy versus
the use of no lapatinib, are reported in Table II. No statistically significant
differences were observed between the two groups, with the
exception of grade 3–4 vomiting.
 | Table IIPooled analysis of other adverse
events. |
Table II
Pooled analysis of other adverse
events.
| Adverse event | Number of
trials | Events, n/total
number of patients | RR | 95% CI | P-value | I2,
% |
|---|
|
|---|
| L arm | No L arm |
|---|
| Vomiting, grades
3–4 | 3 | 17/250 | 6/246 | 2.56 | 1.06–6.15 | 0.04 | 0 |
| Fatigue, grades
3–4 | 3 | 14/250 | 12/246 | 1.12 | 0.54–2.36 | 0.76 | 0 |
| Sensory neuropathy,
grades 3–4 | 3 | 11/250 | 6/246 | 1.82 | 0.71–4.67 | 0.21 | 0 |
| Mucositis, grades
3–4 | 2 | 3/219 | 2/214 | 1.31 | 0.27–6.24 | 0.74 | 31 |
| Febrile
neutropenia | 3 | 14/250 | 11/246 | 1.28 | 0.60–2.75 | 0.52 | 0 |
| Dyspnoea | 2 | 17/204 | 18/210 | 0.97 | 0.52–1.83 | 0.93 | 40 |
| Nausea, grades
3–4 | 2 | 9/204 | 2/210 | 3.91 | 0.99–15.53 | 0.05 | 0 |
| Dehydration | 2 | 10/204 | 6/210 | 1.66 | 0.64–4.34 | 0.30 | 0 |
| CHF | 3 | 2/341 | 7/326 | 0.50 | 0.03–9.48 | 0.65 | 59 |
| LVEF
declinea | 3 | 1/341 | 2/326 | 0.53 | 0.07–3.85 | 0.53 | 0 |
Discussion
The present study, with the inclusion of all
available RCTs regarding lapatinib combined to neoadjuvant therapy,
provides evidence that the addition of lapatinib to neoadjuvant
chemotherapy in HER2-positive breast cancer patients results in a
significant increase in the pCR rate. Neoadjuvant studies using
anti-HER2 agents have revealed that the pCR rate is correlated with
disease-free survival (16,17). The neo-adjuvant Herceptin study
(16), in which patients with
HER2-positive locally advanced or inflammatory breast cancer were
randomly allocated to the chemotherapy or chemotherapy plus
trastuzumab groups, demonstrated a doubling in the pCR rate in the
trastuzumab group compared with the chemotherapy group, and a
strong correlation between the pCR rate and event-free survival was
also identified. The Taxol epirubicin cyclophosphamide Herceptin
neoadjuvant study reported a correlation between pCR and improved
disease-free or overall survival (17). According to these previous studies,
lapatinib combined with neoadjuvant therapy is likely to improve
disease-free survival subsequent to additional follow-up.
The BCS rate demonstrated no statistically
significant difference between the lapatinib and no lapatinib
groups in the present study. However, the BCS rate is not an
appropriate candidate to assess treatment effect, as breast
conservation depends on several parameters, including tumor
location, presence of ductal carcinoma in situ, breast size,
contraindication to radiation therapy and patient willingness.
The use of lapatinib, as expected, was associated
with two well-documented adverse events, diarrhea and
dermatological toxicity (18),
despite the recommended dosage reduction for lapatinib (19), the grade 3–4 diarrhea and
dermatological toxicity were found to be statistically more
frequent in patients receiving lapatinib in the present study.
Notably, the administration of lapatinib in combination with
trastuzumab did not result in an increased risk of cardiotoxicity.
These results are similar to a previous meta-analysis assessing the
administration neoadjuvant chemotherapy containing trastuzumab
compared with no trastuzumab, in which a low cardiotoxicity was
observed (5).
Anti-HER2 therapy is the treatment of choice for
HER2-positive breast cancer. Dramatic clinical success has been
achieved by blocking the HER-2 signaling pathway in women with
breast cancer that overexpresses HER2 (20–22).
The previously reported neoadjuvant study of pertuzumab and
Herceptin in an early regimen evaluation trial of neoadjuvant
chemotherapy investigated dual HER2 blockade using a combination of
two anti-HER2 antibodies, trastuzumab and pertuzumab (23). This combination has also been found
to be highly effective as a first-line treatment for HER2-positive
metastatic breast cancer (24).
Similar to pertuzumab, lapatinib demonstrates a complementary
mechanism of action with trastuzumab, and the two exhibit a
synergistic effect in preclinical models (25). In addition, a more complete HER2
blockade appears to overcome certain mechanisms of resistance to
anti-HER2 agents (26,27). The effectiveness of dual blockade
with trastuzumab and lapatinib has been demonstrated in the
metastatic setting (28). For
HER2-positive breast cancer, a previous study also supported the
indication that a more complete blockade of HER receptors is an
effective strategy that requires further study (29).
A previous study by Valachis et al (30) compared the efficacy and safety of
the addition of lapatinib with the addition of trastuzumab, or a
combination of the two, to neoadjuvant chemotherapy in
HER2-positive breast cancer. This study included similar objectives
and results on this topic to the present study. However, Valachis
et al only analyzed two abstracts of congresses (31,32)
and two full clinical trials (9,11). Due
to the lack of adequate data, subgroup analyses and risk of bias
assessments were not performed. The present meta-analysis provided
more accurate information and identified certain different outcomes
in the adverse effects compared with the study by Valachis et
al.
The present meta-analysis possesses certain
limitations that require discussion. First, the number of studies
and the number of patients included in certain assessed outcomes
are relatively small, which affects the power of the meta-analysis
to reveal statistically significant results. Second, three trials
(9,11,12)
reported a separate pCR rate between breast tissue and breast
tissue plus axilla. Only the pCR rate in the breast tissue plus
axilla was analyzed in the present study. However, all the
available randomized studies and supplementary information
(10) on the topic were
systematically identified.
In conclusion, based on the available evidence, the
present study revealed that the administration of lapatinib in
HER2-positive breast cancer in the neoadjuvant setting improves the
probability of achieving a higher pCR rate, but is also associated
with an increased risk of toxicity. However, the data in the
literature remains to be limited. Therefore, additional follow-ups
and standard randomized trials should be conducted to elucidate the
role of lapatinib in neoadjuvant therapy in patients with
HER2-positive breast cancer.
References
|
1
|
Mauri D, Pavlidis N and Ioannidis JP:
Neoadjuvant versus adjuvant systemic treatment in breast cancer: a
meta-analysis. J Natl Cancer Inst. 97:188–194. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chia S, Swain SM, Byrd DR and Mankoff DA:
Locally advanced and inflammatory breast cancer. J Clin Oncol.
26:786–790. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fisher B, Bryant J, Wolmark N, et al:
Effect of preoperative chemotherapy on the outcome of women with
operable breast cancer. J Clin Oncol. 16:2672–2685. 1998.PubMed/NCBI
|
|
4
|
Kuerer HM, Newman LA, Smith TL, et al:
Clinical course of breast cancer patients with complete pathologic
primary tumor and axillary lymph node response to doxorubicin-based
neoadjuvant chemotherapy. J Clin Oncol. 17:460–469. 1999.PubMed/NCBI
|
|
5
|
Valachis A, Mauri D, Polyzos NP, et al:
Trastuzumab combined to neoadjuvant chemotherapy in patients with
HER2-positive breast cancer: a systematic review and meta-analysis.
Breast. 20:485–490. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Xia W, Mullin RJ, Keith BR, et al:
Anti-tumor activity of GW572016: a dual tyrosine kinase inhibitor
blocks EGF activation of EGFR/erbB2 and downstream Erk1/2 and AKT
pathways. Oncogene. 21:6255–6263. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rusnak DW, Lackey K, Affleck K, et al: The
effects of the novel, reversible epidermal growth factor
receptor/ErbB-2 tyrosine kinase inhibitor, GW2016, on the growth of
human normal and tumor-derived cell lines in vitro and in vivo. Mol
Cancer Ther. 1:85–94. 2001.
|
|
8
|
Amir E, Ocaña A, Seruga B, Freedman O and
Clemons M: Lapatinib and HER2 status: results of a meta-analysis of
randomized phase III trials in metastatic breast cancer. Cancer
Treat Rev. 36:410–415. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Guarneri V, Frassoldati A, Bottini A, et
al: Preoperative chemotherapy plus trastuzumab, lapatinib, or both
in human epidermal growth factor receptor 2-positive operable
breast cancer: results of the randomized phase II CHER-LOB study. J
Clin Oncol. 30:1989–1995. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Holmes FA, Espina V, Liotta LA, et al:
Pathologic complete response after preoperative anti-HER2 therapy
correlates with alterations in PTEN, FOXO, phosphorylated Stat5,
and autophagy protein signaling. BMC Res Notes. 6:5072013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Baselga J, Bradbury I, Eidtmann H, et al:
NeoALTTO Study Team: Lapatinib with trastuzumab for HER2-positive
early breast cancer (NeoALTTO): a randomised, open-label,
multicentre, phase 3 trial. Lancet. 379:633–640. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Robidoux A, Tang G, Rastogi P, et al:
Lapatinib as a component of neoadjuvant therapy for HER2-positive
operable breast cancer (NSABP protocol B-41): an open-label,
randomised phase 3 trial. Lancet Oncol. 14:1183–1192. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Higgins JP, Altman DG, Gøtzsche PC, et al:
Cochrane Bias Methods Group; Cochrane Statistical Methods Group:
The Cochrane Collaboration’s tool for assessing risk of bias in
randomised trials. BMJ. 343:d59282011. View Article : Google Scholar
|
|
14
|
Egger M, Davey Smith G, Schneider M and
Minder C: Bias in meta-analysis detected by a simple, graphical
test. BMJ. 315:629–634. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Begg CB and Mazumdar M: Operating
characteristics of a rank correlation test for publication bias.
Biometrics. 50:1088–1101. 1994. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gianni L, Eiermann W, Semiglazov V, et al:
Neoadjuvant chemotherapy with trastuzumab followed by adjuvant
trastuzumab versus neoadjuvant chemotherapy alone, in patients with
HER2-positive locally advanced breast cancer (the NOAH trial): a
randomised controlled superiority trial with a parallel
HER2-negative cohort. Lancet. 375:377–384. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Untch M, Fasching PA, Konecny GE, et al:
Pathologic complete response after neoadjuvant chemotherapy plus
trastuzumab predicts favorable survival in human epidermal growth
factor receptor 2-overexpressing breast cancer: results from the
TECHNO trial of the AGO and GBG study groups. J Clin Oncol.
29:3351–3357. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Moy B and Goss PE: Lapatinib-associated
toxicity and practical management recommendations. Oncologist.
12:756–765. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dang C, Lin N, Moy B, et al: Dose-dense
doxorubicin and cyclophosphamide followed by weekly paclitaxel with
trastuzumab and lapatinib in HER2/neu-overexpressed/amplified
breast cancer is not feasible because of excessive diarrhea. J Clin
Oncol. 28:2982–2988. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Slamon DJ, Leyland-Jones B, Shak S, et al:
Use of chemotherapy plus a monoclonal antibody against HER2 for
metastatic breast cancer that overexpresses HER2. N Engl J Med.
344:783–792. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Romond EH, Perez EA, Bryant J, et al:
Trastuzumab plus adjuvant chemotherapy for operable HER2-positive
breast cancer. N Engl J Med. 353:1673–1684. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Piccart-Gebhart MJ, Procter M,
Leyland-Jones B, et al: Trastuzumab after adjuvant chemotherapy in
HER2-positive breast cancer. N Engl J Med. 353:1659–1672. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gianni L, Pienkowski T, Im YH, et al:
Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in
women with locally advanced, inflammatory, or early HER2-positive
breast cancer (NeoSphere): a randomised multicentre, open-label,
phase 2 trial. Lancet Oncol. 13:25–32. 2012. View Article : Google Scholar
|
|
24
|
Baselga J, Cortés J, Kim SB, et al:
CLEOPATRA Study Group: Pertuzumab plus trastuzumab plus docetaxel
for metastatic breast cancer. N Engl J Med. 366:109–119. 2012.
View Article : Google Scholar
|
|
25
|
Konecny GE, Pegram MD, Venkatesan N, et
al: Activity of the dual kinase inhibitor lapatinib (GW572016)
against HER-2-overexpressing and trastuzumab-treated breast cancer
cells. Cancer Res. 66:1630–1639. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang YC, Morrison G, Gillihan R, et al:
Different mechanisms for resistance to trastuzumab versus lapatinib
in HER2-positive breast cancers - role of estrogen receptor and
HER2 reactivation. Breast Cancer Res. 13:R1212011. View Article : Google Scholar
|
|
27
|
Katiyar S, Kufareva I, Behera R, et al:
Lapatinib-binding protein kinases in the African trypanosome:
identification of cellular targets for kinase-directed chemical
scaffolds. PLoS One. 8:e561502013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Blackwell KL, Burstein HJ, Storniolo AM,
et al: Overall survival benefit with lapatinib in combination with
trastuzumab for patients with human epidermal growth factor
receptor 2-positive metastatic breast cancer: final results from
the EGF104900 Study. J Clin Oncol. 30:2585–2592. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rimawi MF, Mayer IA, Forero A, et al:
Multicenter phase II study of neoadjuvant lapatinib and trastuzumab
with hormonal therapy and without chemotherapy in patients with
human epidermal growth factor receptor 2-overexpressing breast
cancer: TBCRC 006. J Clin Oncol. 31:1726–1731. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Valachis A, Nearchou A, Lind P and Mauri
D: Lapatinib, trastuzumab or the combination added to preoperative
chemotherapy for breast cancer: a meta-analysis of randomized
evidence. Breast Cancer Res Treat. 135:655–662. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Robidoux A, Tang G, Rastogi P, et al:
Lapatinib as a component of neoadjuvant therapy for HER2-positive
operable breast cancer (NSABP protocol B-41): an open-label,
randomised phase 3 trial. Lancet Oncol. 14:1183–92. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Holmes FA, Nagarwala YM, Espina VA, et al:
Correlation of molecular effects and pathologic complete response
to preoperative lapatinib and trastuzumab, separately and combined
prior to neoadjuvant breast cancer chemotherapy. J Clin Oncol
(Meeting Abstracts). 29:5062011.
|