Introduction
Reactive nodular fibrous pseudotumor (RNFP) is
described as a rare benign tumor-like lesion (1–3), which
was first reported by Yantiss et al in 2003 (1). According to the limited literature,
RNFP predominantly occurs in the gastrointestinal (GI) tract and
peritoneal regions with a male predominance (male/female ratio,
14/5) in adults. Macroscopically, it appears as a single mass or
multiple nodules attached to the outer layer of the bowel wall
(3), mesentery or omentum (2). Microscopically, the lesion is composed
of spindle or stellate cells resembling fibroblasts/myofibroblasts
enmeshed in a collagenous matrix, which is typically hyalinized or
keloidal in nature (1,4). The fact that the lesion often presents
with multiple intra-abdominal masses evidently causes clinical
concern for malignancy, and complete resection remains a routine
treatment. RNFP shows a good prognosis without signs of recurrence
or metastasis, no cases of RNFP recurrence or metastasis have been
reported in the literature. The present study reports and analyzes
a case of RNFP involving the mesentery and greater omentum in a
60-year-old female patient, with the aim of improving the
characterization of RNFP, and identifying distinguishing features
of the lesion to improve its differential diagnosis from other
neoplasms and non-neoplastic lesions involving this anatomical
region. The present study was approved by the ethics committee of
Zhongshan Hospital of Hubei Province (Wuhan, China) and written
informed consent was obtained from the patient.
Case report
In September 2012, a 60-year-old female patient
presented to the Department of Oncology, Zhongshan Hospital of
Hubei Province (Wuhan, China) with abdominal pain that had
gradually developed over six months following abdominal surgery for
leiomyoma of the uterus. Upon laparoscopy, multiple nodules were
identified throughout the mesentery, greater omentum and serosal
surface of the colon, ranging in size between 2.0–10.0 cm at the
largest dimension. Due to the presence of multiple nodules diffused
throughout the abdominal cavity, the lesion was diagnosed as a
metastatic malignant tumor. Thus, a partial colectomy was performed
and all masses were resected prior to macroscopic, histological and
immunohistochemical examination of the specimens.
The resected specimens were fixed in 10% formalin,
embedded in paraffin, sectioned and stained with hematoxylin and
eosin in accordance with routine procedures, and immunostaining was
performed on 4-μm-thick sections using the standard avidin-biotin
complex technique. A panel of antibodies (Table I) was used to evaluate the tumor
samples for the presence of smooth muscle [Desmin and smooth muscle
actin (SMA)], fibroblastic/myofibroblastic (SMA), schwannian
(S-100) and epithelial cell (AE1/AE3) differentiation, and various
immunohistochemical markers typically expressed in GI stromal
tumors [GIST; discovered on GIST (DOG) 1, cluster of
differentiation (CD) 117, CD34], inflammatory myofibroblastic
tumors [anaplastic lymphoma kinase (ALK)], aggressive fibromatosis
(β-catenin) and immunoglobulin (Ig) G4-associated disease (IgG4,
IgG).
 | Table IAntibodies and dilutions used in the
evaluation of reactive nodular fibrous pseudotumor. |
Table I
Antibodies and dilutions used in the
evaluation of reactive nodular fibrous pseudotumor.
| Antibody | Dilution | Supplier |
|---|
| Vimentin | 1:20 | Dako |
| AE1/AE3 | 1:20 | Dako |
| CD117 | 1:40 | Dako |
| SMA | 1:100 | Dako |
| DOG1 | 1:50 | Leica Biosystems |
| CD34 | 1:20 | Dako |
| Desmin | 1:140 | Dako |
| S-100 | 1:1000 | Dako |
| ALK | 1:10 | Dako |
| β-catenin | 1:400 | Dako |
| IgG4 | 1:500 | Leica Biosystems |
| IgG | 1:250 | Zymed |
Sections of healthy colon tissue (Maixin Bio,
Fuzhou, China) served as the positive controls for the expression
of vimentin, SMA, desmin, AE1/AE3, S-100, DOG1, CD34 and CD117, and
an ALK-positive anaplastic large cell lymphoma cell line (Maixin
Bio) served as the positive control for ALK staining. Negative
controls were performed by replacing the primary antibodies with
saline.
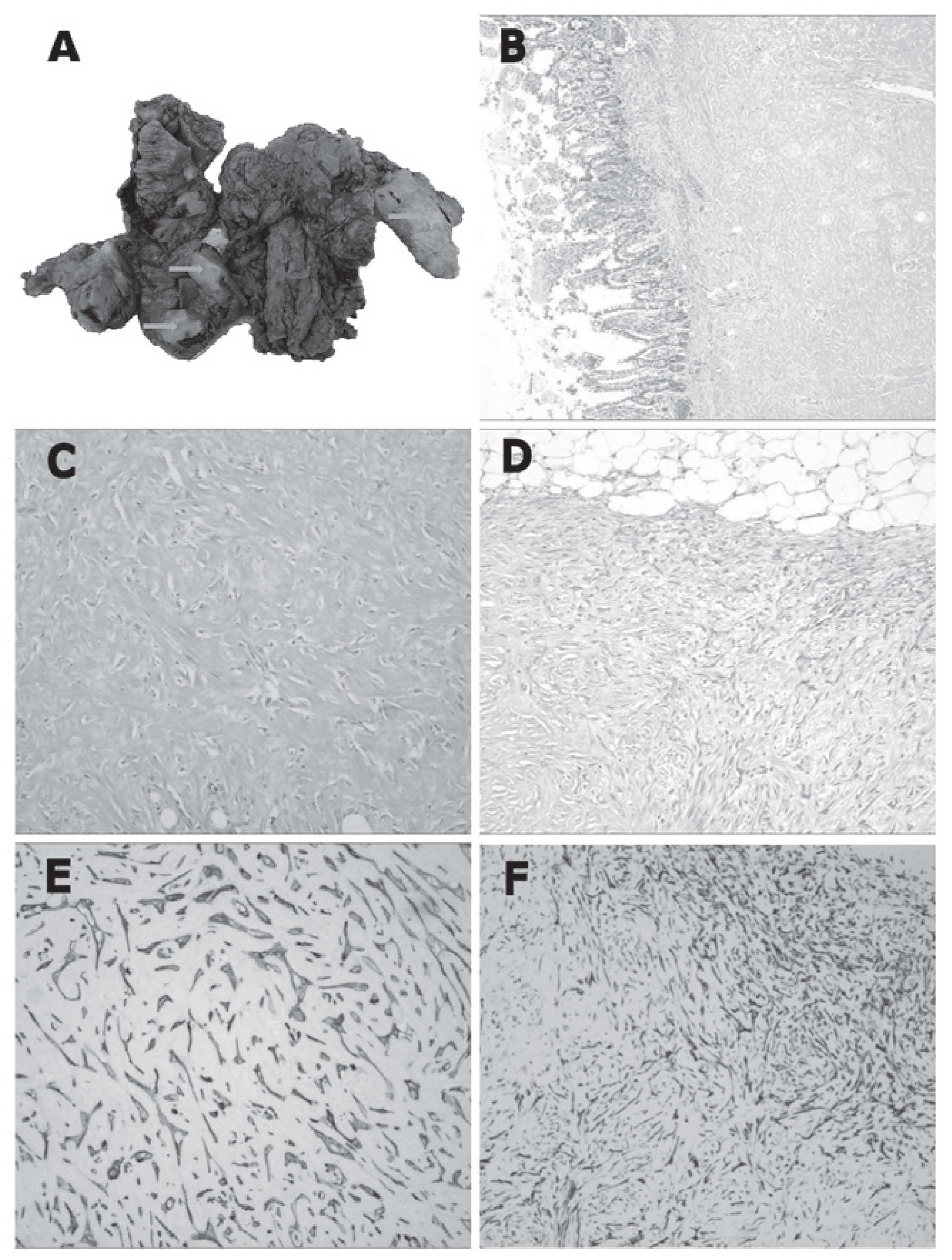
Macroscopically, the specimens were
well-circumscribed, rubbery to hard, white-tan-colored nodules
ranging between 2.0–10.0 cm at the largest dimension, while the cut
surface of the lesions was solid and homogeneous (Fig. 1A). The nodules predominantly
involved the outer layer of the colon, mesentery and omentum, but
were partially associated with the transmural extension of the
colon. Microscopically, the nodules were composed of sparse wavy
spindle or stellate cells within hyalinized keloid-like collagen
(Fig. 1B and C), although certain
nodules contained a more cellular peripheral zone composed of
active fibroblasts (Fig. 1D) or
were surrounded by an inflammatory infiltrate of mononuclear
lymphoid cells.
Immunohistochemically, the spindle or stellate cells
were positive for vimentin, SMA (Fig.
1E) and CD117, focally positive for AE1/AE3 (Fig. 1F) and desmin expression, and
demonstrated no DOG1, CD34, S-100 or β-catenin expression. In
addition, high counts of IgG4-positive plasma cells were not
detected and the ratio of IgG4/IgG-positive cells was determined as
<40%.
The patient was regularly followed up, and at 12
months post-resection the patient remains alive and in good health
with no evidence of recurrence or metastatic disease. The follow-up
continues at approximately eight-week intervals at present.
Discussion
RNFP was initially described in a series by Yantiss
et al (1), and is
characterized by fibroblastic/myofibroblastic spindle or stellate
cells with a hyalinized collagenous background and a lack of atypia
and mitosis. RNFP is considered to be a reactive benign lesion that
is associated with previous surgical procedures or inflammatory
disorders (4).
Immunohistochemically, the spindle cells are typically positive for
vimentin, SMA, desmin and AE1/AE3 expression, and demonstrated no
DOG1, CD34 or S-100 expression. Previous observations of CD117 and
AE1/AE3 expression in RNFP (1,4) appear
to conflict with the results of the present study, which identified
focal positive CD117 and peripheral cellular zonal AE1/AE3
staining. Yantiss et al (1)
observed CD117 expression in four out of five cases of RNFP, while
Daum et al (4) confirmed no
CD117 expression among eight cases of RNFP. However, analysis of
the relevant literature indicates that these conflicting
observations may be the result of methodological differences
(Table II), such as different
antibody clones or antigen retrieval details. Furthermore, the
immunohistochemical profile of the present patient was similar to
the characteristic fibroblastic/myofibroblastic differentiation of
RNFP (5). Considering the specific
marker expression profiles of RNFP spindle cells, including the
coexpression of vimentin and low molecular-weight cytokeratin,
determined by AE1/AE3 expression, it has been proposed that the
cells may be derived from multipotential subserosal cells (4).
 | Table IISummary of the reported cases of
reactive nodular fibrous pseudotumor. |
Table II
Summary of the reported cases of
reactive nodular fibrous pseudotumor.
| First author
(reference) | Year | Cases, n | Positive expression,
protein (n) |
|---|
| Yantiss et al
(1) | 2003 | 5 | Vim (5); CD117 (4);
SMA (3); CD34 (0); CK (0); ALK (ND) |
| Daum et al
(4) | 2004 | 8 | Vim (7); CD117 (0);
SMA (8); CD34 (0); CK (6); ALK (ND) |
| Saglam et al
(2) | 2005 | 1 | Vim (1); CD117 (ND);
SMA (1); CD34 (1); CK (1); ALK (ND) |
| Gauchotte et
al (7) | 2009 | 1 | Vim (1); CD117 (0);
SMA (1); CD34 (0); CK (1); ALK (0) |
| McAteer et al
(6) | 2012 | 1 | Vim (1); CD117 (0);
SMA (1); CD34 (0); CK (1); ALK (0) |
Cases of RNFP arising within the abdominal cavity
(6,7), including the GI tract (3), mesentery and retroperitoneum, are rare
but important due to their propensity to be misdiagnosed as more
aggressive types of tumor, such as metastatic malignant neoplasm,
primary GIST or inflammatory myofibroblastic (IMF) tumor. The case
presented in the present study was initially suspected to be a
metastatic malignant tumor due to the presence of multiple diffuse
nodules within the abdominal cavity. However, histopathological
analysis of the lesion determined rare mitotic figures and no
nuclear atypia or necrosis. Based upon these clinical and
histopathological features, it is proposed that the lesion was a
cohesive case of distinct tumor-like fibroblastic/myofibroblastic
proliferation that represents an acute post-operative response to
surgery.
A recent case series described four patients with
RNFP of the GI tract that possessed abundant IgG4-positive plasma
cells, indicating that RNFP may form part of the IgG4-associated
disease spectrum in specific cases (8,9).
However, in the present case, high counts of IgG4-positive plasma
cells were not observed and the ratio of IgG4/IgG was <40%.
Certain spindle cell lesions arising from the GI
tract should be distinguished from RNFP upon diagnosis. GIST, a
common mesenchymal tumor in this anatomical region (10), is typically more cellular than RNFP
and is immunopositive for DOG1, CD117 and CD34 (11,12).
Although immunopositivity of CD117 and CD34 are the most valuable
factors in the diagnosis of GIST, previous studies have
demonstrated that DOG1, compared with CD117, may be a more specific
and sensitive marker for GIST (13), particularly when RNFP and GIST are
each positive for CD117 expression. In addition, calcifying fibrous
pseudotumor (14,15), which is generally more cellular than
RNFP, including a mixture of granulocytes, plasma cells and
infiltrating lymphocytes, frequently presents with psammomatous or
dystrophic calcifications and is typically positive for CD34, but
exhibits no SMA or desmin expression. Furthermore, IMF (16,17) is
another rare tumor that may be located in the mesentery or the
retroperitoneum. IMF is a hypercellular tumor composed of loosely
arranged fascicles of plump spindle cells with abundant, densely
eosinophilic cytoplasm enmeshed within a variably collagenous,
edematous or myxoid stroma. Additionally, IMF contains an active
inflammatory infiltrate composed of plasma cells and lymphocytes
that are closely associated with the tumor cells, in contrast to
the mononuclear cell infiltrate of RNFP, which is typically sparse
and patchy. Immunohistochemically, ALK-positive staining may also
facilitate in the differential diagnosis of RNFP (18). Therefore, metastatic tumors in GI
tract may be distinguished from RNFP by patient history and
histopathological features.
Non-neoplastic spindle cell lesions that should also
be distinguished from RNFP include aggressive fibromatosis
(19), nodular fasciitis (20) and sclerosing mesenteritis (21). Thus, RNFP may be distinguished from
mesenchymal lesions of the abdomen and GI tract by
clinicopathological features and biological potential.
To conclude, RNFP is a post-operative or
post-inflammatory lesion that is increasingly recognized in the
differential diagnosis of primary and metastatic GI tumors.
Therefore, it is important to differentiate RNFP from similar
lesions with more aggressive phenotypes, as RNFP may be managed
definitively with local resection and surgical follow-up.
References
|
1
|
Yantiss RK, Nielsen GP, Lauwers GY and
Rosenberg AE: Reactive nodular fibrous pseudotumor of the
gastrointestinal tract and mesentery: a clinicopathologic study of
five cases. Am J Surg Pathol. 27:532–540. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Saglam EA, Usubütün A, Kart C, Ayhan A and
Küçükali T: Reactive nodular fibrous pseudotumor involving the
pelvic and abdominal cavity: a case report and review of
literature. Virchows Arch. 447:879–882. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yin SS, Zhang L, Cziffer-Paul A, Divino CM
and Chin E: Reactive nodular fibrous pseudotumor presenting as a
small bowel obstruction. Am Surg. 77:790–791. 2011.PubMed/NCBI
|
|
4
|
Daum O, Vanecek T, Sima R, et al: Reactive
nodular fibrous pseudotumors of the gastrointestinal tract: report
of 8 cases. Int J Surg Pathol. 12:365–374. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Barak S, Wang Z and Miettinen M:
Immunoreactivity for calretinin and keratins in desmoid
fibromatosis and other myofibroblastic tumors: a diagnostic
pitfall. Am J Surg Pathol. 36:1404–1409. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
McAteer J, Huaco JC, Deutsch GH and Gow
KW: Torsed reactive nodular fibrous pseudotumor in an adolescent:
case report and review of the literature. J Pediatr Surg.
47:795–798. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gauchotte G, Bressenot A, Serradori T,
Boissel P, Plenat F and Montagne K: Reactive nodular fibrous
pseudotumor: a first report of gastric localization and
clinicopathologic review. Gastroenterol Clin Biol. 33:1076–1081.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Carruthers MN, Stone JH and Khosroshahi A:
The latest on IgG4-RD: a rapidly emerging disease. Curr Opin
Rheumatol. 24:60–69. 2012. View Article : Google Scholar
|
|
9
|
Chetty R, Serra S, Gauchotte G, Märkl B
and Agaimy A: Sclerosing nodular lesions of the gastrointestinal
tract containing large numbers of IgG4 plasma cells. Pathology.
43:31–35. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu FY, Qi JP, Xu FL and Wu AP:
Clinicopathological and immunohistochemical analysis of
gastrointestinal stromal tumor. World J Gastroenterol.
12:4161–4165. 2006.PubMed/NCBI
|
|
11
|
Novelli M, Rossi S, Rodriguez-Justo M, et
al: DOG1 and CD117 are the antibodies of choice in the diagnosis of
gastrointestinal stromal tumours. Histopathology. 57:259–270. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lee CH, Liang CW and Espinosa I: The
utility of discovered on gastrointestinal stromal tumor 1 (DOG1)
antibody in surgical pathology - the GIST of it. Adv Anat Pathol.
17:222–232. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Espinosa I, Lee CH, Kim MK, et al: A novel
monoclonal antibody against DOG1 is a sensitive and specific marker
for gastrointestinal stromal tumors. Am J Surg Pathol. 32:210–218.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hill KA, Gonzalez-Crussi F and Chou PM:
Calcifying fibrous pseudotumor versus inflammatory myofibroblastic
tumor: a histological and immunohistochemical comparison. Mod
Pathol. 14:784–790. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fetsch JF, Montgomery EA and Meis JM:
Calcifying fibrous pseudotumor. Am J Surg Pathol. 17:502–508. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Coffin CM, Watterson J, Priest JR and
Dehner LP: Extrapulmonary inflammatory myofibroblastic tumor
(inflammatory pseudotumor). A clinicopathologic and
immunohistochemical study of 84 cases. Am J Surg Pathol.
19:859–872. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shatzel J, Wooten K, Ankola A, Cheney RT,
Morrison CD and Skitzki JJ: Inflammatory myofibroblastic tumor of
the mesentery: a clinical dilemma. Int J Clin Oncol. 17:380–384.
2012. View Article : Google Scholar
|
|
18
|
Cook JR, Dehner LP, Collins MH, et al:
Anaplastic lymphoma kinase (ALK) expression in the inflammatory
myofibroblastic tumor: a comparative immunohistochemical study. Am
J Surg Pathol. 25:1364–1371. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ferenc T, Wronski JW, Kopczyński J, et al:
Analysis of APC, alpha-, beta-catenins, and N-cadherin protein
expression in aggressive fibromatosis (desmoid tumor). Pathol Res
Pract. 205:311–324. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Montgomery EA and Meis JM: Nodular
fasciitis. Its morphologic spectrum and immunohistochemical
profile. Am J Surg Pathol. 15:942–948. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Montgomery E, Torbenson MS, Kaushal M,
Fisher C and Abraham SC: Beta-catenin immunohistochemistry
separates mesenteric fibromatosis from gastrointestinal stromal
tumor and sclerosing mesenteritis. Am J Surg Pathol. 26:1296–1301.
2002. View Article : Google Scholar : PubMed/NCBI
|