Introduction
Sarcoidosis is a systemic disorder of unknown
etiology that is characterized by the widespread development of
non-caseating epithelioid cell granulomas in multiple organ systems
(1). The widely accepted pathogenic
hypothesis is that sarcoidosis is promoted in genetically
susceptible individuals by environmental factors (2). Non-caseating granulomas consisting of
epithelioid cells and T lymphocytes are the characteristic lesions
of sarcoidosis (2). The association
between sarcoidosis and malignancy has been reported by several
studies with conflicting results; this is caused by the clinical
and radiographic features of the disease, which exhibit
similarities to malignancies such as lymphoma or lung cancer and
were occasionally in the absence of histological confirmation
(3,4). The present study reports a case of
sarcoidosis mimicking cancer metastasis that was present at the
time of the diagnosis of gastric cancer and discusses the
diagnostic process. The patient provided written informed
consent.
Case report
A 64-year-old female presented to the Lujiang County
Hospital (Hefei, China) with epigastric discomfort and a swallowing
disorder that had persisted for more than one month. During the
course of the disease, the symptoms of night sweating and weight
loss were also present, however, no fever, coughing, expectoration,
hemoptysis, nasal congestion, hematemesis or melena occurred. The
patient had previously suffered from esophageal erosion.
Gastroscopy revealed a gastric lesion that was located in the
cardia, and pathological analysis of the lesion revealed high-grade
intraepithelial neoplasia and local canceration. Bilateral hilar
widening and enlarging of multiple lymph nodes in the mediastinum
and hilum of the lung were revealed by chest X-ray and
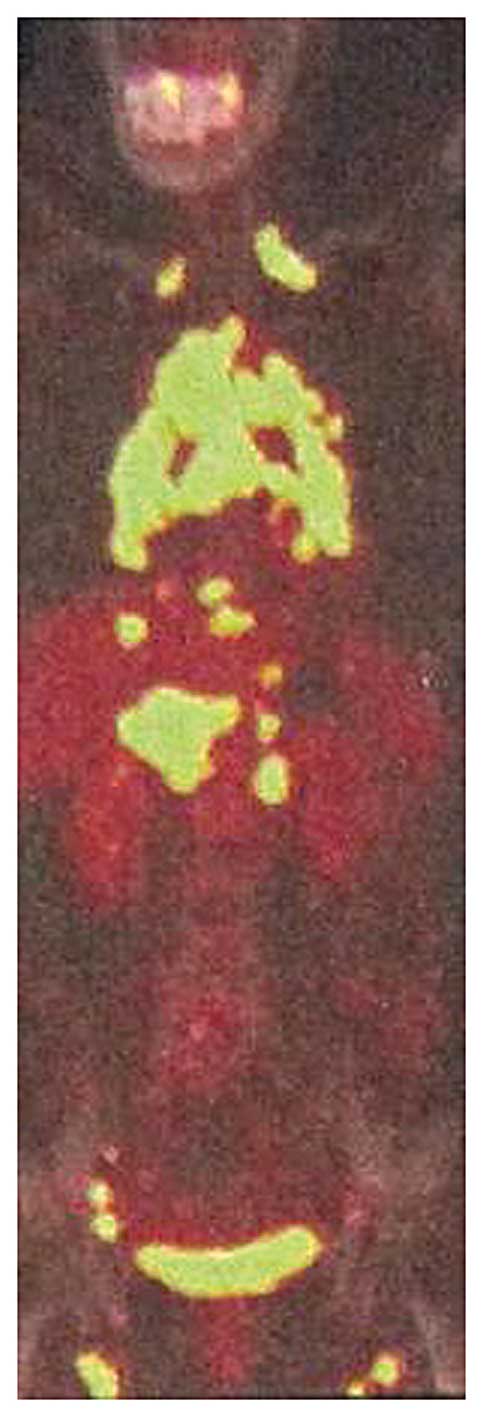
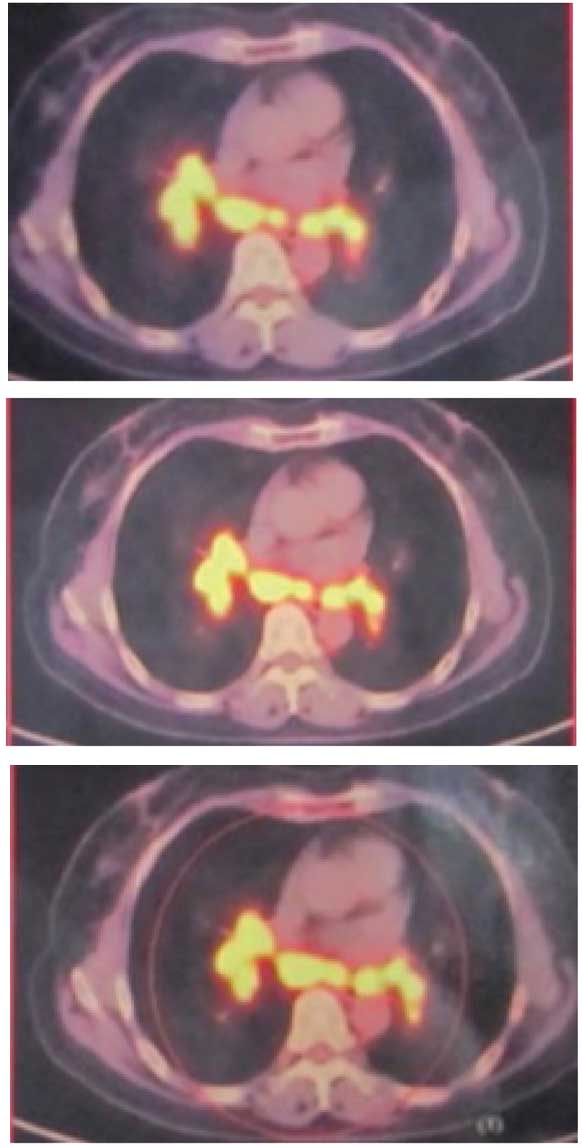
chest-abdomen computed tomography (CT), respectively. Positron
emission tomography (PET)/CT revealed that the fluorodeoxyglucose
metabolism of the lymph nodes in the bilateral supraclavicular,
mediastinal, hilar, retroperitoneal, pelvic and inguinal regions
had increased (Figs. 1 and 2). Based on these results, two types of
disease were considered, lymphoma and a rare digestive tract tumor
with multiple metastases. Lujiang County Hospital diagnosed the
lesion as advanced gastric cancer.
In order to confirm the diagnosis and undergo
treatment, the patient presented to the outpatient department of
the First Affiliated Hospital of Anhui Medical University (Hefei,
China). Upon physical examination, a tenacious lymph node, ~1.0×1.0
cm in size, was identified in the left supraclavicular root of the
neck and several lymph nodes in the right inguinal region. No other
lymph node swelling was observed in the superficial part of the
body. Laboratory investigation indicated that a routine blood
examination (white blood cells, 4.8×109 cells/l, normal
range, 4–10×109 cells/l; hemoglobin, 115 g/l, normal
range, 110–150 g/l; platelet count, 224×109 cells/l,
normal range, 100–300×109 cells/l), liver (aspartate
transaminase, 20 IU/l, normal range, 0–40 IU/l; alanine
transaminase, 22 IU/l, normal range, 0–40 IU/l) and kidney function
(blood urea nitrogen, 4.9 mmol/l, normal range, 2.1–7.9 mmol/l;
creatinine 85μmol/l, normal range, 44–133 μmol/l) tests were
normal, the serum calcium level was 2.75 mmol/l (normal range,
2.25–2.75 mmol/l) and the alkaline phosphatase level was 135 U/l
(normal range, 25–125 U/l). Fine needle aspiration biopsy was
performed on the left supraclavicular lymph node, and revealed
lymphoreticular hyperplasia, as epithelioid cells were present. Due
to the cytology results, a tuberculosis examination was performed,
but the tuberculin testing exhibited a negative result. The biopsy
of the right inguinal lymph node indicated granulomatous
infiltration with occasional multinucleated giant cells (Fig. 3).
The patient was referred to the Department of
General Surgery at the first Affiliated Hospital of Anhui Medical
University, having provided the appropriate informed consent, and
received radical total gastrectomy combined with a Roux-en-Y
procedure (5). A superficial
ulcerative mass was identified inside the small curved side below
the cardia and multiple enlarged lymph nodes were identified around
the stomach and liver duodenum ligament, the largest of which was
2.5×2.0 cm in diameter. The postoperative pathology indicated
erosive medium differentiated adenocarcinoma in the lesser gastric
curvature, ~2.0×1.5 cm in diameter. The cancer cells had invaded
the layer of muscularis mucosae and a number of cells had reached
the submucosa. In total, eight lymph nodes were dissected from the
lesser curvature, one from the greater curvature and 23 from other
areas surrounding the gastric region. The pathology of the total 32
lymph nodes indicated the presence of non-caseating granulomas
(Fig. 4A and B). Therefore, the
patient was diagnosed with gastric erosive medium-differentiated
adenocarcinoma (pT1N0M0) (6) and
sarcoidosis (I) (1). According to
the NCCN guidelines for gastric cancer staging result
determination, the patient did not receive chemotherapy (7). At the four-year checkup, the
sarcoidosis remained stable, and no recurrence of the cancer was
identified.
Discussion
The present study reports a rare case of sarcoidosis
that was present in a patient with gastric cancer at the time of
diagnosis, which highlights the complexity of the diagnostic
process. The present case identifies that clinical presentation and
radiological findings, including PET/CT, may not be able to
differentiate between cancer metastasis and sarcoidosis.
Sarcoidosis has been reported to imitate malignant
neoplasms and has been identified at the time of diagnosis and
during or immediately following chemotherapy, including in cases of
breast (8–10) and lung (11–13)
cancer, Hodgkin’s disease (14,15),
colorectal (16–18) and head and neck cancer (19,20),
melanoma (21–23) and ovarian cancer (24). Although cases of sarcoidosis
mimicking metastatic gastric cancer have been rarely reported,
Konishi et al (25) reported
a case of advanced gastric cancer with sarcoidosis and Blank et
al (26) revealed that four
patients with gastric cancer were identified in their cohort of 425
patients with sarcoidosis.
The association between sarcoidosis and malignancy
remains controversial. Brincker and Wilbek (27) first reported a statistically
significant increase in the incidence of malignant tumors among
patients with sarcoidosis in 1974. A large Japanese study followed
1,411 patients with sarcoidosis for three years and also observed
an increase in mortality from leukemia and uterine cancer, using a
standardized mortality risk (28).
A retrospective cohort study by Askling et al (29) analyzed two cohorts of patients with
sarcoidosis and identified an increased risk of lymphoma and lung,
liver, and skin cancer. Boffetta et al (30) reported that the risks of rectal,
colon and kidney cancer were increased in patients with sarcoidosis
in their cohort study. In addition, 1,045 out of a total of 10,037
hospitalized patients with sarcoidosis were reported to
subsequently develop cancer, with a significant proportion being
reported for skin (squamous cell), kidney and non-thyroid endocrine
tumors and additionally for non-Hodgkin’s lymphoma and leukemia
(31). Furthermore, in the Medical
Center of the University of Heidelberg (Heidelberg, Germany), 61
patients with malignant disease were identified in the cohort of
425 patients with sarcoidosis (26). However, other studies have
hypothesized that malignancy may actually precede the diagnosis of
sarcoidosis. Suen et al (32) reported six cases in which
sarcoidosis was diagnosed an average of nine months following the
development of malignancy and termed this phenomenon
malignancy-sarcoidosis syndrome. Despite the evidence suggesting
that these two entities may be linked, a number of studies have
challenged the existence of an association between sarcoidosis and
malignancy. Rømer et al (33) reviewed the cases of sarcoidosis and
malignancy that Brincker and Wilbek (27) had presented, finding that a number
of cases had been misclassified and identified no increased
occurrence of malignancy in patients with sarcoidosis. The
importance of misclassification was also reported by Seersholm
et al (34), who identified
misclassification in three out of 36 malignancies in 254 patients
with sarcoidosis.
Conflicting results among these various studies are
caused by the similarities in the clinical and radiological
features of sarcoidosis and malignancy and occasionally, the lack
of histological confirmation. PET/CT imaging is a valuable tool for
the diagnosis of cancer and for monitoring cancer metastasis as it
allows for the location of metabolically active malignant tissue to
be determined. However, the specificity of PET/CT is hampered by
non-oncological medical conditions, including sarcoidosis,
Wegener’s granulomatosis, chronic granulomatous disease, and
mycobacterial and aspergillus infections, where glucose consumption
may be observed, particularly in the cellular component of
inflammatory lesions. Therefore, it is important to obtain a
histological diagnosis prior to initiating antineoplastic therapy
based on the imaging findings.
Sarcoidosis must be considered in differential
diagnosis when a cancer patient develops diffuse lymph nodal
involvement. As the currently available imaging techniques fail to
formally distinguish metastasis from sarcoidosis, a pathological
diagnosis must be obtained wherever possible.
References
|
1
|
Newman LS, Rose CS and Maier LA:
Sarcoidosis. N Engl J Med. 336:1224–1234. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wu JJ and Schiff KR: Sarcoidosis. Am Fam
Physician. 70:312–322. 2004.PubMed/NCBI
|
|
3
|
Sato Y, Sasano S, Oyama K, Sakuraba M,
Onuki T and Nitta S: Lung cancer associated with sarcoidosis. Jpn J
Thorac Cardiovasc Surg. 51:21–24. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bouros D, Hatzakis K, Labrakis H and
Zeibecoglou K: Association of malignancy with diseases causing
interstitial pulmonary changes. Chest. 121:1278–1289. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Scott HW Jr and Weidner MG: Total
gastrectomy with Roux-en-Y esophagojejunostomy in treatment of
gastric cancer. Ann Surg. 143:682–696. 1956. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sobin LH, Gospodarowicz MK and Wittekind
C: TNM classification of malignant tumours. 7th edition. Wiley; New
York, NY: 2009
|
|
7
|
NCCN Guidelines version 1.2014 Panel
Members Gastric Cancer. https://www.nccn.org/store/login/login.aspx?ReturnURL=http://www.nccn.org/professionals/physician_gls/pdf/gastric.pdf.
Accessed May 30, 2014
|
|
8
|
Tolaney SM, Colson YL, Gill RR, et al:
Sarcoidosis mimicking metastatic breast cancer. Clin Breast Cancer.
7:804–810. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bush E, Lamonica D and O’Connor T:
Sarcoidosis mimicking metastatic breast cancer. Breast J.
17:533–535. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shin HC, Choe JW, Ryu HS, et al:
Sarcoidosis mimicking metastatic breast cancer in Korean woman with
breast cancer. Breast J. 20:198–199. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Maeda J, Ohta M, Hirabayashi H and Matsuda
H: False positive accumulation in 18F fluorodeoxyglucose positron
emission tomography scan due to sarcoid reaction following
induction chemotherapy for lung cancer. Jpn J Thorac Cardiovasc
Surg. 53:196–198. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Umezu H, Chida M, Inoue T, et al:
Sarcoidosis development during induction chemotherapy for lung
cancer mimicked progressive disease. Gen Thorac Cardiovasc Surg.
58:434–437. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kim JJ, Park JK, Wang YP, Choi SH and Jo
KH: Lung cancer associated with sarcoidosis -A case report-. Korean
J Thorac Cardiovasc Surg. 44:301–303. 2011. View Article : Google Scholar
|
|
14
|
Kyoraku Y, Ashitani J, Sakamoto A, Yanagi
S, Matsumoto N and Nakazato M: A case of Hodgkin’s lymphoma in a
patient with sarcoidosis. Nihon Kokyuki Gakkai Zasshi. 47:900–905.
2009.(In Japanese). PubMed/NCBI
|
|
15
|
Cherk MH, Pham A and Haydon A:
18F-fluorodeoxyglucose positron emission tomography-positive
sarcoidosis after chemoradiotherapy for Hodgkin’s disease: a case
report. J Med Case Rep. 29:2472011. View Article : Google Scholar
|
|
16
|
Kalff V, Hicks RJ, Ware RE, Hogg A, Binns
D and McKenzie AF: The clinical impact of (18)F-FDG PET in patients
with suspected or confirmed recurrence of colorectal cancer: a
prospective study. J Nucl Med. 43:492–499. 2002.PubMed/NCBI
|
|
17
|
Lequoy M, Coriat R, Rouquette A, et al:
Sarcoidosis lung nodules in colorectal cancer follow-up:
sarcoidosis or not? Am J Med. 126:642–645. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Choi JH, Shin JA, Park HK, et al:
Sarcoidosis associated with oxaliplatin-based chemotherapy for
colorectal cancer. Case Rep Oncol Med. 2014:2030272014.PubMed/NCBI
|
|
19
|
Yao M, Funk GF, Goldstein DP, DeYoung BR
and Graham MM: Benign lesions in cancer patients. Case 1
Sarcoidosis after chemoradiation for head and neck cancer. J Clin
Oncol. 23:640–641. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Arana Yi C, McCue P, Rosen M, Machtay M,
Axelrod R and Morris GJ: Sarcoidosis mimicking metastatic bone
disease in head and neck cancer. Semin Oncol. 40:529–534. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hendrickx BW, van Herpen CM, Bonenkamp JJ,
Bulten J and Oyen WJ: Positive positron emission tomography scan in
sarcoidosis and two challenging cases of metastatic cancer. CASE 1
Mediastinal sarcoidosis in a melanoma patient treated with
interferon. J Clin Oncol. 23:8906–8907. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wilgenhof S, Morlion V, Seghers AC, et al:
Sarcoidosis in a patient with metastatic melanoma sequentially
treated with anti-CTLA-4 monoclonal antibody and selective BRAF
Inhibitor. Anticancer Res. 32:1355–1359. 2012.PubMed/NCBI
|
|
23
|
Vogel WV, Guislain A, Kvistborg P,
Schumacher TN, Haanen JB and Blank CU: Ipilimumab-induced
sarcoidosis in a patient with metastatic melanoma undergoing
complete remission. J Clin Oncol. 30:e7–e10. 2012. View Article : Google Scholar
|
|
24
|
Kim MH, Lee K, Kim KU, Park HK, Lee MK and
Suh DS: Sarcoidosis mimicking cancer metastasis following
chemotherapy for ovarian cancer. Cancer Res Treat. 45:354–358.
2013. View Article : Google Scholar
|
|
25
|
Konishi H, Komatsu S, Ichikawa D, et al:
Diagnostic problems in gastric cancer patients with sarcoidosis -
case report and literature review. Gan To Kagaku Ryoho.
39:2330–2332. 2012.PubMed/NCBI
|
|
26
|
Blank N, Lorenz HM, Ho AD and
Witzens-Harig M: Sarcoidosis and the occurrence of malignant
diseases. Rheumatol Int. 2014.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Brincker H and Wilbek E: The incidence of
malignant tumors in patients with sarcoidosis. Ugeskr Laeger.
136:2192–2195. 1974.(In Danish). PubMed/NCBI
|
|
28
|
Yamaguchi M, Odaka M, Hosoda Y, Iwai K and
Tachibana T: Excess death of lung cancer among sarcoidosis
patients. Sarcoidosis. 8:51–55. 1991.PubMed/NCBI
|
|
29
|
Askling J, Grunewald J, Eklund A,
Hillerdal G and Ekbom A: Increased risk for cancer following
sarcoidosis. Am J Respir Crit Care Med. 160:1668–1672. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Boffetta P, Rabkin CS and Gridley G: A
cohort study of cancer among sarcoidosis patients. Int J Cancer.
124:2697–2700. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ji J, Shu X, Li X, Sundquist K, Sundquist
J and Hemminki K: Cancer risk in hospitalized sarcoidosis patients:
a follow-up study in Sweden. Ann Oncol. 20:1121–1126. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Suen JS, Forse MS, Hyland RH and Chan CK:
The malignancy-sarcoidosis syndrome. Chest. 98:1300–1302. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Rømer FK, Hommelgaard P and Schou G:
Sarcoidosis and cancer revisited: a long-term follow-up study of
555 Danish sarcoidosis patients. Eur Respir J. 12:906–912. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Seersholm N, Vestbo J and Viskum K: Risk
of malignant neoplasms in patients with pulmonary sarcoidosis.
Thorax. 52:892–894. 1997. View Article : Google Scholar : PubMed/NCBI
|