Introduction
Tumor lysis syndrome (TLS) is a life-threatening
oncological emergency that is caused by the abrupt and massive
lysis of cancer cells (1–6). The cell lysis leads to the rapid release
of intracellular metabolites, including nucleic acids, proteins,
phosphorus and potassium. This process can potentially cause
hyperuricemia, hyperkalemia, hyperphosphatemia and hypocalcemia,
thereby inducing renal insufficiency, cardiac arrhythmias, seizures
and neurological disorders, ultimately resulting in mortality
(1–6).
However, TLS is preventable at an early stage, and it is essential
to identify patients at risk of TLS and those requiring TLS
management (2,3).
An international TLS expert consensus panel
developed guidelines for a medical decision tree to assign the
classifications of a low, intermediate and high risk of TLS to
patients with cancer (2,3). Patients are assigned to low-,
intermediate- and high-risk groups based on the type of malignancy,
white blood cell count, lactate dehydrogenase level, type of
therapy, presence of renal damage, and levels of uric acid (UA),
phosphate and potassium. This risk stratification enables the
appropriate management of each group (2,3).
The management of TLS includes aggressive hydration
and a reduction in the serum UA (S-UA) level through the
administration of drugs that decrease the production of UA or
degrade UA (2,3,5,6). Hyperuricemia results from the rapid
catabolism of purine-containing nucleic acids from cancer cells,
since purine nucleotides are converted to hypoxanthine, xanthine,
and finally to UA by xanthine oxidase (6). In the aforementioned guidelines, two
UA-decreasing agents, the conventional xanthine oxidase inhibitor
allopurinol and the recombinant uricase rasburicase are recommended
to be employed in the management of TLS. Allopurinol is used to
treat patients at an intermediate risk of TLS and the rasburicase
is used in patients with a high risk (2,3).
Rasburicase (Japan, Rasritek™; Europe, Fasturtec™;
USA, Elitek™; Sanofi, Paris, France) is a recombinant form of an
Aspergillus-derived urate oxidase that is expressed in a
Saccharomyces cerevisiae vector (6–10). Urate
oxidase, which is not present in humans, metabolizes UA to the more
soluble allantoin, carbon dioxide and hydrogen peroxide. Allantoin
is readily excreted by the kidneys. The UA-lowering efficacy of
rasburicase is prompt and potent, and the administration of
rasburicase appears to be extremely well tolerated (6–10). The
approved method of administration in Japan is 0.2 mg/kg once daily,
as an intravenous infusion administered over a 30-min period, for a
maximum of seven days (11).
Nevertheless, smaller doses and a shorter duration of
administration of rasburicase are suggested to be equally
efficacious (12,13). Therefore, the optimal dosing and
duration of rasburicase have not yet been established.
The present retrospective study investigated the
efficacy of rasburicase in patients with hematological malignancies
accompanied by TLS and those at risk of TLS at the University of
Fukui Hospital. A xanthine oxidase inhibitor, either allopurinol or
febuxostat, was used in combination with rasburicase in certain
cases. The UA-lowering efficacy was assessed by the dosage and the
duration of rasburicase, which differed according to the
physicians' decision. In addition, the economics of the reduced use
of rasburicase treatment was evaluated in the present study, due to
the expense of the agent.
Patients and methods
Patients
Patients that were admitted to the University of
Fukui Hospital between March 2011 and February 2013 were
retrospectively evaluated in the present study. The study was
approved by the ethics committee of University of Fukui Hospital,
(Fukui, Japan). All patients had been diagnosed with hematological
malignancies and already exhibited TLS or were at risk of TLS.
These patients received rasburicase for the indicated durations,
with or without the sequential administration of a xanthine oxidase
inhibitor, either febuxostat or allopurinol, during induction
chemotherapy. The patients did not receive any other medications
that may have affected the S-UA level, which included losartan,
fenofibrate, atorvastatin, pyrazinamide and cyclosporine.
Classification of TLS
The risk classification for TLS was made based on
previously reported guidelines (2).
Diseases at an intermediate risk for TLS consisted of acute myeloid
leukemia with a peripheral white blood cell count between
1×104 and 5×104 cells/µl, acute lymphoblastic
leukemia with a peripheral white blood cell count between
5×104 and 1×105 cells/µl, diffuse large B
cell non-Hodgkin's lymphoma, and other hematological malignancies
that would proliferate rapidly with expected rapid response to
therapy (2). Diseases at a high risk
included acute monoblastic leukemia, acute myeloid leukemia with a
peripheral white blood cell count ≥5×104 cells/µl, and
acute lymphoblastic leukemia with a peripheral white blood cell
count ≥1×105 cells/µl (2).
TLS is divided into laboratory TLS and clinical TLS. Laboratory TLS
is defined according to abnormal serum values of UA, potassium,
phosphate or calcium (1,2). Clinical TLS requires the presence of
clinical manifestations, including increased creatinine levels,
cardiac arrhythmia and seizure, in addition to laboratory TLS. The
treatment algorithm was based on the aforementioned guidelines
(2).
Categorizing the type of
hyperuricemia
Hyperuricemia is broadly classified into
UA-overproduction, UA-under-excretion and combined types, according
to the guidelines for the management of hyperuricemia and gout that
were published in Japan in 2010 (14). The urinary UA excretion, UA clearance
and creatinine clearance rates were determined for the
categorization of hyperuricemia. A urinary UA excretion rate of
>0.51 mg/kg/h and a UA clearance rate of >7.3 ml/min was
defined as overproduction type, a urinary UA excretion rate of
<0.48 mg/kg/h or a UA clearance rate of <7.3 ml/min was
classified as under-excretion type, and a UA excretion rate of
>0.51 mg/kg/h and a UA clearance rate of <7.3 ml/min was
classified as combined type.
Treatments and assessments
All patients received rasburicase. The standard
method of administration of rasburicase is 0.2 mg/kg/day for a
maximum of seven days, which is covered by the national health
insurance system in Japan. In the patients analyzed in the present
study, the use of rasburicase was modified depending on the level
of S-UA, according to the physicians' decision. Induction
chemotherapy was initiated with a regimen suitable for each
patient, alongside the administration of rasburicase. In certain
cases, xanthine oxidase inhibitors, such as allopurol or
febuxostat, were used in combination with the other agents. The
primary endpoint was the normalization of the S-UA level to ≤7
mg/dl at the end of rasburicase treatment and on the seventh day
subsequent to the first administration of rasburicase. The S-UA
levels were determined at the University of Fukui Hospital using a
TBA-c16000 automatic analyzer (Toshiba, Otawara, Tochigi, Japan)
(15).
Calculation of the cost of TLS
treatment
The total drug cost for treating TLS was calculated
for each patient. Rasburicase (Rasritek; 7.5 mg) is priced at
49,938 yen in Japan, according to the standard price of these drugs
determined by the Ministry of Health, Labour and Welfare in Japan,
2012. Febuxostat (Feburic™) at weights of 10, 20 and 40 mg costs
31.1, 56.4 and 106.6 yen, respectively. Allopurinol (Zyloric™) at a
weight of 100 mg is 25.3 yen. If Rasritek is used in the standard
regimen of 0.2 mg/kg/day for seven days, the total cost is 699,132
yen for an ordinary adult patient (60 kg).
Statistical analyses
All statistical analyses were performed using
Microsoft Excel 2007 software (Microsoft, Redmond, WA, USA). All
graphs were generated using GraphPad Prism software (version 5.0;
GraphPad Software, Inc., San Diego, CA, USA). The data are reported
as the mean ± standard deviation.
Results
Patient characteristics
A total of 13 patients admitted to the University of
Fukui Hospital between March 2011 and February 2013 were evaluated
retrospectively (Table I). The median
age was 67 years (range, 26–89 years), with eight males and five
females. The diagnoses included diffuse large B-cell lymphoma
(n=4), acute myeloid leukemia (n=7), acute lymphoblastic leukemia
(n=1), and myelodysplastic syndrome (n=1). Patients 1, 3 and 6
already exhibited clinical TLS, while patients 3, 5, 7–9, 11 and 12
demonstrated laboratory TLS (Table
I).
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| No. | Age | Gender | Diagnosis | WBC,
n/µla | LDH,
mg/dlb | S-UA, mg/dl | S-Cr, mg/dl | S-Ca, mg/dl | S-K, mg/dl | S-P, mg/dl | eGFR, ml/min/1.73
m2 | LTLS/CTLS |
|---|
| 1 | 89 | M | AML MDS overt | 86200 | 751 | 22.3 | 4.92 | 10.0 | 5.7 | 13.2 | 9.4 | +/+ |
| 2 | 55 | M | MDS (RAEB-I) | 200 | 720 | 8.0 | 1.92 | 12.0 | 4.3 | 6.2 |
| +/+ |
| 3 | 79 | F | AML M4 | 486400 | 1187 | 8.8 | 1.58 | 9.4 | 2.7 | 2.8 |
| +/– |
| 4 | 82 | F | AML M5 | 128000 | 190 | 6.3 | 1.70 | 8.9 | 3.7 | 4.4 | 22.6 | −/– |
| 5 | 63 | M | DLBCL
(IVc) | 70200 | 1854 | 8.1 | 0.61 | 10.4 | 3.7 | 3.0 |
| +/– |
| 6 | 66 | F | DLBCL
(IIIc) | 19000 | 2287 | 11.0 | 2.77 | 9.7 | 3.4 | 1.9 | 14.1 | +/+ |
| 7 | 73 | M | DLBCL
(IVc) | 7600 | 572 | 15.4 | 1.23 | 10.4 | 3.9 | 3.4 |
| +/– |
| 8 | 67 | M | AML M7 | 15500 | 3324 | 11.8 | 1.45 | 9.2 | 4.9 | 5.1 |
| +/– |
| 9 | 57 | F | AML M5a | 56400 | 2047 | 9.7 | 1.11 | 9.1 | 3.2 | 4.1 |
| +/– |
| 10 | 79 | M | DLBCL
(IVc) | 9800 | 1038 | 11.0 | 1.64 | 10.1 | 3.5 | 3.4 | 32.2 | −/– |
| 11 | 26 | M | ALL L2 | 218400 | 722 | 10.5 | 1.03 | 9.4 | 3.3 | 4.2 |
| +/– |
| 12 | 66 | M | AML M5b | 33900 | 295 | 8.1 | 1.16 | 9.4 | 3.5 | 4.5 | 49.6 | +/– |
| 13 | 71 | F | AML M5b | 152000 | 619 | 4.1 | 1.27 | 9.2 | 2.7 | 0.7 | 32.5 | −/– |
UA-associated parameters
Eleven patients already presented hyperuricemia
(patient numbers 1–3 and 5–12). The parameters associated with UA
were determined in five patients (patients 1, 2, 8, 9 and 12),
which included the urinary UA excretion and the UA clearance rates
(Table II). On the basis of
previously reported criteria (14),
three cases were classified as UA overproducers and two cases were
classified as UA under-excretors (Table
II).
 | Table II.Classification of hyperuricemia. |
Table II.
Classification of hyperuricemia.
| Patient | U-UA,
mg/kga | C-UA,
ml/minb | Type of
hyperuricemia |
|---|
| 1 | 0.12 | 2.8 | Underexcretion |
| 2 | 0.35 | 4.6 | Underexcretion |
| 8 | 0.74 | 7.0 | Overproduction |
| 9 | 0.51 | 5.0 | Overproduction |
| 12 | 0.71 | 3.6 | Overproduction |
Method of administration of
rasburicase
All patients underwent induction chemotherapy for
the treatment of the diagnosed malignancies. Concomitantly,
rasburicase was administered at various doses and durations
(Table III). The median dose was
0.19 mg/kg, and the median duration was four days. Seven patients
received rasburicase alone (patients 1, 5–8, 10 and 11), while six
patients received rasburicase in combination with a xanthine
oxidase inhibitor, either allopurinol (patients 2–4) or febuxostat
(patients 9, 12 and 13) (Table
III).
 | Table III.Chemotherapies and uric acid-lowering
therapies. |
Table III.
Chemotherapies and uric acid-lowering
therapies.
|
|
|
| Rasburicase | Febuxostat | Allopurinol |
|
|---|
|
|
|
|---|
| Patient | TLS risk | Chemotherapy | Dose, mg/kg | Duration,
daysa | Dose, mg/kg | Duration,
daysa | Dose, mg/kg | Duration,
daysa | Cost,
%b |
|---|
| 1 |
| Low-dose ara-C | 0.19 | 6 |
|
|
|
| 14 |
| 2 |
| Ara-C-based | 0.18 | 6–7 |
|
| 200 | 1–7 | 29 |
| 3 |
| Ara-C-based | 0.19 | 1–5 |
|
| 200 | 1–7 | 71 |
| 4 | High | Ara-C-based | 0.19 | 1–5 |
|
| 200 | 1–7 | 36 |
| 5 |
| DEX | 0.13 | 1–4 |
|
|
|
| 29 |
| 6 |
| RCHOP | 0.14 | 1–7 |
|
|
|
| 50 |
| 7 |
| RCHOP | 0.18 | −1–5 |
|
|
|
| 86 |
| 8 |
| Ara-C-based | 0.23 | −1–3 |
|
|
|
| 57 |
| 9 |
| Ara-C-based | 0.21 | −1–1 | 60 | 2–6 |
|
| 29 |
| 10 | Int | RCHOP-like | 0.21 | 1–7 |
|
|
|
| 100 |
| 11 |
| CPA+DNR+PSL | 0.25 | 1,6,7 |
|
|
|
| 43 |
| 12 |
| Ara-C-based | 0.19 | 1–3 | 10 | −7–7 |
|
| 43 |
| 13 | High | Ara-C-based | 0.17 | 1–3 | 10 | −1–7 |
|
| 21 |
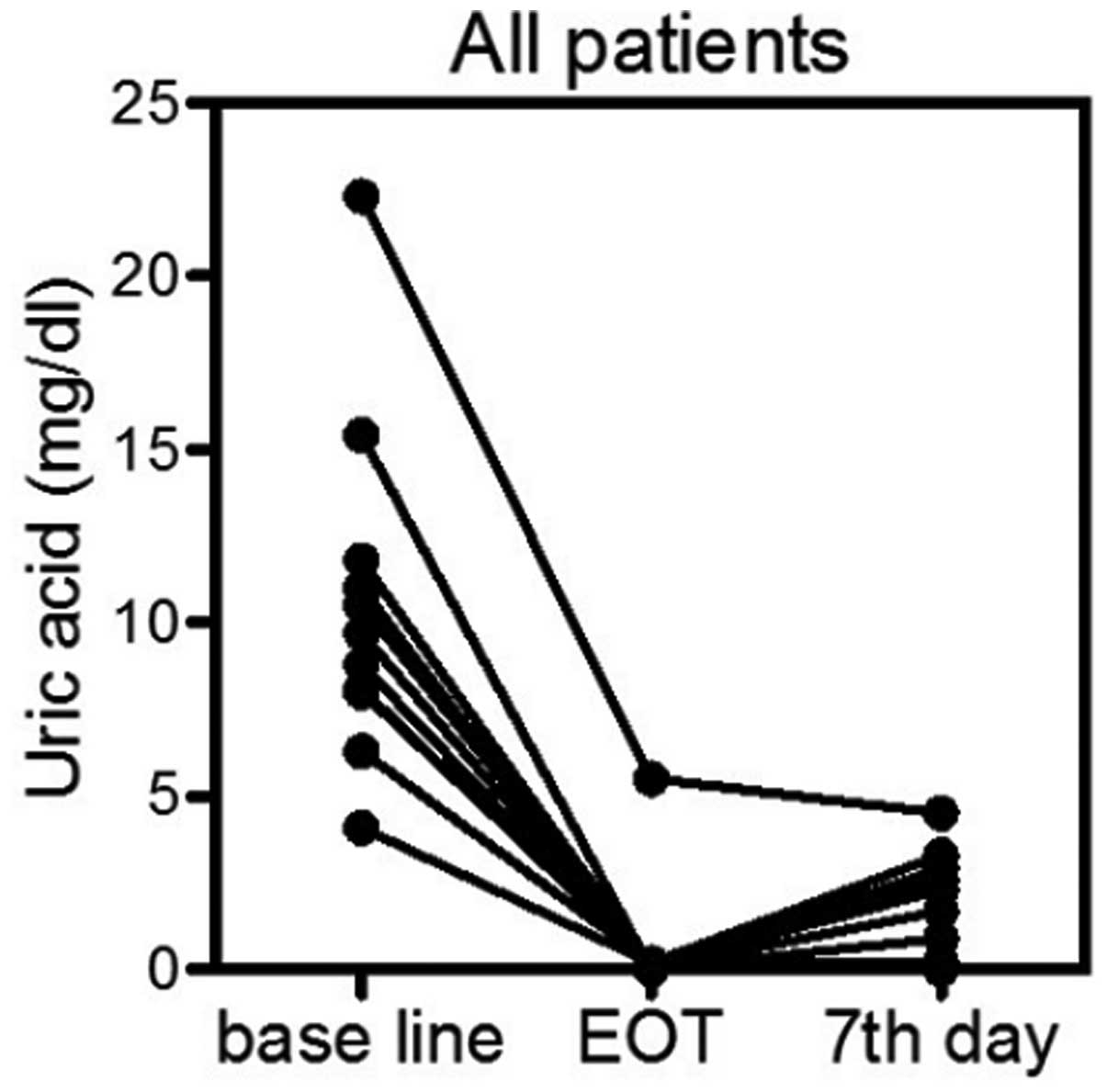
UA-lowering efficacy
The primary estimate of the present retrospective
study was the normalization of the S-UA level at the end of
rasburicase treatment and on the seventh day subsequent to the
first administration of rasburicase. While the S-UA baseline level
was 10.4±4.5 mg/dl, the S-UA level at the end of rasburicase
administration was 0.5±1.5 mg/dl (paired t-test;
P<0.0001) and the S-UA level on day seven was 1.4±1.5 mg/dl
(paired t-test, P<0.0001) (Fig.
1). All patients achieved the response criteria of
normalization of the S-UA level. The patients were divided into two
groups, a short-duration group, in which the patients received
rasburicase for a maximum of three days, and a long-duration group,
in which the patients received rasburicase for more than three
days. The time course of S-UA was compared between these two groups
(Fig. 2A and B). The S-UA level at
baseline was 10.5±6.2 mg/dl for the short-duration group and
10.3±3.0 mg/dl for the long-duration group (P=0.47, Mann-Whitney
test) (Fig. 2C). The S-UA level on
day seven was 1.9±1.8 mg/dl for the short-duration group and
1.0±1.3 mg/dl for the long-duration group (P=0.20, Mann-Whitney
test; Fig. 2D). This suggested that
the S-UA level remained in the normal range in the short-duration
group, although a non-significant re-increase in the S-UA level was
observed.
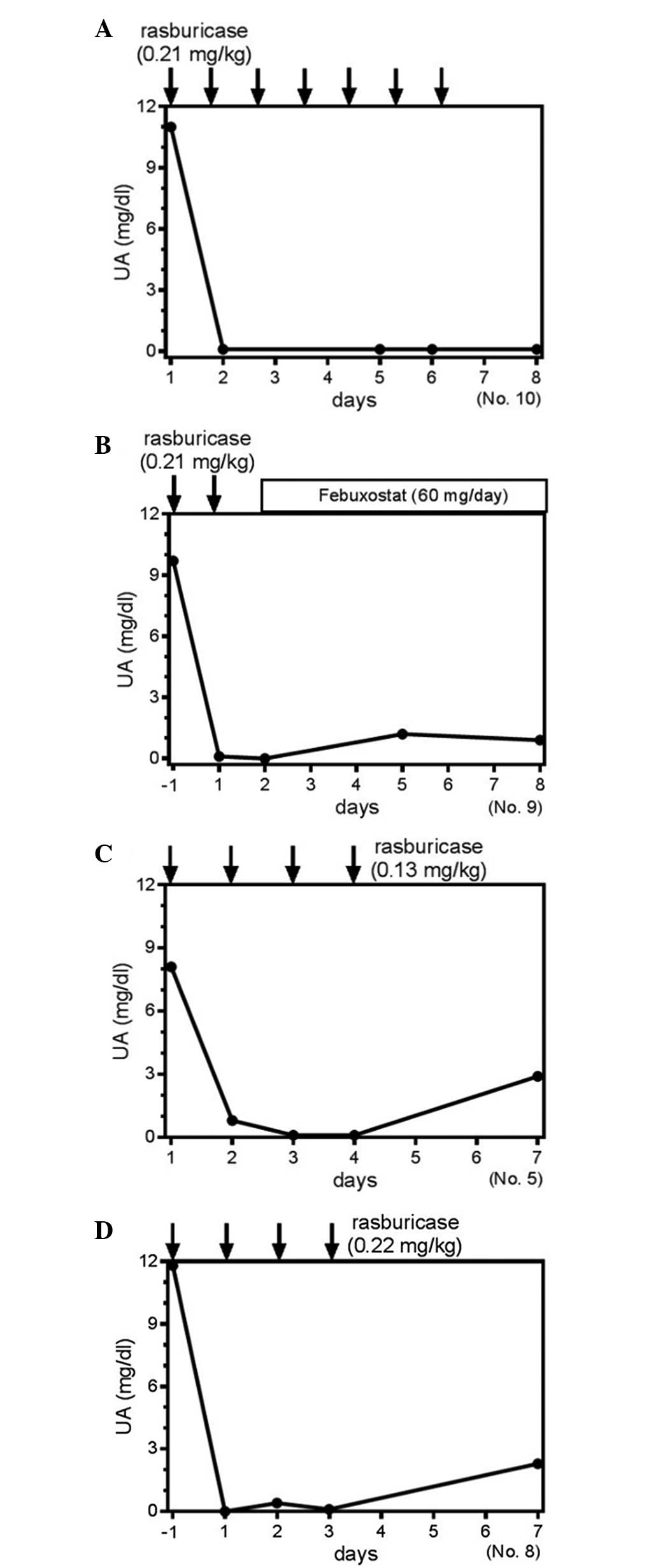
Clinical course of the S-UA level in
certain patients
Patient 10 received rasburicase treatment for seven
days, and the S-UA level was completely suppressed (Fig. 3A). By contrast, patient 9 received two
days of rasburicase treatment followed by the administration of
febuxostat (Fig. 3B). In this case,
despite the short duration of rasburicase use, sequential
administration of febuxostat was suggested to suppress the S-UA
level further (Fig. 3B). For the
comparison between various dosages of rasburicase, patients five
and eight received rasburicase for four days at the doses of 0.13
mg/kg and 0.22 mg/kg, respectively (Fig.
3C and D). These two cases clearly demonstrated an equivalent
time course to achieving normalized S-UA levels (Fig. 3C and D), indicating that a smaller
dose was equally effective for controlling S-UA production. Thus,
the administration of rasburicase at smaller doses or for a shorter
period of administration may be efficacious for managing the S-UA
level in patients receiving chemotherapy with TLS or at risk of
developing TLS. These results suggest that the method of
administration of rasburicase may continue to require
optimization.
Economic considerations
The cost of drugs for treating TLS was calculated in
each patient (Table II). When the
cost for the standard regime of rasburicase treatment, consisting
of 0.2 mg/kg/day for seven days, totaling 699,132 yen, was set as
100%, the mean real drug cost for treating TLS was 326,714 yen
(range, 99,876–699,132 yen), or 47% (range, 14–100%), in all
patients. This suggests that the cost for treating TLS largely
depends on the use of rasburicase, regardless of the addition of
allopurinol or febuxostat.
Discussion
Hyperuricemia in TLS occurs through the catabolism
of purine nucleic acid released from cancer cells upon cell lysis.
It is logical to block xanthine oxidase, which converts purine
metabolites such as hypoxanthine and xanthine to UA, thereby
reducing the production of UA (2,3,8). However, xanthine oxidase inhibitors do
not reduce the already generated UA. Rasburicase effectively
converts UA into much more soluble allantoin (6,16) in the
blood, thereby preventing the crystallization of UA that may lead
to renal damage.
The present retrospective study revealed that
rasburicase was used at various doses for various durations in an
actual clinical setting (Table
III), although the approved method of administration in Japan
is 0.2 mg/kg once daily for a maximum of seven days. Nevertheless,
rasburicase effectively reduced and normalized the S-UA levels
during induction chemotherapy in all of the patients (Figs. 1 and 2).
The re-increase in S-UA subsequent to the discontinuation of
rasburicase administration was further suppressed by the use of
xanthine oxidase inhibitor (Fig. 3).
In addition, the reduction in the dose of rasburicase did not
appear to attenuate the UA-reducing activity (Fig. 3).
Ishizawa et al prospectively compared the
administration of two different doses of rasburicase, 0.15 mg/kg
and 0.2 mg/kg, in Japanese adult patients with leukemia or lymphoma
(17). The primary endpoint was the
normalization of the S-UA level from 48 h subsequent to the first
infusion to 24 h subsequent to the last infusion. The overall
response rate was 100.0% with the administration of 0.15 mg/kg
rasburicase and 96.0% with 0.20 mg/kg rasburicase, indicating that
the two dose levels were equally effective for controlling the S-UA
level. McBride et al evaluated single fixed dosing versus
weight-based dosing strategies for rasburicase to determine the
minimum dose required to mitigate hyperuricemia in the treatment or
prevention of TLS in a total of 373 patients with a hematological
malignancy or solid tumor (18). The
patients were divided into groups receiving 3, 6 or 7.5 mg
rasburicase, or those receiving weight-based dosing. The primary
endpoint was a S-UA level <7.5 mg/dl obtained within 24 h of
receiving rasburicase. McBride et al found that all the
treatments provided comparable UA-lowering efficacy, but the 6 mg
dose resulted in lower sustained UA levels than the 3 mg dose
(18). Vadhan-Raj et al
compared the UA-lowering efficacy of rasburicase (0.15 mg/kg)
administered as a single dose and daily dosing for five days in
adult patients at risk for TLS prospectively (19). The primary endpoint was the
normalization of the S-UA level within 48 h and the persistence of
normal S-UA for five days. In total, 39 out of 40 (98%) patients in
the daily-dose arm and 34 out of 40 (85%) patients in the
single-dose arm demonstrated a sustained S-UA response, although
six patients within the single-dose arm required a second dose for
S-UA levels >7.5 mg/dl (19).
Cortes et al (20) compared
the UA-lowering efficacy between rasburicase at a dose of 0.2 mg/kg
(days 1–5), rasburicase plus allopurinol at a rasburicase dose of
0.2 mg/kg (days 1–3) and an allopurinol dose of 300 mg (days 3–5),
and allopurinol at a dose of 300 mg (days 1–5) for patients at risk
of TLS, using a prospective study design (20). The UA response rate was defined as the
percentage of patients achieving or maintaining S-UA ≤7.5 mg/dl
during days 3–7. The response rates were 87% for rasburicase alone,
78% for rasburicase plus allopurinol, and 66% for allopurinol alone
(20). These studies suggested that a
reduced dose or shorter duration of rasburicase administration
effectively controlled the S-UA level in the majority of cases, but
a re-increase in S-UA may require additional dosing or sequential
use of allopurinol.
In the present retrospective study, rasburicase was
not administered in a uniform regimen. Nevertheless, a reduced dose
or shorter duration of rasburicase administration was efficacious
for managing S-UA in patients with hematological malignancies and
TLS or those at risk of developing TLS. The combination of
rasburicase and febuxostat demonstrated a rapid decrease in S-UA
level and suppressed the re-increase (Fig. 3), suggesting that such a combination
would be a mechanistically more reasonable and cost-effective
approach that treatment with rasburicase alone (Table III). The present study did not
reveal the most appropriate dose and schedule for rasburicase
administration. However, careful monitoring of S-UA levels may
individualize rasburicase therapy for each patient through
adjustment of the dose and the duration, and by combination with a
xanthine oxidase inhibitor.
Glossary
Abbreviations
Abbreviations:
|
TLS
|
tumor lysis syndrome
|
|
S-UA
|
serum uric acid
|
References
|
1
|
Cairo MS and Bishop M: Tumour lysis
syndrome: new therapeutic strategies and classification. Br J
Haematol. 127:3–11. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Coiffier B, Altman A, Pui CH, Younes A and
Cairo MS: Guidelines for the management of pediatric and adult
tumor lysis syndrome: an evidence-based review. J Clin Oncol.
26:2767–2778. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cairo MS, Coiffier B, Reiter A and Younes
A: TLS expert panel: Recommendations for the evaluation of risk and
prophylaxis of tumour lysis syndrome (TLS) in adults and children
with malignant diseases: an expert TLS panel consensus. Br J
Haematol. 149:578–586. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Howard SC, Jones DP and Pui CH: The tumor
lysis syndrome. N Engl J Med. 364:1844–1854. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Firwana BM, Hasan R, Hasan N, Alahdab F,
Alnahhas I, Hasan S and Varon J: Tumor lysis syndrome: a systematic
review of case series and case reports. Postgrad Med. 124:92–101.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wilson FP and Berns JS: Onco-nephrology:
tumor lysis syndrome. Clin J Am Soc Nephrol. 7:1730–1739. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pui CH, Mahmoud HH, Wiley JM, Woods GM,
Leverger G, Camitta B, Hastings C, Blaney SM, Relling MV and Reaman
GH: Recombinant urate oxidase for the prophylaxis or treatment of
hyperuricemia in patients with leukemia or lymphoma. J Clin Oncol.
19:697–704. 2001.PubMed/NCBI
|
|
8
|
McDonnel AM, Lenz KL, Frei-Lahr DA,
Hayslip J and Hall PD: Single-dose rasburicase 6 mg in the
management of tumor lysis syndrome in adults. Pharmacotherapy.
26:806–812. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cammalleri L and Malaguarnera M:
Rasburicase represents a new tool for hyperuricemia in tumor lysis
syndrome and in gout. Int J Med Sci. 4:83–93. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cheuk DK, Chiang AK, Chan GC and Ha SY:
Urate oxidase for the prevention and treatment of tumor lysis
syndrome in children with cancer. Cochrane Database Syst Rev.
16:CD0069452010.
|
|
11
|
Ishizawa K, Ogura M, Hamaguchi M, Hotta T,
Ohnishi K, Sasaki T, Sakamaki H, Yokoyama H, Harigae H and
Morishima Y: Safety and efficacy of rasburicase (SR29142) in a
Japanese phase II study. Cancer Sci. 100:357–62. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Trifilio S, Gordon L, Singhal S, Tallman
M, Evens A, Rashid K, Fishman M, Masino K, Pi J and Mehta J:
Reduced-dose rasburicase (recombinant xanthine oxidase) in adult
cancer patients with hyperuricemia. Bone Marrow Transplant.
37:997–1001. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jeha S, Kantarjian H, Irwin D, Shen V,
Shenoy S, Blaney S, Camitta B and Pui CH: Efficacy and safety of
rasburicase, a recombinant urate oxidase (Elitek), in the
management of malignancy associated hyperuricemia in pediatric and
adult patients: Final results of a multicenter compassionate use
trial. Leukemia. 19:34–38. 2005.PubMed/NCBI
|
|
14
|
The guideline revising committee of
Japanese Society of Gout and Nucleic Acid Metabolism. Digest of the
guideline for management of hyperuricemia and gout. 2nd edition.
Gout Nucleic Acid Metabol. 34:107–143. 2010.(In Japanese).
View Article : Google Scholar
|
|
15
|
Yamauchi T, Negoro E, Lee S, Takai M,
Matsuda Y, Takagi K, Kishi S, Tai K, Hosono N, Tasaki T, Ikegaya S,
Inai K, Yoshida A, Urasaki Y, Iwasaki H and Ueda T: A high serum
uric acid level is associated with poor prognosis in patients with
acute myeloid leukemia. Anticancer Res. 33:3947–3951.
2013.PubMed/NCBI
|
|
16
|
Yeldandi AV, Yeldandi V, Kumar S, Murthy
CV, Wang XD, Alvares K, Rao MS and Reddy JK: Molecular evolution of
the urate oxidase-encoding gene in hominoid primates: Nonsense
mutations. Gene. 109:281–284. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ishizawa K, Ogura M, Hamaguchi M, Hotta T,
Ohnishi K, Sasaki T, Sakamaki H, Yokoyama H, Harigae H and
Morishima Y: Safety and efficacy of rasburicase (SR29142) in a
Japanese phase II study. Cancer Sci. 100:357–362. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
McBride A, Lathon SC, Boehmer L, Augustin
KM, Butler SK and Westervelt P: Comparative evaluation of single
fixed dosing and weight-based dosing of rasburicase for tumor lysis
syndrome. Pharmacotherapy. 33:295–303. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Vadhan-Raj S, Fayad LE, Fanale MA, Pro B,
Rodriguez A, Hagemeister FB, Bueso-Ramos CE, Zhou X, McLaughlin PW,
Fowler N, Shah J, Orlowski RZ, Samaniego F, Wang M, Cortes JE,
Younes A, Kwak LW, Sarlis NJ and Romaguera JE: A randomized trial
of a single-dose rasburicase versus five-daily doses in patients at
risk for tumor lysis syndrome. Ann Oncol. 23:1640–1645. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cortes J, Moore JO, Maziarz RT, Wetzler M,
Craig M, Matous J, Luger S, Dey BR, Schiller GJ, Pham D, Abboud CN,
Krishnamurthy M, Brown A Jr, Laadem A and Seiter K: Control of
plasma uric acid in adults at risk for tumor Lysis syndrome:
efficacy and safety of rasburicase alone and rasburicase followed
by allopurinol compared with allopurinol alone - results of a
multicenter phase III study. J Clin Oncol. 28:4207–4213. 2010.
View Article : Google Scholar : PubMed/NCBI
|