Introduction
Rhabdoid tumors (RTs) are aggressive neoplasms,
initially described by Beckwith and Palmer as a sarcomatoid
rhabdoid variant of Wilms’ tumor (1). Tumors with similar clinicopathological
characteristics have been subsequently reported in a number of
extrarenal sites and associated with an unfavorable prognosis
(2). Of these RTs, colorectal
cancers with rhabdoid features are extremely rare, and to date,
only nine cases have been previously reported (2–9). The
most noteworthy morphological feature is the strongly and
homogeneously acidophilic cytoplasm of the tumor cells (the result
of packing by intermediate filament) with occasional lateral
displacement of the nuclei (10).
On immunohistochemical analysis, the tumor cells are
characteristically positive for vimentin (VMT) and often for
cytokeratin (CK) and epithelial membrane antigen (EMA), but
generally negative for skeletal muscle marker or S-100 protein
(11). Rhabdoid cells in extrarenal
anatomic sites may be divided into specific tissue-based diagnostic
categories, such as poorly differentiated neoplasms, including
sarcomas, carcinomas and carcinosarcomas, and metastatic sarcomas
within a preexisting carcinoma (6).
Adenocarcinoma may also manifest various metaplastic features,
including sarcomatoid dedifferentiation; this distinctive
histological entity has been previously described as adenocarcinoma
with sarcomatoid dedifferentiation, true carcinosarcoma, and poorly
differentiated adenocarcinoma (6).
Adenocarcinoma with rhabdoid features may exhibit similar
morphological characteristics to those of malignant rhabdoid
tumors, and therefore, the existence of malignant extrarenal
rhabdoid tumors as a distinct clinicopathological entity remains
open to discussion (12). The
current study presents the 10th case of poorly differentiated
adenocarcinoma with rhabdoid features arising in the colon and
reviews the previously reported cases. To the best of our
knowledge, this is the first case of colonic carcinoma with
rhabdoid features coinciding with appendiceal mucinous cystadenoma.
The study was approved by the ethics committee of Chosun University
Hospital (Institutional review Board of Chosun university hospital,
Gwangju, Korea), who waived the requirement for written informed
consent due to the nature of the study.
Case report
Clinical summary
A 73-year-old male was admitted to the Department of
Surgery, Chosun University Hospital (Gwangju, Korea) with a 2-week
history of pain in the right lower quadrant. Abdominal computed
tomography (CT) with enhancement by contrast media revealed acute
appendicitis and a cecal edema; based upon this finding and
inflammation, cancer was suspected. Upon a clinical diagnosis of
acute appendicitis, appendectomy was performed. During surgery, a
cecal mass was identified, and an examination of the frozen section
of the cecal lesion revealed malignancy. Therefore, in addition to
appendectomy, a right hemicolectomy with regional lymph node
dissection was conducted.
Pathological findings
A protruding mass of 4.0×3.0×1.5 cm in size, with
central ulceration and necrosis was identified in the cecum
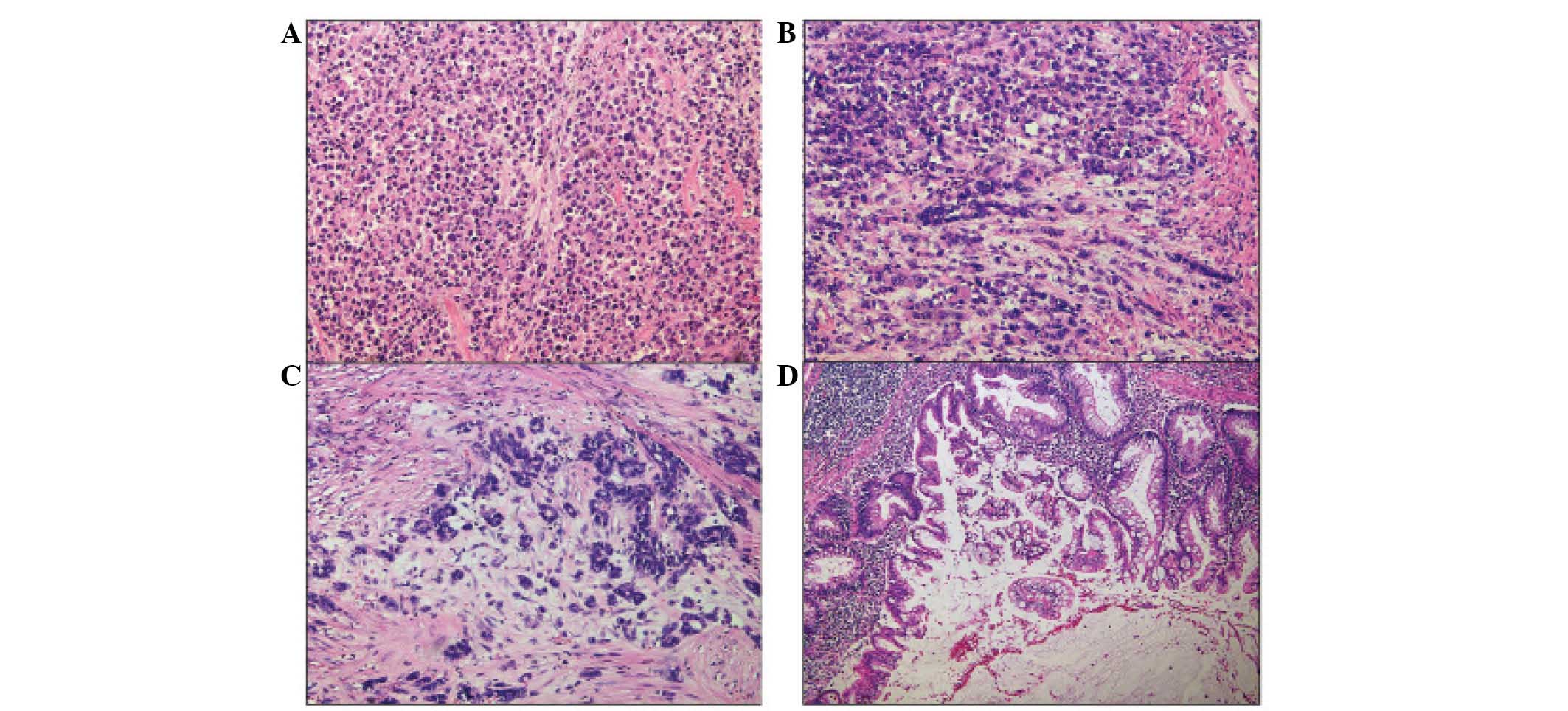
(Fig 1). Microscopically, the tumor
was composed of loosely cohesive, rhabdoid cells which grew in a
diffuse, solid and focal alveolar pattern (Fig. 2A). Transition of the gland-forming
adenocarcinoma to the area or malignancy demonstrating prominent
rhabdoid features was identified (Fig.
2B); the amount of adenocarcinoma component forming the
glandular structure was <1% of the total tumor area (Fig. 2C). The most noteworthy feature of
these rhabdoid tumor cells was the strongly and homogeneously
acidophilic cytoplasm of the tumor cells, with lateral displacement
of the nuclei (Fig. 2A). Extensive
necrosis was observed and regional lymph node metastasis was also
identified in four out of 45 regional lymph nodes, pN2a. The
metastatic lesion was entirely composed of rhabdoid tumor cells.
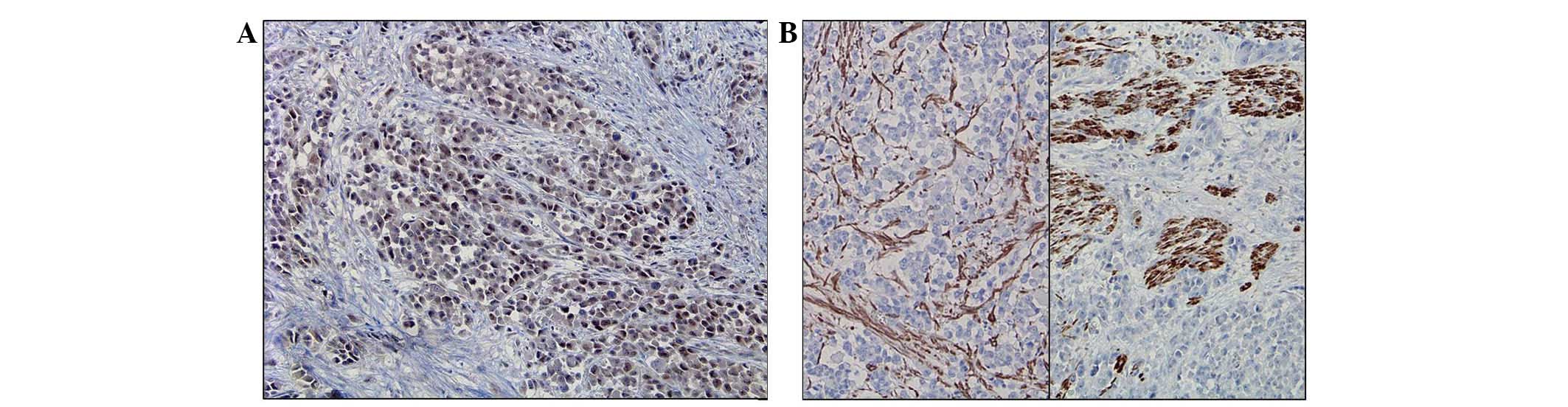
Immunohistochemically, the tumor cells of the adenocarcinoma and
rhabdoid components were positive for CK (Fig. 3A, adenocarcinoma component; Fig. 3B, rhabdoid component), VMT (Fig. 3C, adenocarcinoma component; Fig. 3D, rhabdoid component) and MLH-1
(Fig. 4A), but negative for
skeletal muscle marker, desmin and smooth muscle actin (Fig. 4B and C). In addition to the
malignant tumor, separated appendiceal mucinous cystadenoma was
also identified (Fig. 2D). The
final diagnosis was poorly differentiated adenocarcinoma with
prominent rhabdoid features, combined with appendiceal mucinous
cystadenoma. At two months following surgery the patient succumbed
to peritoneal seeding and metastasis of liver and bone.
Discussion
RT was originally described as a primary renal
neoplasm (13), however, examples
of a morphologically similar neoplasms have been subsequently
identified in a number of other sites, including soft tissues
(14). Of these, RTs of the colon
are extremely rare, and to the best of our knowledge, only nine
cases have been previously reported in the English language
literature (2–9). Histologically, RT is characterized by
the unique morphological feature of proliferating rhabdoid cells,
which have an abnormally located large nucleus and prominent
nucleoli, and a typical eosinophilic inclusion of aggregated
intermediate filament (12,15). Only two types of RT have been
reported: One is the pure type and the other is described as the
composite type (7). Chetty et
al (8) proposed that, in the
composite type of RT showing malignant rhabdoid cells coexisting
with adenocarcinoma, the rhabdoid cells may have been derived from
sarcomatoid dedifferentiation of malignant epithelial cells. This
is in contrast to the pure type, where the tumors are composed
exclusively of rhabdoid cells, without any other epithelial
component. In total, five of the nine cases previously reported
were composite type, and four cases were pure type (2–9). The
present case was determined to be composite type RT, concurrent
with mucinous adenoma of appendix. A marginal volume of
gland-forming adenocarcinoma was identified, which accounted for
<1% of the total tumor volume. Transition of adenocarcinoma
component to the rhabdoid area was also observed.
The previously reported cases were diagnosed as
malignant extrarenal rhabdoid tumors (MERTs) or adenocarcinoma with
rhabdoid features. Although MERTs have been well-defined and
characterized as a clinicopathological entity (ICD-O 8963/3)
(16), the existence of a composite
carcinoma consisting of an epithelial component associated with
rhabdoid cells indicates that rhabdoid cells may be the result of
sarcomatous dedifferentiation (12). Furthermore, the rhabdoid cells of
the pure and composite forms of RT exhibit similar morphological
and immunohistochemical characteristics. This feature also suggest
that rhabdoid cells may have dedifferentiated from epithelial tumor
cells, and not from metastatic sarcoma or metastatic malignant
renal rhabdoid tumors (6).
All rhabdoid colorectal tumors (RCTs), including
those previously reported and the present case, are listed in
Table I (2–9). All
RCTs have similar clinicopathological features. The majority of
MERT cases affect infants, whereas RCTs exclusively affect elderly
patients; the mean age at diagnosis was 73.5 years. The predominant
site involved was the cecum, six out of 10 cases; the other sites
of involvement were the transverse colon, two out of 10 cases;
sigmoid colon, one out of 10 cases; rectum, one out of 10 cases. No
gender predilection was evident. (male:female ratio, 6:4). In
total, eight out of 10 cases exhibited regional lymph node
metastasis at diagnosis, and four out of 10 cases showed hepatic
metastasis. The biological behavior was very aggressive; seven
patients succumbed to the disease within eight month (mean, eight
months and two weeks). The patient in the present case succumbed to
the disease two months following surgery.
 | Table IReported cases of colorectal tumor
with prominent rhabdoid feature. |
Table I
Reported cases of colorectal tumor
with prominent rhabdoid feature.
| Author | Age/gender | Site | Size, cm | Histology | LN metastasis | Outcome | Other |
|---|
| Chetty et al
(8) | 72/F | Cecum | 6×5 | Composite | + | STD (3 mo) | None |
| Yang et al
(3) | 75/M | Transverse | 10×10 | Pure | + | STD (2 wk) | None |
| Marcus et al
(4) | 84/F | Transverse | 7×6 | Pure | − | Alive (12 mo) | None |
| Nakamura et al
(5) | 76/M | Cecum | 14×8 | Pure | + | STD (12 wk) | None |
| Kono et al
(6) | 66/M | Cecum | 13×13 | Composite | + | STD (6 wk) | None |
| Pancione et al
(2) | 71/M | Cecum | 10×10 | Pure | − | STD (8 mo) | None |
| Remo et al
(7) | 73/F | Cecum | 10×8 | Composite | + | STD (6 mo) | PC |
| Lee et al
(9) | 62/M | Sigmoid | 4.5×4.0 | Composite | + | Alive (36 mo) | None |
| 83/F | Rectum | 6.5×4.3 | Composite | + | STD (1 mo) | None |
| Present case | 73/M | Cecum | 4×3 | Composite | + | Alive (4 wk) | Adenoma |
Several genetic abnormalities were reported in
previously published cases. Kono et al (6) reported a case of cecal adenocarcinoma,
which showed prominent rhabdoid features on immunohistochemical,
ultrastructural and molecular analyses, and the authors observed
strong expression of human mutL homolog 1 (hMLH1) protein in the
nuclei of the rhabdoid cells. However, microsatellite instability
(MSI) at five polymorphic markers (BAT25, BAT26, D2S123, D5S346,
D17S250) was not observed in the rhabdoid cells. Pancione et
al (2) reported a novel case of
colon rhabdoid carcinoma associated with a positive CpG island
methylator phenotype and BRAF mutation. The authors revealed that
the promoter regions of four out of five specific genes that define
the CpG island methylator phenotype, including MLH1, were
methylated. Additionally, MSI was detected. Furthermore, a mutation
in BRAF V600E was detected, however, no KRAS mutation was
identified. This indicated that genetic and epigenetic events may
be involved in the occurrence and progression of this rare and
aggressive phenotype, revealing a potential implication for its
management. In the study by Remo et al (7), all neoplastic cells were observed to
express hMSH2 protein but were negative for hMLH1; a BRAF V600E
mutation was identified, but no KRAS mutation was present, which is
consistent with the study by Pancione et al. Remo et
al (7) also reported that the
promoter regions of the characteristic subset of genes for the CIMP
status (NEUROG1, IGF2, RUNX3, SOCS1, including MLH1) were
hypermethylated, suggesting the presence of a CIMP+ and MSI-high
tumor. In the present case, tumor cells were immunoreactive for
MLH1, which indicates that a similar genetic event may have
occurred, causing this abnormality.
In conclusion, the current study reports the 10th
case of RCT (composite type) with a review of the previously
reported RCTs. In the present case, separate appendiceal mucinous
cystadenoma was concomitant with RCT. All RCT cases exhibit similar
clinicopathological features, as well as the characteristic
histological feature of sarcomatous dedifferentiation of rhabdoid
cells, which appears to indicate aggressive biological behavior.
Further investigations into this highly aggressive colonic
carcinoma showing rhabdoid feature are required in order to
determine specific and effective treatment for this tumor type.
References
|
1
|
Beckwith JB and Palmer NF: Histopathology
and prognosis of Wilms tumors: results from the First National
Wilms Tumor Study. Cancer. 41:1937–1948. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pancione M, Di Blasi A, Sabatino L, et al:
A novel case of rhabdoid colon carcinoma associated with a positive
CpG island methylator phenotype and BRAF mutation. Hum Pathol.
42:1047–1052. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yang AH, Chen WY and Chiang H: Malignant
rhabdoid tumour of colon. Histopathology. 24:89–91. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Marcus VA, Viloria J, Owen D and Tsao MS:
Malignant rhabdoid tumor of the colon. Report of a case with
molecular analysis. Dis Colon Rectum. 39:1322–1326. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nakamura I, Nakano K, Nakayama K, et al:
Malignant rhabdoid tumor of the colon: report of a case. Surg
Today. 29:1083–1087. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kono T, Imai Y, Imura J, et al: Cecal
adenocarcinoma with prominent rhabdoid feature: report of a case
with immunohistochemical, ultrastructural, and molecular analyses.
Int J Surg Pathol. 15:414–420. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Remo A, Zanella C, Molinari E, et al:
Rhabdoid carcinoma of the colon: a distinct entity with a very
aggressive behavior: a case report associated with a polyposis coli
and review of the literature. Int J Surg Pathol. 20:185–190. 2012.
View Article : Google Scholar
|
|
8
|
Chetty R and Bhathal PS: Caecal
adenocarcinoma with rhabdoid phenotype: an immunohistochemical and
ultrastructural analysis. Virchows Arch A Pathol Anat Histopathol.
422:179–182. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lee SH, Seol H, Kim WY, et al: Rhabdoid
colorectal carcinomas: reports of two cases. Korean J Pathol.
47:372–377. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Frierson HF Jr, Mills SE and Innes DJ Jr:
Malignant rhabdoid tumor of the pelvis. Cancer. 55:1963–1967. 1985.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kodet R, Newton WA Jr, Sachs N, et al:
Rhabdoid tumors of soft tissues: a clinicopathologic study of 26
cases enrolled on the Intergroup Rhabdomyosarcoma Study. Hum
Pathol. 22:674–684. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wick MR, Ritter JH and Dehner LP:
Malignant rhabdoid tumors: a clinicopathologic review and
conceptual discussion. Semin Diagn Pathol. 12:233–248.
1995.PubMed/NCBI
|
|
13
|
Berry PJ and Vujanic GM: Malignant
rhabdoid tumour. Histopathology. 20:189–193. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tsuneyoshi M, Daimaru Y, Hashimoto H, et
al: Malignant soft tissue neoplasms with the histologic features of
renal rhabdoid tumors: an ultrastructural and immunohistochemical
study. Hum Pathol. 16:1235–1242. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Haas JE, Palmer NF, Weinberg AG and
Beckwith JB: Ultrastructure of malignant rhabdoid tumor of the
kidney. A distinctive renal tumor of children. Hum Pathol.
12:646–657. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Flatcher C, Unni K and Mertens F:
Pathology and Genetics of Tumours of Soft tissue and Bond. IARC
Press; Lyon: 2002
|