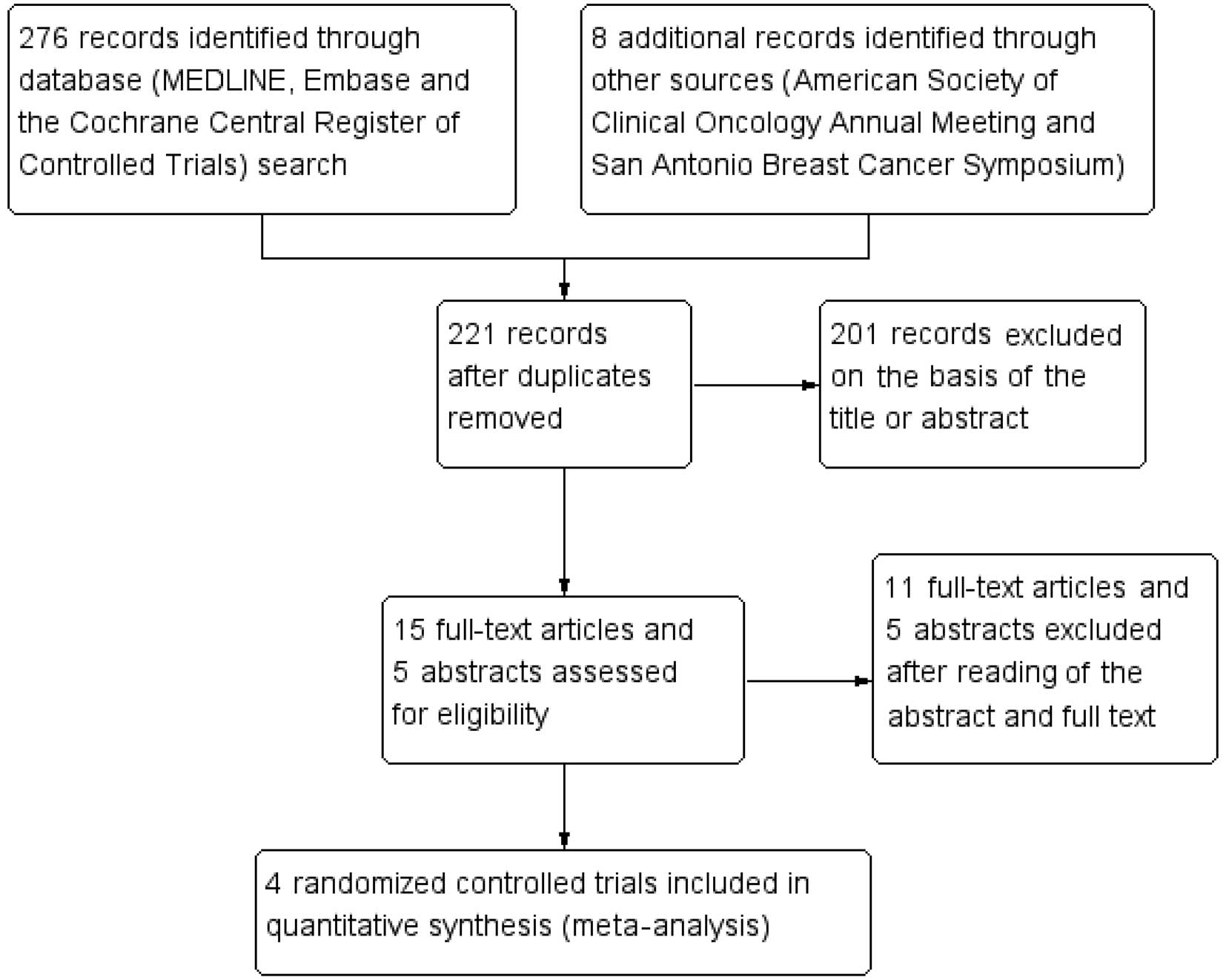
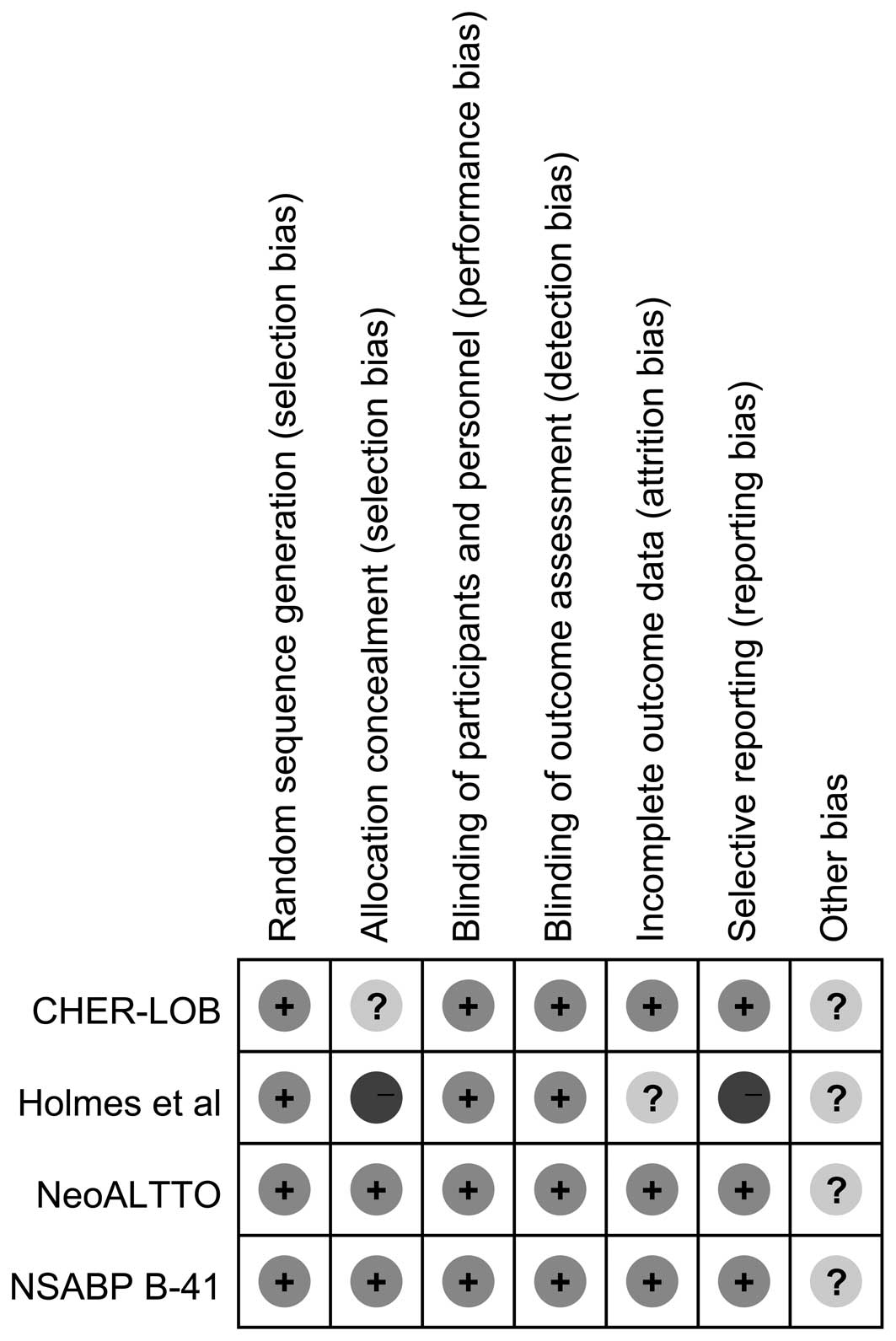
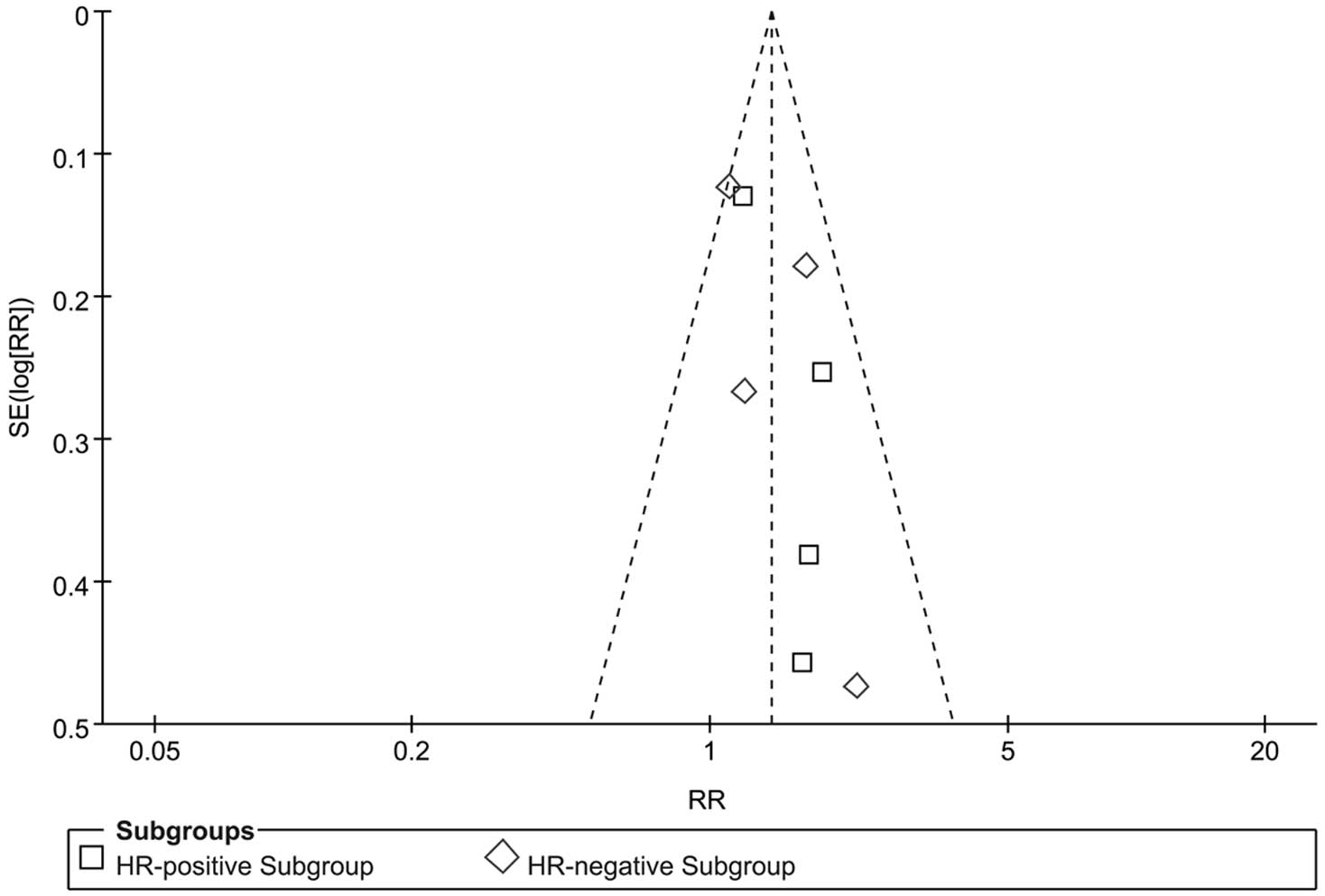
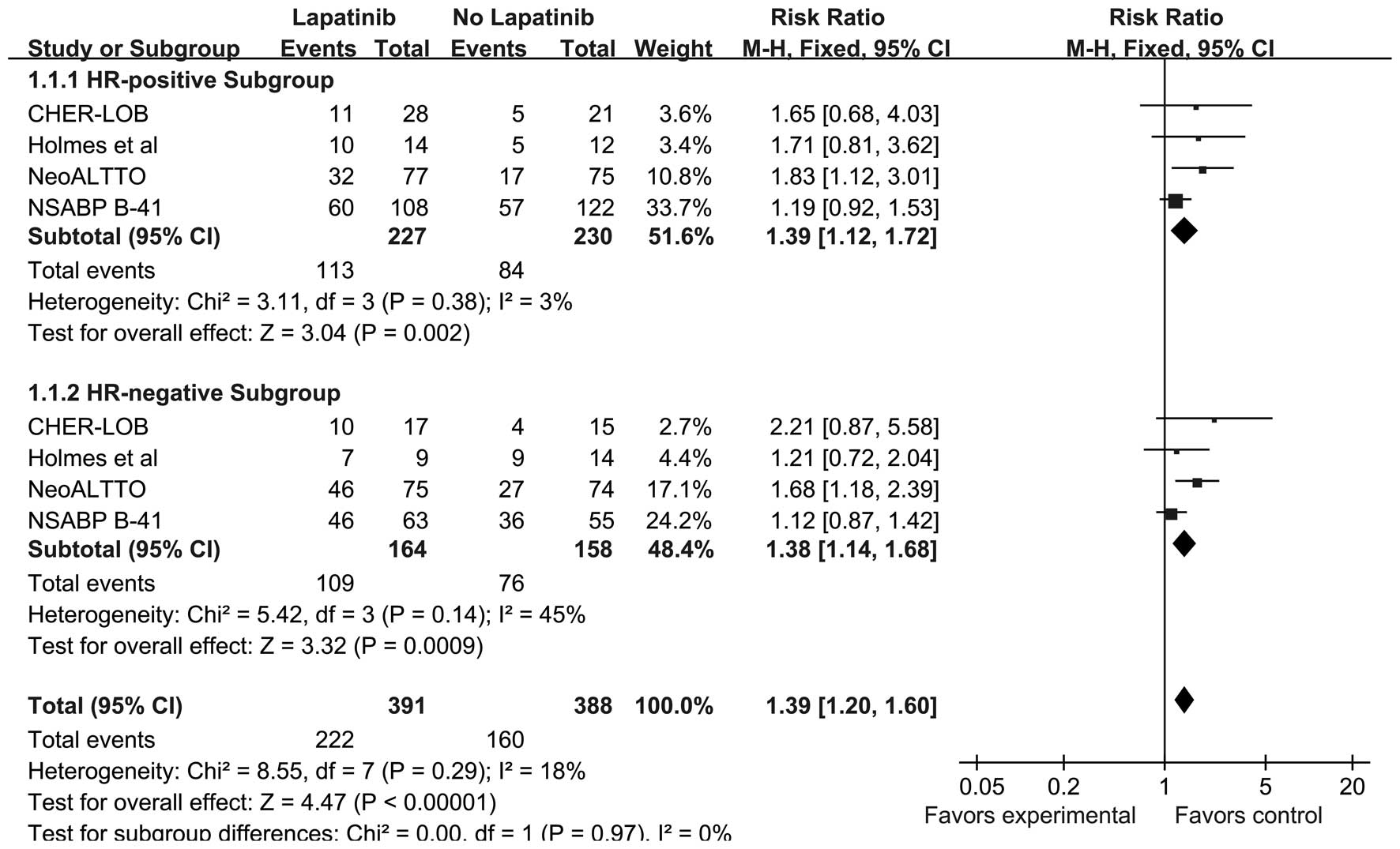
|
1
|
Mauri D, Pavlidis N and Ioannidis JP:
Neoadjuvant versus adjuvant systemic treatment in breast cancer: a
meta-analysis. J Natl Cancer Inst. 97:188–194. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chia S, Swain SM, Byrd DR and Mankoff DA:
Locally advanced and inflammatory breast cancer. J Clin Oncol.
26:786–790. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fisher B, Bryant J, Wolmark N, et al:
Effect of preoperative chemotherapy on the outcome of women with
operable breast cancer. J Clin Oncol. 16:2672–2685. 1998.PubMed/NCBI
|
|
4
|
Kuerer HM, Newman LA, Smith TL, et al:
Clinical course of breast cancer patients with complete pathologic
primary tumor and axillary lymph node response to doxorubicin-based
neoadjuvant chemotherapy. J Clin Oncol. 17:460–469. 1999.PubMed/NCBI
|
|
5
|
Valachis A, Mauri D, Polyzos NP, et al:
Trastuzumab combined to neoadjuvant chemotherapy in patients with
HER2-positive breast cancer: a systematic review and meta-analysis.
Breast. 20:485–490. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Xia W, Mullin RJ, Keith BR, et al:
Anti-tumor activity of GW572016: a dual tyrosine kinase inhibitor
blocks EGF activation of EGFR/erbB2 and downstream Erk1/2 and AKT
pathways. Oncogene. 21:6255–6263. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rusnak DW, Lackey K, Affleck K, et al: The
effects of the novel, reversible epidermal growth factor
receptor/ErbB-2 tyrosine kinase inhibitor, GW2016, on the growth of
human normal and tumor-derived cell lines in vitro and in vivo. Mol
Cancer Ther. 1:85–94. 2001.
|
|
8
|
Amir E, Ocaña A, Seruga B, Freedman O and
Clemons M: Lapatinib and HER2 status: results of a meta-analysis of
randomized phase III trials in metastatic breast cancer. Cancer
Treat Rev. 36:410–415. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Guarneri V, Frassoldati A, Bottini A, et
al: Preoperative chemotherapy plus trastuzumab, lapatinib, or both
in human epidermal growth factor receptor 2-positive operable
breast cancer: results of the randomized phase II CHER-LOB study. J
Clin Oncol. 30:1989–1995. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Holmes FA, Espina V, Liotta LA, et al:
Pathologic complete response after preoperative anti-HER2 therapy
correlates with alterations in PTEN, FOXO, phosphorylated Stat5,
and autophagy protein signaling. BMC Res Notes. 6:5072013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Baselga J, Bradbury I, Eidtmann H, et al:
NeoALTTO Study Team: Lapatinib with trastuzumab for HER2-positive
early breast cancer (NeoALTTO): a randomised, open-label,
multicentre, phase 3 trial. Lancet. 379:633–640. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Robidoux A, Tang G, Rastogi P, et al:
Lapatinib as a component of neoadjuvant therapy for HER2-positive
operable breast cancer (NSABP protocol B-41): an open-label,
randomised phase 3 trial. Lancet Oncol. 14:1183–1192. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Higgins JP, Altman DG, Gøtzsche PC, et al:
Cochrane Bias Methods Group; Cochrane Statistical Methods Group:
The Cochrane Collaboration’s tool for assessing risk of bias in
randomised trials. BMJ. 343:d59282011. View Article : Google Scholar
|
|
14
|
Egger M, Davey Smith G, Schneider M and
Minder C: Bias in meta-analysis detected by a simple, graphical
test. BMJ. 315:629–634. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Begg CB and Mazumdar M: Operating
characteristics of a rank correlation test for publication bias.
Biometrics. 50:1088–1101. 1994. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gianni L, Eiermann W, Semiglazov V, et al:
Neoadjuvant chemotherapy with trastuzumab followed by adjuvant
trastuzumab versus neoadjuvant chemotherapy alone, in patients with
HER2-positive locally advanced breast cancer (the NOAH trial): a
randomised controlled superiority trial with a parallel
HER2-negative cohort. Lancet. 375:377–384. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Untch M, Fasching PA, Konecny GE, et al:
Pathologic complete response after neoadjuvant chemotherapy plus
trastuzumab predicts favorable survival in human epidermal growth
factor receptor 2-overexpressing breast cancer: results from the
TECHNO trial of the AGO and GBG study groups. J Clin Oncol.
29:3351–3357. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Moy B and Goss PE: Lapatinib-associated
toxicity and practical management recommendations. Oncologist.
12:756–765. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dang C, Lin N, Moy B, et al: Dose-dense
doxorubicin and cyclophosphamide followed by weekly paclitaxel with
trastuzumab and lapatinib in HER2/neu-overexpressed/amplified
breast cancer is not feasible because of excessive diarrhea. J Clin
Oncol. 28:2982–2988. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Slamon DJ, Leyland-Jones B, Shak S, et al:
Use of chemotherapy plus a monoclonal antibody against HER2 for
metastatic breast cancer that overexpresses HER2. N Engl J Med.
344:783–792. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Romond EH, Perez EA, Bryant J, et al:
Trastuzumab plus adjuvant chemotherapy for operable HER2-positive
breast cancer. N Engl J Med. 353:1673–1684. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Piccart-Gebhart MJ, Procter M,
Leyland-Jones B, et al: Trastuzumab after adjuvant chemotherapy in
HER2-positive breast cancer. N Engl J Med. 353:1659–1672. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gianni L, Pienkowski T, Im YH, et al:
Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in
women with locally advanced, inflammatory, or early HER2-positive
breast cancer (NeoSphere): a randomised multicentre, open-label,
phase 2 trial. Lancet Oncol. 13:25–32. 2012. View Article : Google Scholar
|
|
24
|
Baselga J, Cortés J, Kim SB, et al:
CLEOPATRA Study Group: Pertuzumab plus trastuzumab plus docetaxel
for metastatic breast cancer. N Engl J Med. 366:109–119. 2012.
View Article : Google Scholar
|
|
25
|
Konecny GE, Pegram MD, Venkatesan N, et
al: Activity of the dual kinase inhibitor lapatinib (GW572016)
against HER-2-overexpressing and trastuzumab-treated breast cancer
cells. Cancer Res. 66:1630–1639. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang YC, Morrison G, Gillihan R, et al:
Different mechanisms for resistance to trastuzumab versus lapatinib
in HER2-positive breast cancers - role of estrogen receptor and
HER2 reactivation. Breast Cancer Res. 13:R1212011. View Article : Google Scholar
|
|
27
|
Katiyar S, Kufareva I, Behera R, et al:
Lapatinib-binding protein kinases in the African trypanosome:
identification of cellular targets for kinase-directed chemical
scaffolds. PLoS One. 8:e561502013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Blackwell KL, Burstein HJ, Storniolo AM,
et al: Overall survival benefit with lapatinib in combination with
trastuzumab for patients with human epidermal growth factor
receptor 2-positive metastatic breast cancer: final results from
the EGF104900 Study. J Clin Oncol. 30:2585–2592. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rimawi MF, Mayer IA, Forero A, et al:
Multicenter phase II study of neoadjuvant lapatinib and trastuzumab
with hormonal therapy and without chemotherapy in patients with
human epidermal growth factor receptor 2-overexpressing breast
cancer: TBCRC 006. J Clin Oncol. 31:1726–1731. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Valachis A, Nearchou A, Lind P and Mauri
D: Lapatinib, trastuzumab or the combination added to preoperative
chemotherapy for breast cancer: a meta-analysis of randomized
evidence. Breast Cancer Res Treat. 135:655–662. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Robidoux A, Tang G, Rastogi P, et al:
Lapatinib as a component of neoadjuvant therapy for HER2-positive
operable breast cancer (NSABP protocol B-41): an open-label,
randomised phase 3 trial. Lancet Oncol. 14:1183–92. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Holmes FA, Nagarwala YM, Espina VA, et al:
Correlation of molecular effects and pathologic complete response
to preoperative lapatinib and trastuzumab, separately and combined
prior to neoadjuvant breast cancer chemotherapy. J Clin Oncol
(Meeting Abstracts). 29:5062011.
|