Introduction
Gastric cancer (GC) remains the second leading cause
of cancer-associated mortality worldwide (1,2), and
approximately one-half of worldwide gastric cancer cases occur in
China (3). Similar to other
malignancies, the mechanism of gastric carcinogenesis involves a
complex multi-stage and multi-factorial process that depends on
gene-environment interactions (4). In
previous epidemiological studies, genetic factors were demonstrated
to play an important role in the development of gastric cancer
(5–8).
The expression of human leukocyte antigen (HLA)
class I antigens on the surface of tumor cells is critical for the
recognition of these cells by antigen-specific cytotoxic T
lymphocytes (CTLs), not only for the initiation of CTL-associated
inflammation but also for the anticancer immune response. An
HLA-class I restricted immune response has been reported to
eliminate GC cells, and the expression of HLA-class I antigens may
be essential for the host immune response against cancer (9). Furthermore, downregulation of the
expression of the HLA-class I antigen in GC is associated with
tumor progression and tumor histology (10). Therefore, HLA-class I antigens may
play a crucial role in the development and progression of GC.
Several steps lead to the generation of the tumor
antigen-derived peptides that are presented by HLA class I antigens
to cognate CTLs. In particular, tumor antigens are degraded into
short peptides by the proteasome and immunoproteasome subunits low
molecular weight protein (LMP)2 and LMP7. The resulting peptides
are transported into the endoplasmic reticulum (ER) lumen by
transporter associated with antigen presentation (TAP). In the ER,
newly synthesized HLA class I heavy chains assemble with
β2-microglobulin and peptides. Upon peptide binding, the HLA class
I heterotrimeric complex is released from the ER and transported to
the cell surface via the constitutive secretory pathway (11,12).
Therefore, the integrality of LMP2 and LMP7 function plays an
important role in the processing of GC cell antigens. The present
study hypothesized that the processing of gastric tumor antigens
may be influenced by structural differences encoded by the LMP
alleles.
Several studies have reported an association between
the genetic variants of Arg to His substitution at codon 60 of LMP2
(LMP2-60) and Gln to Lys substitution at codon 145 of LMP7
(LMP7-145) and the risk of numerous malignant diseases, including
ovarian cancer, colon cancer and esophageal carcinoma (13–15).
However, the association between LMP2 and LMP7 polymorphisms and
the risk of GC has not been investigated to date. The present study
evaluated this association by conducting a hospital-based
case-control study.
Materials and methods
Ethical approval and patient
consent
The present study was approved by the Ethics
Committee of the First Affiliated Hospital of Nanjing Medical
University (Nanjing, Jiangsu, China). Written informed consent was
obtained from each subject prior to enrollment in the present
study.
Subjects
The present hospital-based case-control study
included 502 GC cases and 502 cancer-free controls. All
participants were genetically unrelated and resided in Jiangsu or
the surrounding regions. All GC patients were consecutively
recruited at the First Affiliated Hospital of Nanjing Medical
University between May 2010 and April 2014. The diagnosis of GC was
confirmed by endoscopic biopsy or surgical specimens in all
patients. The cancer-free control subjects were matched with the
patients by gender and possessed an age difference of less than
five years. The control subjects were randomly selected from the
First Affiliated Hospital of Nanjing Medical University during the
same selection period as the patients with GC. Subjects with
secondary recurrent malignancies, genetic diseases or those who
underwent non-self blood transfusions were excluded from the
present study, as were patients that received chemotherapy or
radiotherapy. The data on the age, gender, smoking status, urban or
rural residence, hypertension, diabetes and personal medical
history of the patients were collected by questionnaire or medical
records. Individuals that formerly or continued to smoke ≥10
cigarettes per day for at least two years were defined as smokers.
Former smokers that had successfully stopped smoking for >2
years were defined as non-smokers.
Genotyping and haplotype
construction
Genomic DNA was extracted from 2 ml of peripheral
blood using standard methods, and the protocol used for the genomic
DNA extraction was described in a previous study (16). LMP2 and LMP7 gene polymorphisms were
genotyped using the polymerase chain reaction-restriction fragment
length polymorphism (PCR-RFLP) assay. PCR reactions were performed
in a total volume of 20 µl of reaction mixtures containing 2 µl 10X
PCR buffer (Fermentas, Waltham, MA, USA), 1.75 mmol/l
MgCl2, 0.15 mmol/l dNTP, 1 unit Taq polymerase
(Fermentas), 150 ng genomic DNA and 0.25 µmol/l of each primer. The
primers used were as follows: LMP2-60 forward,
5′-CTCCACTTTACAGATGCAGA-3′ and reverse, 5′-ACTTGGTGACTGTTGACTCC-3′;
and LMP7-145 forward, 5′-TCATGGCGCTACTAGATGTATG-3′ and reverse,
5′-AACTCTTTGTCCTAACTTGCAC-3′. For PCR amplification, an initial
denaturation was performed at 95°C for 2 min, followed by 30 cycles
of denaturation at 95°C for 35 sec, annealing at 56.5°C for
LMP2-60, or 57.5°C for LMP7-145, for 30 sec and elongation at 72°C
for 45 sec, with a final elongation step at 72°C for 10 min.
For RFLP, the 330-bp and 351-bp PCR products for the
LMP2-60 and LMP7-145 polymorphisms were digested using the 5 units
each of the restriction enzymes HhaI and BsmI (New
England Biolabs, Ipswich, MA, USA) for 16 h at 37°C and 65°C,
respectively, followed by electrophoresis on a 3% agarose gel. The
Arg/Arg genotype at LMP2-60 was resolved as bands of DNA 79 and 251
bp in length. The His/His genotype yielded a 330-bp band and the
Arg/His genotype yielded 79, 251 and 330-bp bands on agarose
electrophoresis (Fig. 1A). The
Gln/Gln genotype at LMP7-145 yielded 146 and 205-bp bands, the
Lys/Lys genotype yielded a 351-bp band and the Gln/Lys genotype
produced bands 146, 205 and 351 bp in length on agarose
electrophoresis (Fig. 1B). In total,
~10% of the samples were randomly selected and subjected to
repeated assays, and the results were all concordant. The DNA
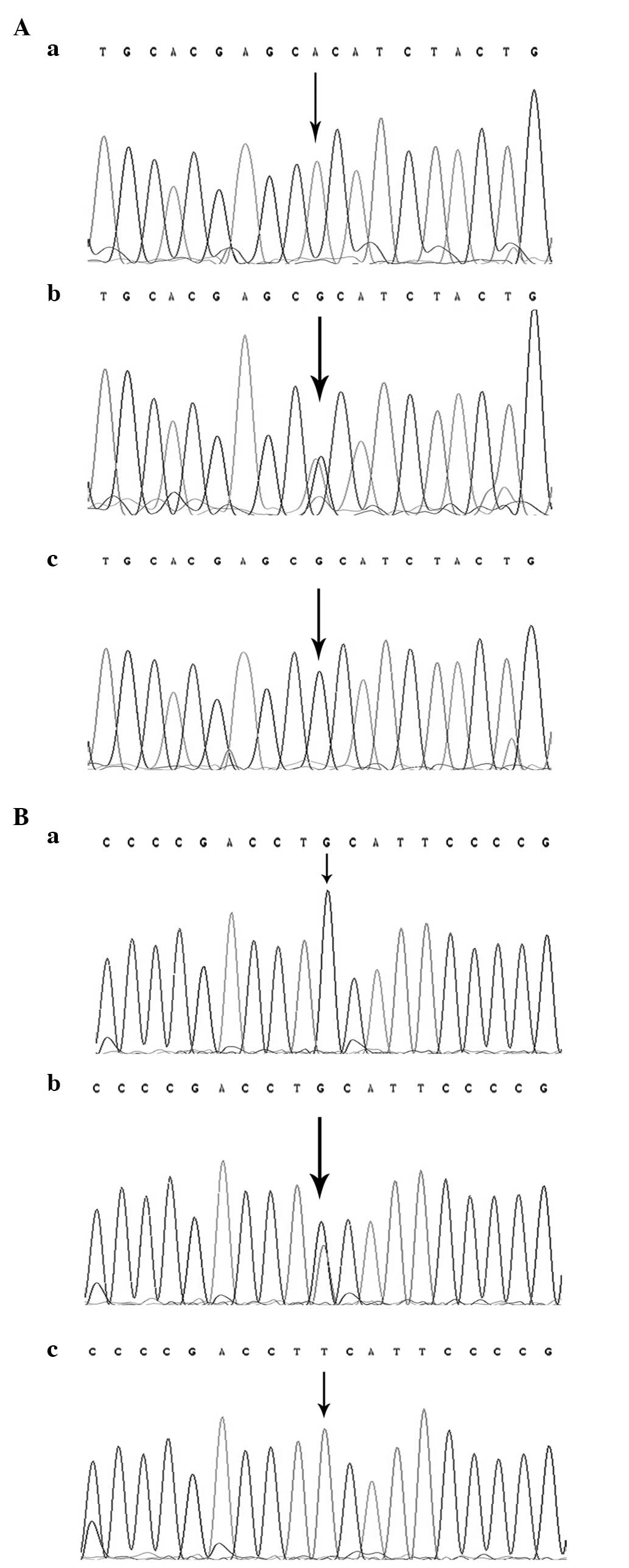
direct sequencing method was used to confirm the genotypes of
LMP2-60 and LMP7-145 (Fig. 2), and
the results were 100% concordant.
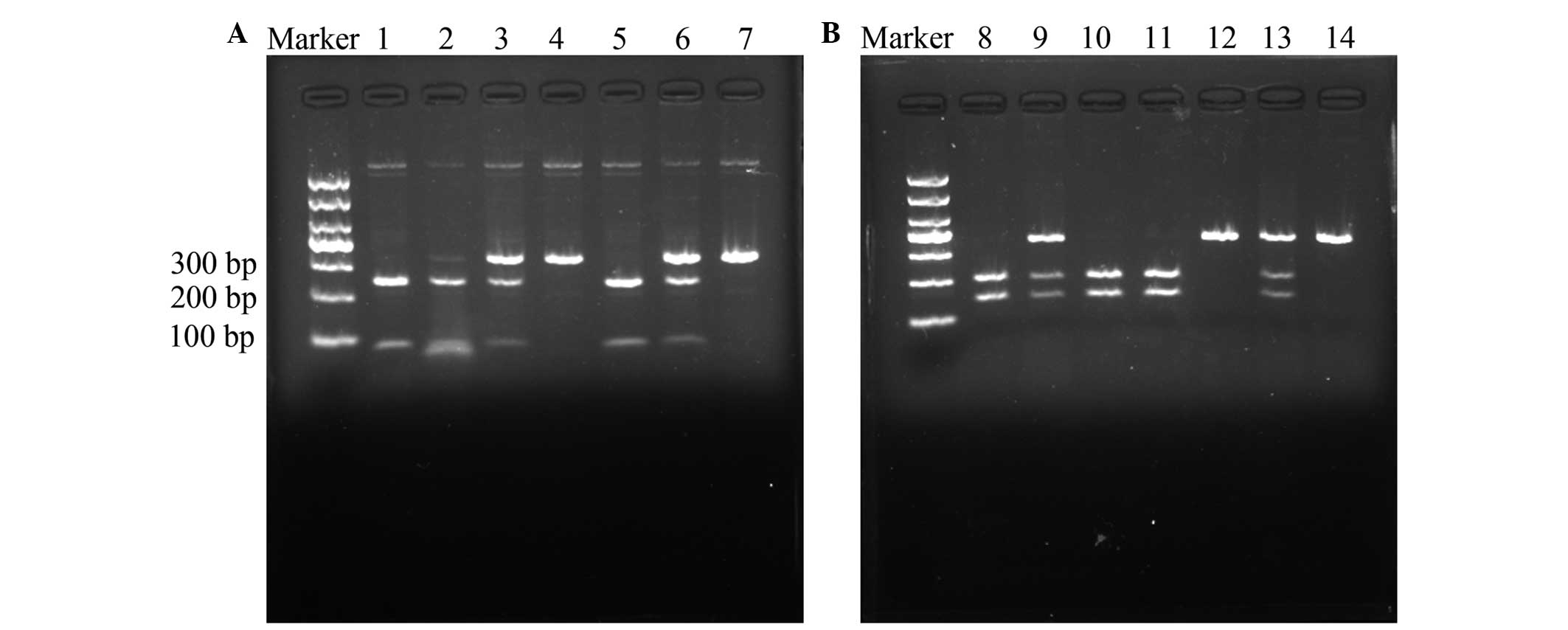 | Figure 1.Digestion of polymerase chain reaction
products by restriction enzymes. (A) Arg to His genotype variation
at codon 60 of LMP2. Lanes 1, 2 and 5, Arg/Arg homozygous cells,
yielding bands of 250 and 79 bp; lanes 3 and 6, Arg/His
heterozygous cells, yielding bands of 329, 250 and 79 bp; and lanes
4 and 7, His/His homozygous cells, yielding a band of 329 bp. (B)
Glyn to Lys genotype variation at codon 145 of LMP7. Lanes 8, 10
and 11, Gln/Gln homozygous cells, yielding bands of 205 and 146 bp;
lanes 9 and 13, Gln/Lys heterozygous cells, yielding bands of 351,
205 and 146 bp; and lanes 12 and 14, Lys/Lys homozygous cells,
yielding a band of 351 bp. LMP, low molecular weight protein. |
The haplotype frequencies of LMP2 and LMP7 gene
polymorphisms were estimated using PHASE2.1 software, based on the
Bayesian algorithm (Matthew Stephens Lab, University of Chicago,
Chicago, IL, USA) (17).
Statistical analysis
Statistical analysis was performed using SPSS
software, version 20.0 (IBM, Armonk, NY, USA). All P-values were
two-tailed and P<0.05 was considered to indicate a statistically
significant difference. Quantitative variables departing from the
normal distribution were summarized as medians and analyzed by the
Mann-Whitney U test. Distributions of categorical variables, and
allele and genotype frequencies in cases and controls were compared
using the Pearson's χ2 test. The Hardy-Weinberg
equilibrium was used to assess the controls using the χ2
goodness of fit test. The odds ratio (OR) and 95% confidence
interval (CI) were used to evaluate the association between
polymorphisms or haplotypes and the risk of GC. The crude OR was
calculated using the Woolf approximation method and the adjusted OR
was computed by multivariate analysis with unconditional logistic
regression. The data were adjusted for age, gender, hypertension,
diabetes, smoking status and residence.
Results
Demographic information
The present cohort comprised 502 gastric cancer
patients and 502 age and gender-matched cancer-free control
individuals. The demographic characteristics of the study subjects
are shown in Table I. No significant
differences in the distribution of age, gender, hypertension,
diabetes and residence were observed between the GC and control
groups. However, the proportion of smokers was significantly higher
in patients with GC compared with control individuals
(P=0.003).
 | Table I.Demographic characteristics of
patients with gastric cancer and control individuals (n=502). |
Table I.
Demographic characteristics of
patients with gastric cancer and control individuals (n=502).
|
| Value per group, n
(%) |
|
|---|
|
|
|
|
|---|
| Characteristics | Gastric cancer | Control | P-value |
|---|
| Gender |
|
| 0.188 |
| Male | 364 (72.5) | 345 (68.7) |
|
|
Female | 138 (27.5) | 157 (31.3) |
|
| Median age, years
(25th-75th percentiles) | 60 (52–66) | 59 (49–69) | 0.916 |
| Hypertension |
|
| 0.439 |
| Yes | 129 (25.7) | 140 (27.9) |
|
| No | 373 (74.3) | 362 (72.1) |
|
| Diabetes |
|
| 0.236 |
| Yes | 52 (10.4) | 64 (12.7) |
|
| No | 450 (89.6) | 438 (87.3) |
|
| Smoking |
|
| 0.003 |
|
Smoker | 123 (24.5) | 85 (16.9) |
|
|
Non-smoker | 379 (75.5) | 417 (83.1) |
|
| Residence |
|
| 0.200 |
|
Rural | 289 (57.6) | 265 (52.8) |
|
|
Urban | 213 (42.4) | 237 (47.2) |
|
Polymorphism of LMP2 and LMP7,
haplotype analysis and the association with gastric cancer
The distribution of the polymorphisms of LMP2 and
LMP7 was within the Hardy-Weinberg equilibrium (P>0.05; Table II). The allele and variant genotypes
of the LMP7 gene were significantly different between the patients
with GC and control subjects. The frequency of the Lys allele was
markedly increased in patients with GC compared with the control
subjects (P=0.004; OR, 1.39; 95% CI, 1.11–1.74). Compared with the
Gln/Gln genotype, the frequency of the LMP7-145 Gln/Lys
heterozygotic genotype was significantly increased in patients with
GC compared with control subjects (P=0.049; adjusted OR, 1.32; 95%
CI, 1.00–1.73). Similarly, the frequency of the Lys/Lys homozygous
genotype was increased in GC patients compared with control
individuals (P=0.041; adjusted OR, 2.13; 95% CI, 1.03–4.39). In
addition, with the Gln/Gln genotype as a reference, the variant
genotypes (Gln/Lys + Lys/Lys) were associated with an increased
susceptibility to GC (P=0.017; adjusted OR, 1.38; 95% CI,
1.06–1.80) subsequent to adjustment of the data for age, gender,
smoking status, hypertension, diabetes and residence. However, no
significant association between the LMP2-60 polymorphism and GC was
observed in the present study (P>0.05; Table II).
 | Table II.Association between polymorphisms in
the LMP2 and LMP7 genes and the risk of gastric cancer. |
Table II.
Association between polymorphisms in
the LMP2 and LMP7 genes and the risk of gastric cancer.
|
| Incidence, n (%) |
|
|
|
|
|---|
|
|
|
|
|
|
|
|---|
| Genotype | Gastric cancer | Control | Crude OR (95%
CI) | P-value | Adjusted OR (95%
CI)a | P-value |
|---|
| Total | 502 (100.00) | 502 (100.00) |
|
|
|
|
| LMP2–60 |
|
|
|
|
|
|
|
Arg/Arg | 338 (67.3) | 335 (66.7) | 1 |
| 1 |
|
|
Arg/His | 151 (30.1) | 155 (30.9) | 0.97
(0.74–1.27) | 0.799 | 0.97
(0.74–1.27) | 0.819 |
| His/
His | 13 (2.6) | 12 (2.4) | 1.07
(0.48–2.39) | 0.861 | 1.08
(0.48–2.41) | 0.857 |
| Arg/His
+ His/His | 164 (32.7) | 167 (33.3) | 0.97
(0.75–1.27) | 0.840 | 0.98
(0.75–1.27) | 0.856 |
| Allelic |
|
|
|
|
|
|
|
Arg | 827 (82.4) | 825 (82.3) | 0.99
(0.78–1.24) | 0.907 |
|
|
|
His | 177 (17.6) | 179 (17.8) |
|
|
|
|
| HWE | 0.424 | 0.228 |
|
|
|
|
| LMP7–145 |
|
|
|
|
|
|
|
Gln/Gln | 310 (61.8) | 349 (69.5) | 1 |
| 1 |
|
|
Gln/Lys | 169 (33.7) | 141 (28.1) | 1.35
(1.03–1.77) | 0.030 | 1.32
(1.00–1.73) | 0.049 |
|
Lys/Lys | 23 (4.6) | 12 (2.4) | 2.16
(1.06–4.41) | 0.035 | 2.13
(1.03–4.39) | 0.041 |
| Gln/Lys
+ Lys/Lys | 192 (38.3) | 153 (30.5) | 1.41
(1.09–1.84) | 0.010 | 1.38
(1.06–1.80) | 0.017 |
| Allelic |
|
|
|
|
|
|
|
Gln | 789 (78.6) | 839 (83.6) | 1.39
(1.11–1.74) | 0.004 |
|
|
|
Lys | 215 (21.4) | 165 (16.4) |
|
|
|
|
| HWE | 0.995 | 0.613 |
|
|
|
|
Each individual haplotype derived from polymorphism
of the LMP2 and LMP7 genes was constructed using PHASE2.1 software
based on the Bayesian algorithm. When compared with the most
frequent Arg-Gln haplotype, the Arg-Lys haplotype was associated
with the risk of GC (P=0.013; adjusted OR, 1.34; 95% CI,
1.06–1.70). However, no association was detected between the other
two haplotypes and the risk of GC (Table III).
 | Table III.Associated risk analysis between the
gastric cancer and the haplotypes constructed by LMP2/LMP7 gene
polymorphisms. |
Table III.
Associated risk analysis between the
gastric cancer and the haplotypes constructed by LMP2/LMP7 gene
polymorphisms.
|
| Incidence, n |
|
|
|
|
|---|
|
|
|
|
|
|
|
|---|
| Haplotype | Gastric cancer | Control | Crude OR (95%
CI) | P-value | Adjusted OR (95%
CI)a | P-value |
|---|
| LMP2 (Arg)-LMP7
(Gln) | 616 | 659 | 1 |
| 1 |
|
| LMP2 (Arg)-LMP7
(Lys) | 211 | 165 | 1.37
(1.09–1.72) | 0.008 | 1.34
(1.06–1.70) | 0.013 |
| LMP2 (His)-LMP7
(Gln) | 173 | 178 | 1.04
(0.82–1.32) | 0.746 | 1.04
(0.82–1.32) | 0.751 |
| LMP2 (His)-LMP7
(Lys) | 4 | 2 | 2.14
(0.39–11.72) | 0.381 | 2.13
(0.38–11.86) | 0.387 |
Stratified analysis of polymorphisms
and gastric cancer risk
The results of the stratified analysis of the LMP2
and LMP7 polymorphisms according to the median age of controls (59
years), gender, smoking status and residence are summarized in
Table IV. The variant genotypes of
LMP7-145, consisting of the Gln/Lys and Lys/Lys variants,
significantly increased the risk of GC in subjects aged >59
years (P=0.024; adjusted OR, 1.57; 95% CI, 1.06–2.31), males
(P=0.015; adjusted OR, 1.48; 95% CI, 1.08–2.03) and non-smokers
(P=0.010; adjusted OR, 1.48; 95% CI, 1.10–1.99). However, the
association was not statistically significant in subjects aged ≤59,
females and smokers. No statistically significant association was
identified between the location of residence and the presence of
polymorphisms or the susceptibility to GC. Furthermore, no
significant association was observed between the LMP2-60
polymorphisms and age, gender, smoking status and residence.
 | Table IV.Stratified analyses of the genotypes
of the LMP2 and LMP7 genes in patients with gastric cancer and
control individuals. |
Table IV.
Stratified analyses of the genotypes
of the LMP2 and LMP7 genes in patients with gastric cancer and
control individuals.
|
| LMP2-60 Arg/His +
His/His vs. Arg/Arg |
|
| LMP7-145 Gln/Lys +
Lys/Lys vs. Gln/Gln |
|
|
|---|
|
|
|
|
|
|
|
|
|---|
| Variable | Gastric cancer, n
(%) | Control, n (%) | Allelic OR (95%
CI)a | P-value | Gastric cancer, n
(%) | Control, n (%) | Allelic OR (95%
CI)a | P-value |
|---|
| Median age,
years |
|
|
≤59 | 91 (18.1)/156
(31.1) | 89 (17.7)/172
(34.3) | 1.13
(0.78–1.63) | 0.508 | 97 (19.3)/150
(29.9) | 88 (17.5)/173
(34.5) | 1.25
(0.87–1.80) | 0.235 |
|
>59 | 73 (14.5)/182
(36.3) | 78 (15.5)/163
(32.5) | 0.83
(0.56–1.22) | 0.336 | 95 (18.9)/160
(31.9) | 65 (12.9)/176
(35.1) | 1.57
(1.06–2.31) | 0.024 |
| Gender |
|
|
|
|
|
|
|
|
|
Females | 45 (9.0)/93
(18.5) | 52 (10.4)/105
(20.9) | 0.97
(0.59–1.60) | 0.918 | 46 (9.2)/92
(18.3) | 47 (9.4)/110
(21.9) | 1.21
(0.73–2.00) | 0.465 |
|
Males | 119 (23.7)/245
(48.8) | 115 (22.9)/230
(45.8) | 0.97
(0.71–1.33) | 0.842 | 146 (29.1)/218
(43.4) | 106 (21.1)/239
(47.6) | 1.48
(1.08–2.03) | 0.015 |
| Smoking status |
|
|
|
|
|
|
|
|
|
Smokers | 42 (8.4)/81
(16.1) | 28 (5.6)/57
(11.4) | 1.07
(0.59–1.95) | 0.822 | 49 (9.8)/74
(14.7) | 32 (6.4)/53
(10.6) | 1.15
(0.64–2.08) | 0.634 |
|
Non-smokers | 122 (24.3)/257
(51.2) | 139 (27.7)/278
(55.4) | 0.95
(0.71–1.28) | 0.756 | 143 (28.5)/236
(47.0) | 121 (24.1)/296
(59.0) | 1.48
(1.10–1.99) | 0.010 |
| Residence |
|
|
|
|
|
|
|
|
|
Rural | 91 (18.1)/198
(39.4) | 86 (17.1)/179
(35.7) | 0.95
(0.66–1.36) | 0.767 | 110 (21.9)/179
(35.7) | 82 (16.3)/183
(36.5) | 1.29
(0.90–1.85) | 0.160 |
|
Urban | 73 (14.5)/140
(27.9) | 81 (16.1)/156
(31.1) | 0.99
(0.67–1.47) | 0.951 | 82 (16.3)/131
(26.1) | 71 (14.1)/166
(33.1) | 1.46
(0.98–2.17) | 0.061 |
The effects of the LMP2 and LMP7 polymorphisms were
evaluated subsequent to stratification of the GC patients by
clinicopathological characteristics (Table V), which revealed no significant
association between the SNPs and the depth of tumor infiltration,
grade of differentiation, lymph node metastasis or location of the
primary cancer.
 | Table V.Association between the variant
genotypes of the LMP2 and LMP7 genes and the clinicopathological
characteristics of gastric cancer. |
Table V.
Association between the variant
genotypes of the LMP2 and LMP7 genes and the clinicopathological
characteristics of gastric cancer.
|
| LMP2-60 | LMP7-145 |
|---|
|
|
|
|
|---|
| Variable | Arg/His + His/His,
n | Arg/Arg, n | Allelic OR (95%
CI)a | P-value | Gln/Lys + Lys/Lys,
n | Gln/Gln, n | Allelic OR (95%
CI)a | P-value |
|---|
| Tumor
differentiation |
|
|
|
|
|
|
|
|
| Well | 5 | 18 | 1 |
| 8 | 15 | 1 |
|
| Moderate | 44 | 81 | 1.84
(0.62–5.48) | 0.269 | 44 | 81 | 1.00
(0.38–2.66) | 0.993 |
| Poor | 115 | 239 | 1.73
(0.62–4.83) | 0.292 | 140 | 214 | 1.12
(0.46–2.75) | 0.804 |
| Depth of tumor
infiltration |
|
|
|
|
|
|
|
|
| T1 | 26 | 54 | 1 |
| 35 | 45 | 1 |
|
| T2 | 19 | 38 | 1.27
(0.58–2.77) | 0.551 | 17 | 40 | 0.48
(0.22–1.06) | 0.070 |
| T3 | 59 | 119 | 1.01
(0.57–1.81) | 0.965 | 70 | 108 | 0.87
(0.50–1.50) | 0.615 |
| T4 | 60 | 127 | 0.90
(0.50–1.61) | 0.712 | 70 | 117 | 0.71
(0.41–1.23) | 0.216 |
| Lymph node
metastasis |
|
|
|
|
|
|
|
|
| Negative | 59 | 115 | 1 |
| 71 | 103 | 1 |
|
| Positive | 105 | 223 | 0.90
(0.60–1.33) | 0.579 | 121 | 207 | 0.85
(0.58–1.24) | 0.399 |
| Localization |
|
|
|
|
|
|
|
|
| Cardia | 75 | 129 | 1 |
| 73 | 131 | 1 |
|
| Non-cardia | 89 | 209 | 0.71
(0.49–1.04) | 0.081 | 119 | 179 | 1.19
(0.82–1.73) | 0.365 |
Discussion
Genetic and environmental factors influence the type
of malignant tumor that develops and the growth of the tumor
(18). In the present study, the
association between LMP2 and LMP7 polymorphisms and the risk of GC
was investigated. The LMP2 and LMP7 genes are located in a short,
800-kb region of the short arm of chromosome 6, which encodes the
proteins essential for sequential peptide processing in the pathway
of major histocompatibility complex (MHC)-I antigen presentation
(19,20).
Cancer cells escape immune recognition through
reduced MHC expression, as the presentation of immunogenic tumor
peptides by HLA class I antigens is a prerequisite for a successful
antitumor immune response (21). The
efficient expression of peptide-HLA complexes at the cell surface
depends on the type and quantity of the peptides that are produced
and processed (14). Overexpression
of the LMP2 and LMP7 gene may lead to the production of an
insufficient peptide level, which may allow cancer cells to escape
immune processing, leading to GC development. It was hypothesized
that the Arg to His polymorphism at LMP2-60 and Gln to Lys
polymorphism at LMP7-145 may lead to functional alterations that
affect the development and progression of gastric cancer.
To verify the hypothesis that LMP2 and LMP7
polymorphisms may be associated with the risk of GC, a
hospital-based case-control study that consisted of 502 gastric
cancer patients and 502 healthy control individuals was performed.
For LMP7, the data revealed that the frequency of the Lys/Lys
genotype at LMP7-145 was significantly increased in gastric cancer
patients compared with control individuals (P=0.041; adjusted OR,
2.13; 95% CI, 1.03–4.39). However, the Gln/Lys genotype was more
common in patients with GC compared with control individuals
(P=0.049; adjusted OR, 1.32; 95% CI, 1.00–1.73). Compared with the
common genotype Gln/Gln, patients possessing the Gln/Lys + Lys/Lys
genotype demonstrated a 38% increased risk of developing GC
(P=0.017; adjusted OR, 1.38; 95% CI, 1.06–1.80). Separate analysis
of the Lys allele revealed that this allele was more prevalent in
patients with GC compared with control individuals (21.4 vs. 16.4%;
P=0.004; OR, 1.39; 95% CI, 1.11–1.74). These results indicated that
the Gln/Lys + Lys/Lys genotypes at LMP7-145, in which Gln is
substituted with Lys, were significantly associated with an
increased risk of GC and the Lys allele may be an independent risk
factor of GC.
No association was identified between the LMP2-60
genotypes or alleles and the risk of GC. These results were
consistent with those reported in the majority of previous studies
that investigated the association between polymorphisms in LMP2 and
LMP7, and other diseases (11–13,22,23).
However, Ozbas-Gerceker et al reported that LMP2 plays a
role in the development of acute myeloid leukemia and multiple
myeloma, whereas LMP7 is not a risk factor for hematological
malignancies (24). The discrepancy
between these results and the present findings may be attributed to
the fact that the same polymorphism may exert different genetic
effects on various types of cancer (25,26).
Overall, the present statistical analysis revealed a
significant association between the risk of GC and polymorphisms of
LMP7, but not polymorphisms of LMP2, and these findings are in
agreement with the LMP2/7 gene expression levels in human GC cells.
According to Kang et al, MHC class I surface expression is
associated, in the majority of cases, with the expression of the
LMP7 gene, regardless of the LMP2, TAP1, TAP2 or MHC class I genes
(27).
Based on the two polymorphisms of LMP2 and LMP7
being located close to each other in the MHC class II region, four
haplotypes were constructed. The present results indicated that
compared with the most frequent Arg-Gln haplotype, the Arg-Lys
haplotype was more frequent in patients with GC compared with
control individuals (P=0.013; adjusted OR, 1.34; 95% CI,
1.06–1.70). This was consistent with the effects of the single LMP7
SNP. However, the OR was low compared with the OR of the Lys allele
of the LMP7 polymorphism alone. In addition, no association was
identified between the incidence of the His-Lys haplotype and the
occurrence if GC. These results suggest that the LMP2 polymorphism
may weaken the susceptibility of the haplotype to GC. However,
further fine mapping studies are required to clarify this
mechanism.
In the stratified analysis of the present study, the
data revealed that polymorphisms at LMP7-145 were associated with
an increased risk of GC in patients aged >59 years, but not in
those aged ≤59 years. Carcinogenesis is an accumulation of genetic
events during aging, and the age-dependent increase in the
incidence of GC demonstrates a steep slope (28). The increased risk of GC in the elderly
indicates that gene-environment interactions may be involved in
carcinogenesis, and the LMP7-145 genotype effects tended to be
age-specific. A significantly elevated risk was also identified in
male subjects. A previous study performed using a Chinese cohort
reported that non-cardia gastric cancer is more common in males
than females, with a ratio of ~2:1, and that gastric cardia cancer
has an increased male to female ratio of ~4.1:1 (29). These findings suggest that LMP7-145
polymorphisms play an important role in men with GC. In the present
stratified analysis of smoking status, an evident association
between polymorphisms at LMP7-145 and the risk of GC was observed
in non-smokers, but not in smokers. Tobacco smoke is a known
independent risk factor for GC (3).
The association between the incidence of polymorphism and GC risk
may be masked by the overwhelming accumulated exposure to tobacco
carcinogens in smokers, resulting in a more significant association
among non-smokers (30). However,
additional studies are required to clarify the association between
the LMP7-145 polymorphism and GC in men, non-smokers and elderly
patients.
The present study demonstrates several limitations.
Firstly, the relatively small sample size may have limited the
statistical power of the data. Secondly, the consecutive enrollment
of subjects from the same hospital and during the same period may
have led to a selection bias. However, the genotype distribution in
the healthy control individuals in the present study was compatible
with the Hardy-Weinberg expectations. Thirdly, Helicobacter
pylori is an independent risk factor for gastric cancer, but
the variable was not tested for, as it was unethical to perform the
test for Helicobacter pylori on each subject, particularly
in the control individuals. Additionally, in the stratified
analysis, the risk association with Lauren's classifications
(31) and the consumption of alcohol
was not evaluated. Future studies should include these variables in
the stratified analysis. Lastly, due to the analysis being limited
to individuals from the Chinese population, extrapolation of the
present results to other regions and ethnicities should be
performed with caution.
In conclusion, to the best of our knowledge, the
present study is the first to demonstrate that LMP7 polymorphisms
are associated with an increased risk of GC in a Chinese
population, particularly in older individuals, non-smokers and
males. Additional studies with larger sample sizes and the
inclusion of various populations, as well as functional studies,
are required to verify the present initial findings.
Acknowledgements
This study was supported by grants from the Medical
Zhong Dian Ren Cai Project of Jiangsu Province (grant no.,
RC2011059), Natural Science Foundation of Jiangsu Province [grant
no., BK20131447 (DA13)], Six RenCai Gaofeng, 333 Project Leader of
Jiangsu Province to L. Yang and A Project Funded by the Priority
Academic Program Development of Jiangsu Higher Education
Institutions (PAPD) (grant no., JX10231801).
References
|
1
|
Danaei G, Vander Hoorn S, Lopez AD, Murray
CJ and Ezzati M: Comparative Risk Assessment collaborating group
(Cancers): Causes of cancer in the world: Comparative risk
assessment of nine behavioural and environmental risk factors.
Lancet. 366:1784–1793. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pisani P, Parkin DM, Bray F and Ferlay J:
Erratum: Estimates of the worldwide mortality from 25 cancers in
1990. Int. J. Cancer. 83:18–29. 1999.
Int J Cancer. 83:870–873. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Parkin DM: International variation.
Oncogene. 23:6329–6340. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wu MS, Chen CJ and Lin JT:
Host-environment interactions: Their impact on progression from
gastric inflammation to carcinogenesis and on development of new
approaches to prevent and treat gastric cancer. Cancer Epidemiol
Biomarkers Prev. 14:1878–1882. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhu H, Yang L, Zhou B, Yu R, Tang N and
Wang B: Myeloperoxidase G-463A polymorphism and the risk of gastric
cancer: A case-control study. Carcinogenesis. 27:2491–2496. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sun Q, Gu H, Zeng Y, Xia Y, Wang Y, Jing
Y, Yang L and Wang B: Hsa-mir-27a genetic variant contributes to
gastric cancer susceptibility through affecting miR-27a and target
gene expression. Cancer Sci. 101:2241–2247. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang L, Gu HJ, Zhu HJ, Sun QM, Cong RH,
Zhou B, Tang NP and Wang B: Tissue inhibitor of metalloproteinase-2
G-418C polymorphism is associated with an increased risk of gastric
cancer in a Chinese population. Eur J Surg Oncol. 34:636–641. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yang L, Zhu H, Zhou B, Gu H, Yan H, Tang
N, Dong H, Sun Q, Cong R, Chen G, et al: The association between
the survivin C-31G polymorphism and gastric cancer risk in a
Chinese population. Dig Dis Sci. 54:1021–1028. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Suzuki K, Sahara H, Okada Y, Yasoshima T,
Hirohashi Y, Nabeta Y, Hirai I, Torigoe T, Takahashi S, Matsuura A,
et al: Identification of natural antigenic peptides of a human
gastric signet ring cell carcinoma recognized by HLA-A31-restricted
cytotoxic T lymphocytes. J Immunol. 163:2783–2791. 1999.PubMed/NCBI
|
|
10
|
Ishigami S, Natsugoe S, Nakajo A, Arigami
T, Kitazono M, Okumura H, Matsumoto M, Uchikado Y, Setoyama T, et
al: HLA-class I expression in gastric cancer. J Surg Oncol.
97:605–608. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ferris RL, Hunt JL and Ferrone S: Human
leukocyte antigen (HLA) class I defects in head and neck cancer:
Molecular mechanisms and clinical significance. Immunol Res.
33:113–133. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Grommé M and Neefjes J: Antigen
degradation or presentation by MHC class I molecules via classical
and non-classical pathways. Mol Immunol. 39:181–202. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Song L, Ma N, Han L, Yan H, Yan B, Yuan Z
and Cao B: Association between LMP2/LMP7 genetic variability and
the metastasis risk of ovarian cancer in Chinese women in Beijing.
Hum Immunol. 75:239–244. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fellerhoff B, Gu S, Laumbacher B, Nerlich
AG, Weiss EH, Glas J, Kopp R, Johnson JP and Wank R: The LMP7-K
allele of the immunoproteasome exhibits reduced transcript
stability and predicts high risk of colon cancer. Cancer Res.
71:7145–7154. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cao B, Tian X, Li Y, Jiang P, Ning T, Xing
H, Zhao Y, Zhang C, Shi X, Chen D, et al: LMP7/TAP2 gene
polymorphisms and HPV infection in esophageal carcinoma patients
from a high incidence area in China. Carcinogenesis. 26:1280–1284.
2005.PubMed/NCBI
|
|
16
|
Zhu H, Yang L, Zhou B, Yu R, Tang N and
Wang B: Myeloperoxidase G-463A polymorphism and the risk of gastric
cancer: A case-control study. Carcinogenesis. 27:2491–2496. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Stephens M and Donnelly P: A comparison of
bayesian methods for haplotype reconstruction from population
genotype data. Am J Hum Genet. 73:1162–1169. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hemminki K, Försti A and Bermejo JL:
Gene-environment interactions in cancer: do they exist? Ann NY Acad
Sci. 1076:137–148. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Basler M, Kirk CJ and Groettrup M: The
immunoproteasome in antigen processing and other immunological
functions. Curr Opin Immunol. 25:74–80. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hussong SA, Roehrich H, Kapphahn RJ,
Maldonado M, Pardue MT and Ferrington DA: A novel role for the
immunoproteasome in retinal function. Invest Ophthalmol Vis Sci.
52:714–723. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lee YM, Leu SY, Chiang H, Fung CP and Liu
WT: Human papillomavirus type 18 in colorectal cancer. J Microbiol
Immunol Infect. 34:87–91. 2001.PubMed/NCBI
|
|
22
|
Lv Y, Yan B, Yang H, Liu J, Zhong W, Li K,
Chen Z and Xu C: LMP2/LMP7 gene variant: A risk factor for
intestinal Mycobacterium tuberculosis infection in the Chinese
population. J Gastroenterol Hepatol. 26:1145–1150. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Camarena A, Aquino-Galvez A,
Falfán-Valencia R, Sánchez G, Montaño M, Ramos C, Juárez A,
García-de-Alba C, Granados J and Selman M: PSMB8 (LMP7) but not
PSMB9 (LMP2) gene polymorphisms are associated to pigeon breeder's
hypersensitivity pneumonitis. Respir Med. 104:889–894. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ozbas-Gerceker F, Bozman N, Kok S,
Pehlivan M, Yilmaz M, Pehlivan S and Oguzkan-Balci S: Association
of an LMP2 polymorphism with acute myeloid leukemia and multiple
myeloma. Asian Pac J Cancer Prev. 14:6399–6402. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yu Z, Liu Q, Huang C, Wu M and Li G: The
interleukin 10 −819C/T polymorphism and cancer risk: A HuGE review
and meta-analysis of 73 studies including 15,942 cases and 22,336
controls. OMICS. 17:200–214. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang YM, Zhou XC, Xu Z and Tang CJ:
Meta-analysis of epidemiological studies of association of two
polymorphisms in the interleukin-10 gene promoter and colorectal
cancer risk. Genet Mol Res. 11:3389–3397. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kang JK, Yoon SJ, Kim NK and Heo DS: The
expression of MHC class I, TAP1/2, and LMP2/7 gene in human gastric
cancer cell lines. Int J Oncol. 16:1159–1163. 2000.PubMed/NCBI
|
|
28
|
Milne AN, Carvalho R, Morsink FM, Musler
AR, de Leng WW, Ristimäki A and Offerhaus GJ: Early-onset gastric
cancers have a different molecular expression profile than
conventional gastric cancers. Mod Pathol. 19:564–572. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kelley JR and Duggan JM: Gastric cancer
epidemiology and risk factors. J Clin Epidemiol. 56:1–9. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gu H, Yang L, Sun Q, Zhou B, Tang N, Cong
R, Zeng Y and Wang B: Gly82Ser polymorphism of the receptor for
advanced glycation end products is associated with an increased
risk of gastric cancer in a Chinese population. Clin Cancer Res.
14:3627–3632. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lauren P: The two histological main types
of gastric carcinoma: Diffuse and so-called intestinal-type
carcinoma An attempt at a histo-clinical classification. Acta
Pathol Microbiol Scand. 64:31–49. 1965.PubMed/NCBI
|