Introduction
Resveratrol (RES) exhibits anti-inflammatory and
anti-oxidant effects (1,2) and is a potential chemopreventive
agent, which is able to inhibit various stages of carcinogenesis,
including the initiation, promotion and progression of tumors
(3). RES has been found to induce
apoptotic cell death in various cancer cell lines and experimental
tumor models (4–7).
Previously, it has been reported that high doses of
RES (3 g/kg/day) cause moderate liver toxicity (8). However, the absorption of RES was
shown to be poor due to its water insoluble properties (9). Furthermore, the insolubility of RES
has been demonstrated to hamper the in vitro and in
vivo biological induction of RES activity (10). Research in nanomedicine has not only
become a frontier movement, but is also a revolutionary drug
delivery field. Bovine serum albumin (BSA) has been used as a
vehicle used for diagnostic and therapeutic agents (11,12),
as it is non-toxic, safe, economical and exhibits good
biocompatibility and no immunogenicity. Previous studies have
explored BSA as a drug carrier (13,14).
RES-BSA nanoparticles (RES-BSANP) are synthesized by
protein desolvation chemical crosslinking. Previous studies have
demonstrated the antiproliferative effects of RES on the growth of
human SKOV3 cell lines in in vivo and in vitro
studies (15–17). The prepared RES-BSANP exhibited an
altered distribution. In addition, it was demonstrated that
RES-BSANP-treated tumors exhibited a similar apoptotic index to RES
control tumors. The cells in the therapeutic groups exhibited
apoptotic and necrotic characteristics. The mechanism resulting in
these properties may be the release of cytochrome c (Cyto
c) and the upregulation of the dynamic expression of
caspase-9 and -3, indicating that the mitochondrial apoptotic
pathway was activated (16,17).
Programmed cell death (PCD) is a specific mechanism
that initiates cell death. Caspases are involved in PCD. PCD may be
divided into two types, caspase-dependent and caspase-independent
PCD, according to the involvement of caspase in PCD.
Caspase-dependent PCD presents typical apoptosis.
Caspase-independent PCD includes autophagy, paraptosis, mitotic
catastrophe, apoptosis-like PCD and necrosis-like PCD.
RES-BSANP has been found to inhibit various stages
of tumor growth, however, the molecular mechanism of its anticancer
activity remains unclear, particularly in ovarian cancer. The aim
of the present study was to elucidate the molecular events that
occur during RES-BSANP-induced apoptotic cell death in human
ovarian SKOV3 cells.
Materials and methods
Reagents
The human ovarian cancer SKOV3 cell line was
obtained from the Tumor Research Institute of Harbin Medical
University (Harbin Medical University, Harbin, China). RES was
purchased from Xian Huacui Biology Co., Ltd. (Xian, China),
(purity, ≥99.9%) and dissolved in dimethyl sulfoxide (DMSO) as a
stock solution of 100 mmol/l. RES was further diluted in Dulbecco’s
modified Eagle’s medium (DMEM) with 10% fetal bovine serum (FBS) to
the appropriate final concentrations. RES-BSANP was prepared at the
Life Science Laboratory of Northeast Forestry University (Harbin,
China).
The general caspase inhibitor, Z-VAD-FMK
[Z-Val-Ala-Asp(OMe)-CH2F], and caspase-9 [Z-LEHD-FMK,
Z-Leu-Glu(OMe)-His-Asp(OMe)-CH2F] were obtained from
Calbiochem (La Jolla, CA, USA). Stock solutions of the caspase
inhibitors (10 mmol/l each) were prepared in DMSO and diluted in
DMEM with 10% FBS to a final concentration of 100 μmol/l.
Polyclonal rabbit anti-rat antibodies against apoptosis-inducing
factor (AIF), Cyto c and B-cell lymphoma 2
(Bcl-2)-associated X protein (Bax) were obtained from Beijing
Zhongshan Golden Bridge Biotechnology Co., Ltd. (Beijing, China).
DMEM, penicillin (1 mg/ml) and streptomycin (1 mg/ml) were obtained
from Invitrogen Life Technologies (Carlsbad, CA, USA) and FBS was
purchased from HyClone Laboratories, Inc.,(Logan, UT, USA). The
2.5% trypsin/EDTA solution was purchased from Invitrogen Life
Technologies and diluted to 0.5% for trypsinizing attached
cells.
Cell culture
The ovarian cancer SKOV3 cell line was cultured in
RPMI 1640 (Gibco-BRL, Carlsbad, CA, USA) supplemented with 10%
heat-inactivated fetal calf serum (FCS; Gibco-BRL) in a humidified
incubator at 37°C, with an atmosphere of 5% CO2. A total
of 2.5 g/l trypsin and 0.2 g/l EDTA were used for subculture.
Morphological study
To evaluate apoptotic cell death, the cells were
seeded at a density of 5×105 cells/ml in 15-mm diameter
wells and incubated for 12 h until the cells had adhered to the
bottom of the wells. DNA ladder formation was examined an hour
following the start of treatment with the compounds. For
morphological examination of the apoptotic changes, the cells were
stained with Hoechst 33342 (5 μg/ml; Invitrogen Life Technologies)
at 37°C for 30 min, washed twice with phosphate-buffered saline,
pipetted drop-wise onto a glass slide, and examined by fluorescence
microscopy using an Olympus microscope (Olympus Corporation, Tokyo,
Japan) equipped with an epi-illuminator and appropriate
filters.
DNA fragmentation
DNA fragmentation was conducted as previously
described (18). Following various
treatments, the cells were collected and lysed with lysis buffer
[0.5% Triton X-100, 5 mmol/l Tris Buffer (pH 7.4), 20 mmol/l EDTA].
RNA was removed by incubation with RNase A (0.8 mg/ml) at 37°C for
30 min. DNA was extracted by phenol/chloroform and precipitated
with 1/10 volume of 3 mol/l sodium acetate (pH 5.2) along with 20μl
of 100% ethanol. DNA pellets obtained by centrifugation at 10,000 ×
g for 20 min at 4°C, were dried and re-suspended in 25 ml 1xTAE (40
mmol/l Tris-acetate and 1 mmol/l EDTA). Samples were then separated
on 2% agarose gels and the DNA ladder was detected by incubation of
the gels with ethidium bromide (1 g/ml) for 20 min followed by
de-staining with distilled H2O.
Inhibition of apoptosis by the
pan-caspase inhibitor
The tripeptide pan-caspase inhibitor, Z-VAD-FMK was
added 12 h prior to treatment with RES-BANP. The optimal
concentration of the inhibitor was determined from a dose-response
curve using the extent of cell death. The inhibition of apoptosis
by Z-VAD-FMK was evaluated by investigating the inhibition of
nucleosomal DNA fragmentation, which was observed as DNA ladder
formation.
Western blot analysis
Western blot analysis was performed to detect the
protein expression of AIF, Cyto c and Bax. Cell lysate was
prepared by lysis buffer protein extraction [40 mmol/l Tris-Cl (pH
8.0), 120 mmol/l NaCl and 0.1% NP40] supplemented with protease
inhibitors. Proteins were separated by SDS-PAGE and transferred to
nitrocellulose membranes (Bio-Rad, Hercules, CA, USA). The
membranes were blocked with 5% skimmed milk in Tris-buffered saline
and incubated with the appropriate primary antibodies for 1 h at
room temperature. The blots were developed with
peroxidase-conjugated secondary antibody, and the proteins were
visualized with an enhanced chemiluminescence kit (GE Healthcare,
Piscataway, NJ, USA) according to the manufacturer’s
instructions.
Statistical analysis
Results are expressed as the mean ± standard
deviation. For multiple comparisons, results were analyzed by
one-way analysis of variance. The least significant difference was
analyzed by the Bonferroni correction test to identify significant
differences between the individual cell groups. P<0.05 was
considered to indicate a statistically significant difference. All
statistical analyses were performed using SPSS software, version
11.5 (SPSS, Inc., Chicago, IL, USA).
Results
Cell detection via microscopic
observations
Following observation under an inverted phase
contrast microscope (IX73-F22FL/PH, Olympus Corp., Tokyo, Japan)
and a fluorescence microscope (CKX41, Olympus Corp.), numerous
cells were found in the culture flask of the control group. The
cells had adhered to the flask wall. The level of cell latency and
refraction was high and the proliferation rate of the tumor cells
was also high. Treatment with 10, 50 and 80 μmol/l RES-BSANP for 2,
4, 6, 8, 12 and 24 h resulted in morphological cell changes, as
observed by apoptotic body/cell nucleus DNA staining. The size of
the cell bodies in the experimental groups treated with 50 μmol/l
RES-BSANP for 8 h were reduced significantly and had become round
in shape. The latency and refractivity of the cells was weakened,
with vacuoles appearing in the cytoplasm. As the concentration of
RES-BSANP and the exposure time increased, the cells began to
shrink and rupture. The morphology of the SKOV3 cells became
irregular, with surrounding debris, and the culture medium became
turbid. A large number of cells collapsed, floated and died. The
difference between apoptosis and necrosis induced by 50 μmol/l
RES-BSANP is shown in Table I.
 | Table IEffects of resveratrol-bovine serum
albumin nanoparticles (80 μmol/l) on apoptotic body/cell nucleus
DNA staining. |
Table I
Effects of resveratrol-bovine serum
albumin nanoparticles (80 μmol/l) on apoptotic body/cell nucleus
DNA staining.
| Duration of
treatment, h |
|---|
|
|
|---|
| Cell count | 2 | 4 | 6 | 8 | 12 | 24 |
|---|
| Apoptotic
bodies | 22±0.21 | 25±2.6 | 47±3.51 | 56±0.8 | 59±2.6 | 63±6.4 |
| Necrosis | 0.44±0.5 | 64±0.6a | 87±5.6a | 102±4.5b | 130±4.7a | 178±1.3b |
DNA fragmentation
To further verify the apoptotic response, DNA
fragmentation was examined. DNA ladder formation was observed in
SKOV3 cells treated with 50 μmol/l RES and 20 μmol/l RES-BSANP for
4 h. Treatment duration was then extended to 6 h. Large scales of
DNA ladder were detected, in contrast to the 50 μmol/l Z-VAD-FMK
control group. These results indicated that the marked suppression
of cell growth by RES and RES-BSANP was attributable to apoptotic
cell death. Gel electrophoresis exhibited DNA ladders and smears in
the high-dose RES-BSANP groups (80 and 100 μmol/l RES-BSANP) and
exhibited only a smear in the Z-VAD-FMK control group (Fig. 1).
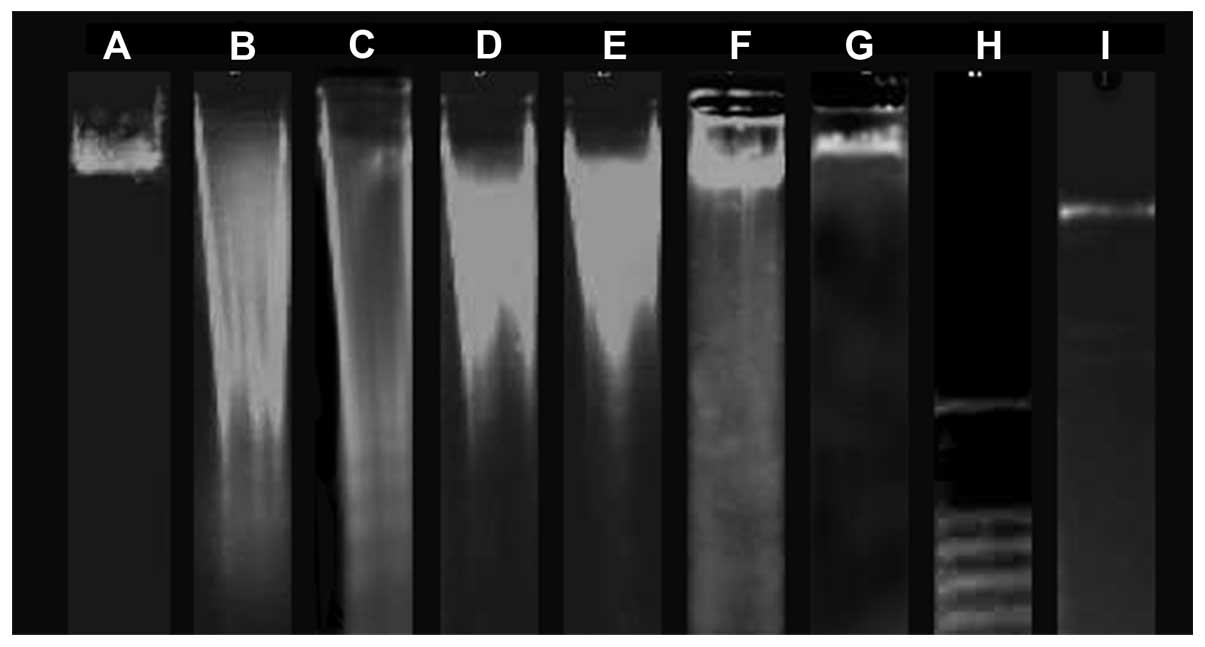 | Figure 1DNA Fragmentation. A, control; B, 20
μmol/l RES-BSNAP; C, 50 μmol/l RES-BSNAP; D, 80 μmol/l RES-BSNAP;
E, 100 μmol/l RES-BSNAP; F, 50 μmol/l Z-VAD-FMK and RES-BSANP; G,
50 μmol/l Z-VAD-FMK; H, λDNA/HindIII; I, 100-base pair DNA
ladder. RES-BSANP, resveratrol-bovine serum albumin
nanoparticles. |
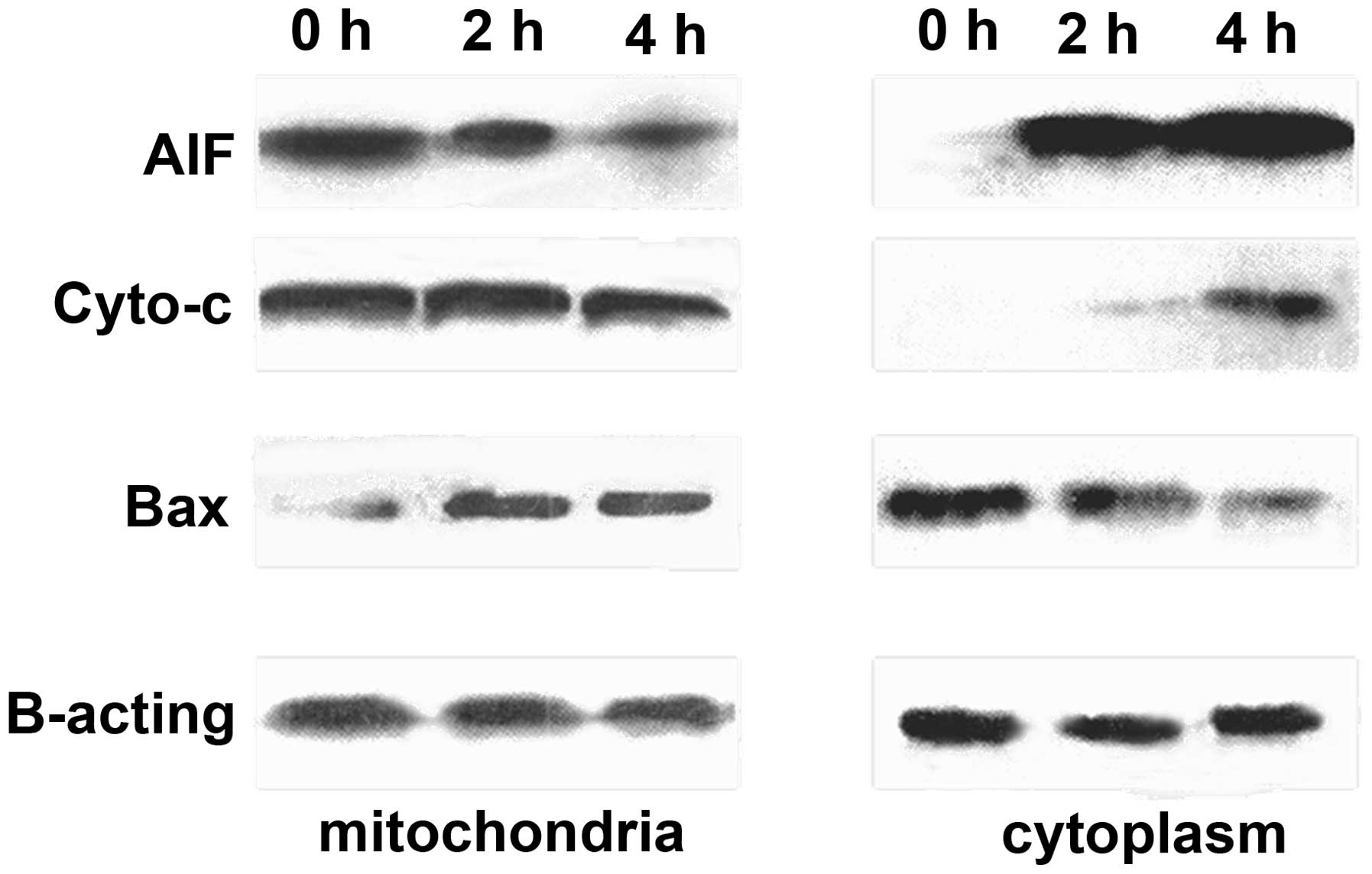
Western blot analysis of AIF, Cyto c and
Bax expression in SKOV3 cells
Induction of apoptosis by RES-BSANP may involve the
translocation of Cyto c and AIF. This translocation was
investigated by biochemically fractionating different subcellular
compartments and quantifying the expression of Cyto c and
AIF by western blot analysis. Cells treated with 80 μmol/l
RES-BSANP for 2 h showed a spatiotemporal release of AIF from the
mitochondria into the nucleus (Fig.
2). Similarly, it appeared that Cyto c was also released
from the mitochondria, however, notably, this was not accompanied
by a concomitant cytoplasmic increase at 4 h of treatment. The
translocation of AIF from mitochondria to cytoplasm occurred
earlier than that of Cyto c. These results indicated that
RES-BSANP-elicited cell death may not occur via a classical Cyto
c mitochondria cytosol translocation mechanism but rather, a
caspase-independent mechanism of cell death via the nucleus
directed shuttling of AIF and Cyto c.
Activation of Bax and its mitochondrial
translocation are the key regulatory events during induction of
mitochondrial membrane depolarization. Treatment of macrophages
with macrophage inflammatory protein supernatant resulted in
mitochondrial translocation of Bax as early as 2 h following
treatment. Reduction of AIF and Cyto c, resulted in
increased mitochondrial Bax. The binding of Bax to the mitochondria
was required for the release of AIF and Cyto c from
mitochondria.
Discussion
Previous studies have identified two primary forms
of cell death, namely apoptosis and necrosis. Apoptosis was
initially considered the primary physiological and programmed form
of cell death. The apoptotic proteins involved with the
caspase-dependent pathway include Cyto c, caspase-9 and
caspase-3 (16,17). Cell death via this pathway is
associated with the activation of caspases. However, it is now
widely recognized that PCD may also occur in the absence of caspase
activation (18–22). The existence of non-caspase PCD
pathways has been found to be associated with caspase-independent
elimination, including the use of mitochondrial protein AIF
(19,20,23,24).
The current study revealed that the caspase-independent pathway,
mediated by AIF, may be involved in the necrotic PCD pathway,
particularly following treatment with high doses of RES-BSANP in
cell culture.
Caspase activation is regarded as a vital factor in
RES-BSANP-induced cell death in in vitro and in vivo
studies. However, previous studies have indicated that RES-induced
cell death in human ovarian cancer cells is caspase-independent
(17,18); the treatment of these cells with
RES-BSANP resulted in the release of Cyto c and the
activation of caspase-3. Apoptotic cell death is induced by
RES-BSANP and appears to be caspase-independent, as caspase
inhibitors fail to attenuate induced cell death by RES treatment.
It has previously been reported that AIF mediates cell death via a
caspase-independent pathway (25).
Mitochondrial AIF translocates to the nucleus as a result of cell
death stimuli and thus initiates nuclear condensation (26,27).
Once the nucleus condenses, this leads to large-scale chromatin
fragmentation, followed by cell death. Consistent with these
observations, the current study demonstrated translocation of AIF
from the mitochondria to the nucleus following RES-BSANP treatment
of the SKOV3 cells. AIF translocation and nuclear condensation were
detectable within 24 h of RES-BSANP treatment. Whilst AIF protein
is considered to be a potent factor in caspase-independent
apoptosis, the mechanism by which AIF causes DNA ladder formation
remains unclear.
It has been reported that AIF protein release is
localized around the cell nuclei and is partly translocated into
these nuclei following treatment with the apoptogenic
dolichylmonoposphate in SKOV3 cells. Caspase-3 and -8 inhibitors
prevent not only DNA fragmentation, but also AIF migration and
chromatin condensation (28). In
the current study, the pan-caspase inhibitor prevented induced
apoptotic cell death following RES-BSANP treatment, indicating that
AIF protein release, rather than caspase release, may be pivotal in
DNA ladder formation. These results are consistent with previous
studies of RES-BSANP-induced apoptosis.
To the best of our knowledge, this is the first
study to investigate the caspase-independent signaling mechanism of
RES-BSANP-induced apoptosis in SKOV3 cells. Further studies are
required to elucidate the precise signaling pathways involved in
the RES-BSANP-induced apoptotic death of ovarian cancer SKOV3
cells. Notably, RES-BSANP exhibited a potent effect on the SKOV3
cells, which indicates that polyphenol compounds may also be a
candidate for a chemo-preventive and chemotherapeutic agent, as the
doses of the compounds used can be considerably lower than the
chemotherapy drugs commonly in use currently to exhibit the same
activity as RES.
It has been reported that a member of the
Bcl-family, Bax, is associated with the release of AIF from the
mitochondria (29,30), and the release of Ca2+
from the endoplasmic reticulum appears to be important in this
process (31). Results of previous
studies indicate that the release of AIF, with a decrease in
membrane potential and modulation of the expression of Bax may be
partly responsible for RES-BSANP-induced apoptosis in SKOV3 cells.
The polyphenol compounds have been shown to affect activation of
caspase-independent cell death pathways and thus exhibit an
anti-cancer activity. Recently, the understanding of how RES
induces cell death in human ovarian cancers has markedly improved.
RES usually inhibits signaling via the mitogen-activated protein
kinase and phosphatidylinositol 3-kinase/AKT pathways (32–36).
Consistently, RES has been found to suppress the activity of the
downstream transcription factors AP-1 and nuclear factor-κB
(RelA/p65) (35–38). Genes that are considered to be
transcriptionally affected by RES with an impact on apoptosis
include cyclins, cyclin-dependent kinases, caspases, p53, p21
(Cip1/WAF1), p300, NF-κB, Bcl-2, Bax and inhibitors of apoptosis
(39,40). Consequently, further studies
investigating RES-BSANP’s role in cell death, excluding the
caspase-dependent and caspase-independent pathways, are required. A
great deal remains be determined with regard to the mechanism of
cell necrosis or cell death by treatment with RES-BSANP.
Taken together, these results indicate that the main
signal transduction pathway of RES-BSANP induced apoptosis in SKOV3
cells is mediated by the activation of caspase. Concurrently,
additional intracellular signaling pathways may also be involved.
Further research is required to clarify the association between the
early response signal and the apoptotic signal. Thus, RES-BSANP,
which is a constituent of an anti-tumor compound, may be a
potentially effective candidate for chemoprevention.
Acknowledgements
This study was supported by The Third Hospital of
Harbin Medical University (Harbin, China). The authors would like
to thank the Tumor Research Institute of Harbin Medical University
for providing technical assistance. The study was funded by the
State Traditional Chinese Medicine Foundation (grant no.
06-07ZP15), the Harbin Youth Scientific and Technological
Innovation Foundation (grant no. 2011RFQYS087) and the China
Postdoctoral Science Foundation (grant no. 20110491102).
References
|
1
|
Koul D, Shen R, Bergh S, Sheng X,
Shishodia S, Lafortune TA, Lu Y, de Groot JF, Mills GB and Yung WK:
Inhibition of Akt survival pathway by a small-molecule inhibitor in
human glioblastoma. Mol Cancer Ther. 5:637–644. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Harikumar KB and Aggarwal BB: Resveratrol:
a multitargeted agent for age-associated chronic diseases. Cell
Cycle. 7:1020–1035. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Delmas D, Lançon A, Colin D, Jannin B and
Latruffe N: Resveratrol as a chemopreventive agent: a promising
molecule for fighting cancer. Curr Drug Targets. 7:423–442. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kundu JK and Surh YJ: Cancer
chemopreventive and therapeutic potential of resveratrol:
mechanistic perspectives. Cancer Lett. 269:243–261. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yang CS, Landau JM, Huang MT and Newmark
HL: Inhibition of carcinogenesis by dietary polyphenolic compounds.
Annu Rev Nutr. 21:381–406. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jang M, Cai L, Udeani GO, Slowing KV,
Thomas CF, Beecher CW, Fong HH, Farnsworth NR, Kinghorn AD, Mehta
RG, et al: Cancer chemopreventive activity of resveratrol, a
natural product derived from grapes. Science. 275:218–220. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tessitore L, Davit A, Sarotto I and
Caderni G: Resveratrol depresses the growth of colorectal aberrant
crypt foci by affecting bax and p21(CIP) expression.
Carcinogenesis. 21:1619–1622. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hebbar V, Shen G, Hu R, Kim BR, Chen C,
Korytko PJ, Crowell JA, Levine BS and Kong AN: Toxicogenomics of
resveratrol in rat liver. Life Sci. 76:2299–2314. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Soleas GJ, Angelini M, Grass L, Diamandis
EP and Goldberg DM: Absorption of trans-resveratrol in rats.
Methods Enzymol. 335:145–154. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Singh G and Pai RS: In-vitro/in-vivo
characterization of trans-resveratrol-loaded nanoparticulate drug
delivery system for oral administration. J Pharm Pharmacol.
66:1062–1076. 2014.PubMed/NCBI
|
|
11
|
Croy SR and Kwon GS: Polymeric micelles
for drug delivery. Curr Pharm Des. 12:4669–4684. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Escorcia FE, McDevitt MR, Villa CH and
Scheinberg DA: Targeted nanomaterials for radiotherapy.
Nanomedicine (Lond). 2:805–815. 2007. View Article : Google Scholar
|
|
13
|
Li F, Zhang X, Li H, Xiang L and Chen Y:
Preparation of self-assembled nanoparticles of chitosan
oligosaccharide-graft-polycaprolactone as a carrier of bovine serum
albumin drug. Biomed Mater Eng. 24:2041–2048. 2014.PubMed/NCBI
|
|
14
|
Yu Z, Yu M, Zhang Z, Hong G and Xiong Q:
Bovine serum albumin nanoparticles as controlled release carrier
for local drug delivery to the inner ear. Nanoscale Res Lett.
9:3432014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Guo LY, Yao JP and Sui LH: Preparation and
Effects of Resveratrol-Bovine Serum Albumin Nanoparticles on
Proliferation of Human Ovarian Carcinoma Cell SKOV3. Chemical
Journal of Chinese Universities. 30:474–477. 2009.
|
|
16
|
Guo LY, Peng Y, Li YL, Yao JP, Wang J,
Zhang GM, Chen J and Sui LH: Mechanisms of resveratrol-bovine serum
albumin nanoparticle-induced cell death in human ovarian cancer
SKOV3 cells. Nan Fang Yi Ke Da Xue Xue Bao. 30:2440–2442. 2010.(In
Chinese). PubMed/NCBI
|
|
17
|
Guo L, Peng Y, Yao J, Sui L, Gu A and Wang
J: Anticancer activity and molecular mechanism of
resveratrol-bovine serum albumin nanoparticles on subcutaneously
implanted human primary ovarian carcinoma cells in nude mice.
Cancer Biother Radiopharm. 25:471–477. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Anastasiadis PZ, Jiang H, Bezin L, Kuhn DM
and Levine RA: Tetrahydrobiopterin enhances apoptotic PC12 cell
death following withdrawal of trophic support. J Biol Chem.
276:9050–9058. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jäättelä M: Programmed cell death: many
ways for cells to die decently. Ann Med. 34:480–488. 2002.
View Article : Google Scholar
|
|
20
|
Jäättelä M and Tschopp J:
Caspase-independent cell death in T lymphocytes. Nat Immunol.
4:416–423. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lorenzo HK and Susin SA: Mitochondrial
effectors in caspase-independent cell death. FEBS Lett. 557:14–20.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Blagosklonny MV: Cell death beyond
apoptosis. Leukemia. 14:1502–1508. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Moubarak RS, Yuste VJ, Artus C, Bouharrour
A, Greer PA, Menissier-de Murcia J and Susin SA: Sequential
activation of poly(ADP-ribose) polymerase 1, calpains, and Bax is
essential in apoptosis-inducing factor-mediated programmed
necrosis. Mol Cell Biol. 27:4844–4862. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zanna C, Ghelli A, Porcelli AM, Martinuzzi
A, Carelli V and Rugolo M: Caspase-independent death of Leber’s
hereditary optic neuropathy cybrids is driven by energetic failure
and mediated by AIF and Endonuclease G. Apoptosis. 10:997–1007.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Miyake K, Bekisz J, Zhao T, Clark CR and
Zoon KC: Apoptosis-inducing factor (AIF) is targeted in
IFN-α2a-induced Bid-mediated apoptosis through Bak activation in
ovarian cancer cells. Biochim Biophys Acta. 1823:1378–1388. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhan ZL and Chen LY: Changes in the
expression of the apoptosis of esophageal cancer EC9706 cells
during nuclear matrix proteins induced by curcumin. Zhong Guo Sheng
Wu Hua Xue Yu Fen Zi Sheng Wu Xue Bao. 546–555. 2010.
|
|
27
|
Wu B and Gao Q: Microscope laser
cytoskeleton changes in the process of cell apoptosis confocal.
Dian Zi Xian Wei Xue Bao. 361–366. 2010.
|
|
28
|
Byun HS, Song JK, Kim YR, Piao L, Won M,
Park KA, Choi BL, Lee H, Hong JH, Park J, et al: Caspase-8 has an
essential role in resveratrol-induced apoptosis of rheumatoid
fibroblast-like synoviocytes. Rheumatology (Oxford). 47:301–308.
2008. View Article : Google Scholar
|
|
29
|
Arnoult D, Parone P, Martinou JC,
Antonsson B, Estaquier J and Ameisen JC: Mitochondrial release of
apoptosis-inducing factor occurs downstream of cytochrome c release
in response to several proapoptotic stimuli. J Cell Biol.
159:923–929. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cregan SP, Fortin A, MacLaurin JG,
Callaghan SM, Cecconi F, Yu SW, Dawson TM, Dawson VL, Park DS,
Kroemer G and Slack RS: Apoptosis-inducing factor is involved in
the regulation of caspase-independent neuronal cell death. J Cell
Biol. 158:507–517. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bröker LE, Kruyt FA and Giaccone G: Cell
death independent of caspases: a review. Clin Cancer Res.
11:3155–3162. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hall JB, Dobrovolskaia MA, Patri AK and
McNeil SE: Characterization of nanoparticles for therapeutics.
Nanomedicine (Lond). 2:789–803. 2007. View Article : Google Scholar
|
|
33
|
Dorrie J, Gerauer H, Wachter Y and Zunino
SJ: Resveratrol induces extensive apoptosis by depolarizing
mitochondrial membranes and activating caspase-9 in acute
lymphoblastic leukemia cells. Cancer Res. 61:4731–4739.
2001.PubMed/NCBI
|
|
34
|
Tinhofer I, Bernhard D, Senfter M, Anether
G, Loeffler M, Kroemer G, Kofler R, Csordas A and Greil R:
Resveratrol, a tumor-suppressive compound from grapes, induces
apoptosis via a novel mitochondrial pathway controlled by Bcl-2.
FASEB J. 15:1613–1615. 2001.PubMed/NCBI
|
|
35
|
Howells LM, Moiseeva EP, Neal CP, Foreman
BE, Andreadi CK, Sun YY, Hudson EA and Manson MM: Predicting the
physiological relevance of in vitro cancer preventive activities of
phytochemicals. Acta Pharmacol Sin. 28:1274–1304. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Athar M, Back JH, Tang X, Kim KH,
Kopelovich L, Bickers DR and Kim AL: Resveratrol: a review of
preclinical studies for human cancer prevention. Toxicol Appl
Pharmacol. 224:274–283. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Pervaiz S: Chemotherapeutic potential of
the chemopreventive phytoalexin resveratrol. Drug Resist Updat.
7:333–344. 2004. View Article : Google Scholar
|
|
38
|
Signorelli P and Ghidoni R: Resveratrol as
an anticancer nutrient: molecular basis, open questions and
promises. J Nutr Biochem. 16:449–466. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Le Corre L, Chalabi N, Delort L, Bignon YJ
and Bernard-Gallon DJ: Resveratrol and breast cancer
chemoprevention: molecular mechanisms. Mol Nutr Food Res.
49:462–471. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sheridan C, Delivani P, Cullen SP and
Martin SJ: Bax- or Bak-induced mitochondrial fission can be
uncoupled from cytochrome C release. Mol Cell. 31:570–585. 2008.
View Article : Google Scholar : PubMed/NCBI
|