Introduction
Primary salivary gland-type tumors of the lung
account for ~0.1–1% of all primary lung carcinomas; among them, the
most frequently observed histological subtype is mucoepidermoid
carcinoma, followed by adenoid cystic carcinoma (1,2).
Epithelial-myoepithelial carcinoma (EMC) is less common and
accounts for approximately 4 and 8% of the primary salivary
gland-type tumors in Korea and China, respectively (1,2). There
have been ~50 cases of pulmonary EMC reported to date (3).
The presence of EMC in the salivary gland was first
described by Donath et al in 1972 (4). EMCs have now been demonstrated to
account for 1.0% of all salivary gland tumors (5). Despite its predilection for the parotid
gland, EMC also occurs less frequently in other locations,
including the minor salivary glands or seromucous gland sites, such
as the upper and lower respiratory tract (5). EMC of the salivary gland is considered
to derive from the intercalated duct (6). It is speculated that the tumor
originates from the ductal structure of the bronchial gland, which
is a counterpart of the lungs (7).
Each tumor nest presents with a characteristic morphology comprised
of a biphasic pattern with an inner layer of epithelial cells and
an outer layer of myoepithelial cells. The characteristics of its
counterpart in the lung remain to be elucidated due to the limited
number of cases. A number of pathologists have proposed the term
pulmonary epithelial-myoepithelial tumor to describe this type of
tumor, which has unproven malignant potential (8); however, there have been at least 3
reported cases of high-grade pulmonary EMC that presented with
metastasis in the regional lymph nodes (9), bone (2)
and chest wall (3).
The sequential development of high-grade
malignancies inside pre-existing tumors has recently been defined
as ‘high-grade transformation’ (HGT). The term ‘dedifferentiation’
once included HGT; however, it is now only applied in situations in
which a high-grade neoplasm that has progressed from a low-grade
neoplasm loses the histological characteristics of its original
lineage (10). Salivary EMCs and
numerous other histological subtypes have been reported to undergo
HGT (10). Although EMC is generally
considered to be a low-grade malignancy with good clinical
prognosis (5,11), a number of studies have revealed that
EMC with HGT is exceptionally aggressive, and exhibits poor
prognosis with a high rate of distant metastasis (12). Although >20 cases of EMC with HGT
concentrated in the salivary glands have previously been reported
(13), to the best of our knowledge,
pulmonary EMCs with HGT have yet to be documented. In the present
case report, a case of EMC is presented that demonstrates
progression of the myoepithelial overgrowth into a myoepithelial
carcinomatous proliferation and exhibits HGT-like features.
Case report
In February 2014, a 72-year-old woman was admitted
to Fujieda Municipal General Hospital (Fujieda, Shizuoka, Japan)
for evaluation of a mass detected on a chest radiograph during a
routine health examination. The patient had no history of smoking
and her past medical history was unremarkable. Subsequent enhanced
computed tomography demonstrated an enhancing lobular mass
measuring 34×42×30 mm in size, with multiple focal areas of low
attenuation in the S8 segment of the left lung (Fig. 1A). The proximal bronchovascular bundle
adjacent to the left hilum was involved by the mass. In the coronal
view, the left B8 segment of the lung was observed to be occluded
by the tumor (Fig. 1B). No
mediastinal lymph node metastasis or other organ metastases were
observed. No abnormalities were observed in the salivary glands. A
transbronchial lung biopsy of the area was conducted, and the
tissue was demonstrated to contain atypical cells that were not
observed in normal bronchopulmonary tissue. The serum levels of the
tumor markers were as follows, with reference ranges in brackets:
Carcinoembryonic antigen, 3.4 ng/ml (0–5.0 ng/ml); carbohydrate
antigen 19-9, 9.1 U/ml (0–37 U/ml); squamous cell carcinoma
antigen, 0.3 ng/ml (0–1.5 ng/ml); cytokeratin 19 fragment, 1.7 U/ml
(0–3.5 U/ml); and pro-gastrin-releasing peptide, 87.9 pg/ml (0–80.0
pg/ml). Video-assisted thoracoscopic left lower pneumonectomy was
performed. Although a definitive diagnosis of the tumor was not
possible during intraoperative examination due to unfamiliar
histology, gross observation of the resected tissue strongly
indicated malignancy. Consequently, a lymph node dissection was
performed at N2a-1, and no lymph node metastasis was identified. No
recurrence has been observed for 4 months.
The surgically resected specimen was fixed with 10%
buffered formalin (Formaldehyde Solution; Wako Pure Chemical
Industries, Ltd., Osaka, Japan) for ~24 h. Then, 5-mm thick tissue
slices were embedded in paraffin to prepare paraffin blocks.
Sections (2.5-µm thick) were cut from each paraffin block for
hematoxylin and eosin staining (New Hematoxylin Type G and Eosin Y;
Muto Pure Chemicals Co., Ltd., Tokyo, Japan); 4-µm sections were
used for immunohistochemistry (IHC). A Bench-Mark XT automated
slide stainer (Ventana Medical Systems, Tucson, AZ, USA) was used
to perform IHC. The primary antibodies used in the IHC analysis are
listed in Table I. The ultraView
Universal DAB Detection Kit (Ventana Medical Systems) was used for
visualization.
 | Table I.Antibodies used in the present
study. |
Table I.
Antibodies used in the present
study.
| Antibody | Clone | Dilution | Catalogue no. | Antigen
retrieval | Manufacturer |
|---|
| AE1/AE3 | AE1 and AE3 | 1:100 | AE1/ AE3-L-CE | HIER | Novocastra
Laboratories, Newcastle upon Tyne, UK |
| αSMA | αsm-1 | 1:50 | SMA-CE | None | Novocastra
Laboratories, Newcastle upon Tyne, UK |
| p63 | 7JUL | 1:100 | P63-L-CE | HIER | Novocastra
Laboratories, Newcastle upon Tyne, UK |
| p53 | DO-7 | Prediluted | 760–2542 | HIER | Ventana Medical
Systems, Tucson, Arizona, USA |
| Ki-67 | MIB-1 | 1:100 | IR626/IS626 | HIER | Dako, Glostrup,
Denmark |
| Cyclin D1 | SP4-R | Prediluted | 790–4508 | HIER | Ventana Medical
Systems, Tucson, Arizona, USA |
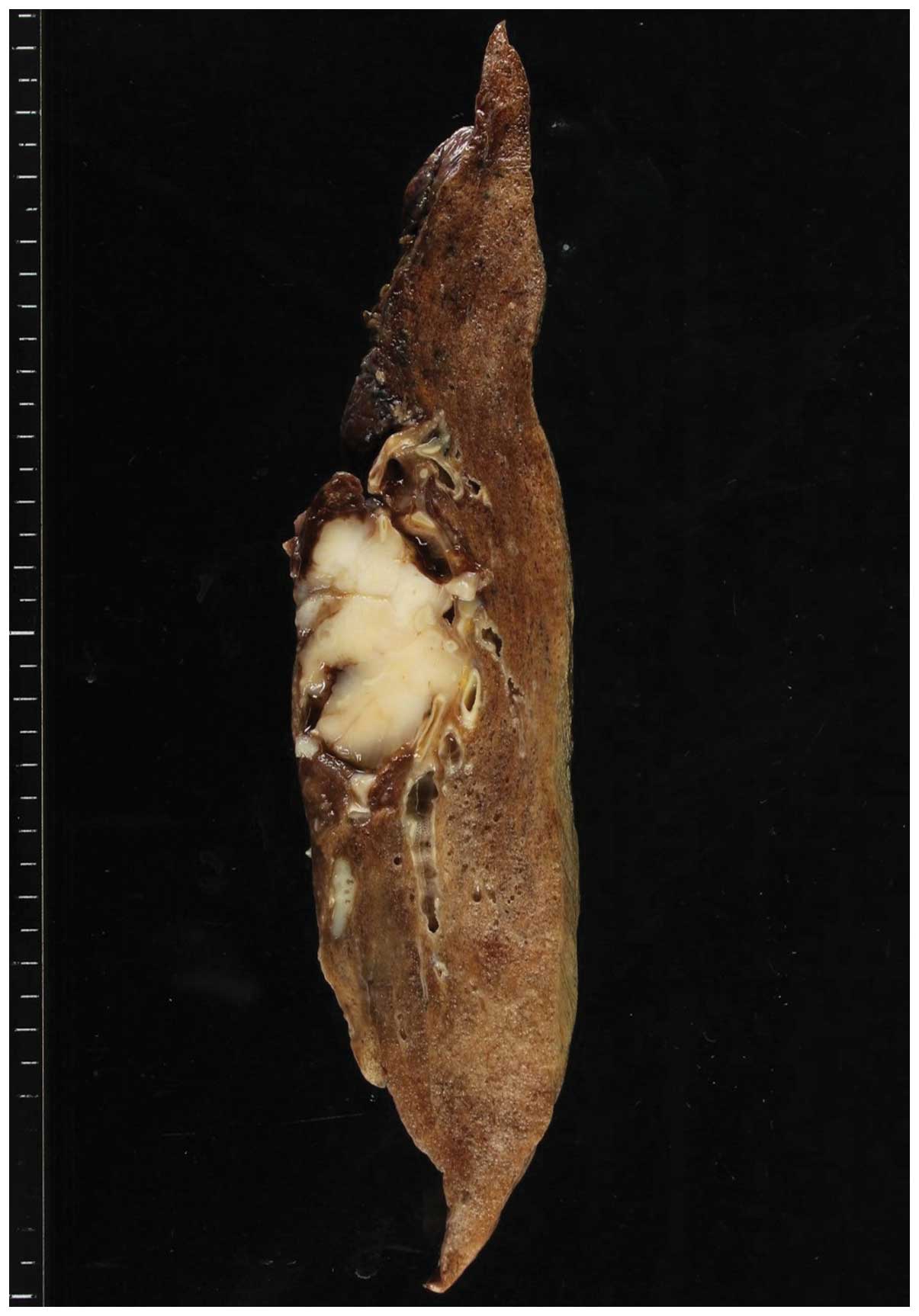
Macroscopically, the tumor was solid and white-ish
in color; it measured ~38×30 mm on the cut surface, which was
lobulated and well-delineated without a capsule (Fig. 2). No necrotic and hemorrhagic foci
were observed.
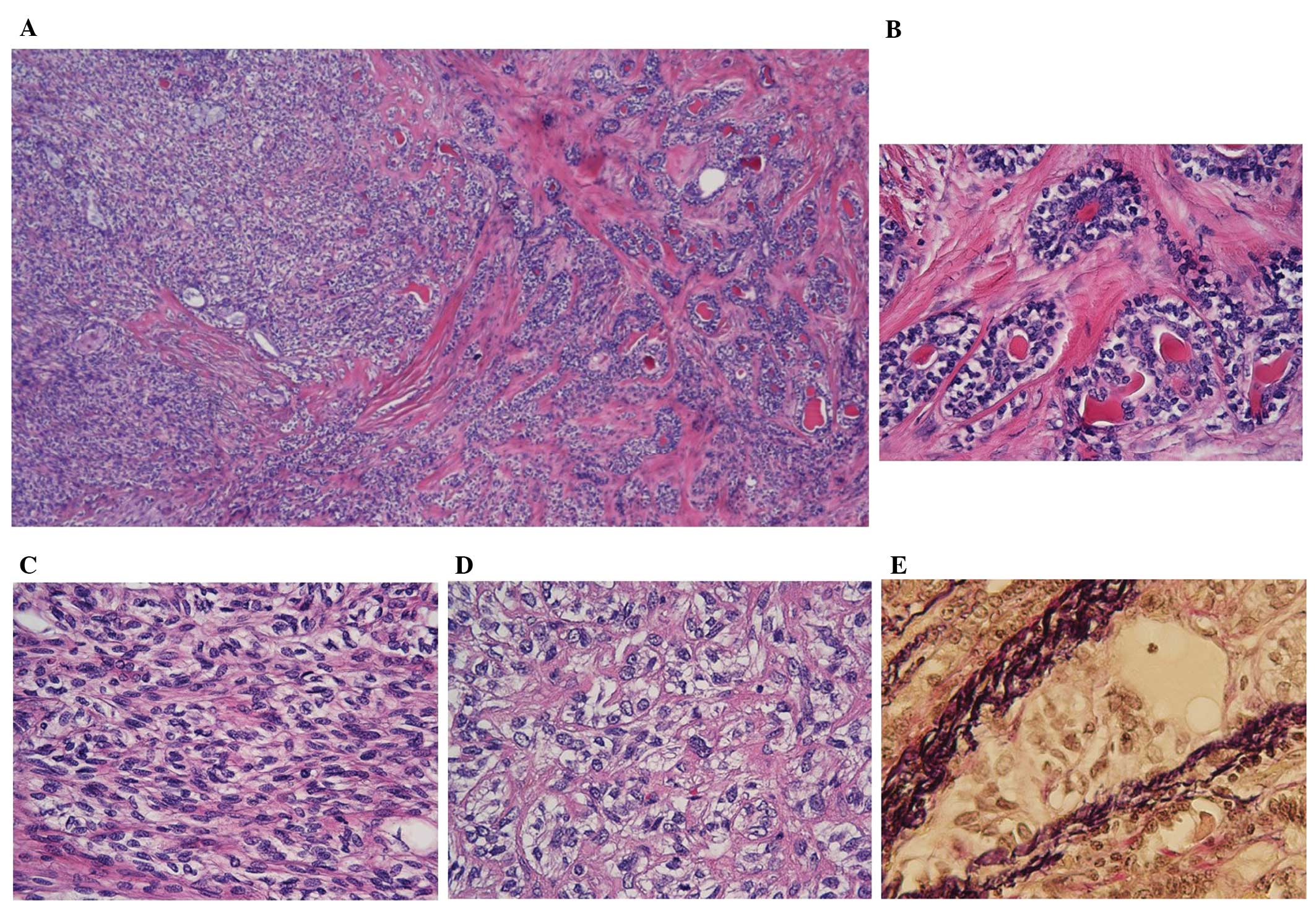
Histopathological examination at low-power
magnification (Olympus BX 51; Olympus Corporation, Tokyo, Japan)
demonstrated relatively homogenous cellular proliferation overall,
with differences in scattered areas (Fig.
3A). Closer visual assessment demonstrated the presence of a
focal bilayered ductal component (10% of tumoral tissue; Fig. 3B), which was overwhelmed by admixed
spindle-shaped and polygonal-shaped cell components presenting with
clear to weakly eosinophilic cytoplasm (70% of tumoral tissue;
Fig. 3C). The remainder of the tumor
mass consisted of relatively pleomorphic polygonal cells with
increased nuclear atypia and clear cytoplasm situated at the
advancing edge of the tumor (20% of tumoral tissue; Fig. 3D). The latter two components
demonstrated a reciprocal gradual transition. The ductal structure
was composed of an inner layer of glandular cells with eosinophilic
cytoplasm, and an outer multilayer of polygonal cells with clear
cytoplasm. Cells within the substructures demonstrated mild nuclear
atypia; no mitotic cells were observed. The majority of the tumor
cells did not present with increased nuclear atypia and mitotic
cells were not easily detectable (<1/10 high-power fields), with
the exception of a noteworthy component, 20% of which was somewhat
pleomorphic and contained a few mitotic cells (≤3/10 high-power
fields); indicating that this component was a higher grade. The
tumor infiltrated the pulmonary parenchyma at multiple sites,
irrespective of the degree of its cellular atypia. Venous invasion
was demonstrated in the higher-grade component beyond the line of
circumscription of the tumor (Fig.
3E). True perineural invasion was not apparent, although
peripheral nerves were involved in the tumor-infiltrative area, and
destructive invasion of the nerves was observed. No necrosis was
observed.
IHC analyses are presented in Fig. 4: The inner cells of the ductal
structure were strongly positive for AE1/AE3, while the outer cells
were largely negative (Fig. 4A). By
contrast, a positive reaction for α-smooth muscle actin (αSMA) and
p63 was evident in the outer cells (Fig.
4D and G). This component was regarded to comprise
epithelial-myoepithelial biphasic nests. The overwhelming component
in the admixture of spindle and polygonal cells was weakly positive
for AE1/AE3 and αSMA (Fig. 4B and E),
but demonstrated a relatively strong reaction for p63 (Fig. 4H); thus, it was established to be an
overgrowth of myoepithelial cells. The higher-grade component was
observed to be weakly immunoreactive for AE1/AE3 and αSMA (Fig. 4C and F); however, as it maintained p63
reactivity (Fig. 4I), this component
was established to be a myoepithelial carcinoma.
 | Figure 4.Immunohistochemical findings at 400X
magnification. (A–C) Immunopositivity for AE1/AE3. Glandular cells
in (A) EMC demonstrate strongly positive immunoreactivity for
AE1/AE3. (D–F) Immunoreactivity for αSMA. Glandular cells in (D)
EMC demonstrate negative immunoreactivity for αSMA. (G–I)
Immunostaining for p63. In each component, the majority of the
cells are positive for p63 with the exception of (G) the glandular
cells in MO. (J–L) Immunostaining for p53. Accumulation of p53 in
the nuclei is sparse, and no significant variations are
demonstrated between the three components. (M–O) Immunostaining for
Ki-67. In (M) EMC and (N) MO, a few cells are labeled with Ki-67.
Labeling indexes of the former and the latter component are 1.6 and
2.8%, respectively. In (O) MC, the index is high, correlating with
higher nuclear atypia (14.2%). When counting the cells, other
fields were included. (P–R) Immunostaining for cyclin D1. Cyclin
D1-stained cells are scattered with weak intensity in (P) EMC and
(Q) MO. In (R) MC, scattered, intensely positive cells are
observed, indicating overexpression of cyclin D1. EMC,
epithelial-myoepithelial component; MO, myoepithelial overgrowth;
MC, myoepithelial carcinoma. |
In order to predict biological behavior, each
component of the tumor was evaluated histopathologically by using
several antibodies. As a tumor marker, the tumor suppressor p53 was
not densely accumulated in the nuclei and few cells displayed
positive staining (Fig. 4J–L). The
accumulation profiles did not vary markedly among the three
components. To observe the proliferative activity of the tumor,
Ki-67 was selected, and labeling indexes were calculated for the 3
components. A noticeable difference was observed; per 1,000 cells
of the epithelial-myoepithelial component, myoepithelial overgrowth
and myoepithelial carcinoma, the indices were 1.6, 2.8 and 14.2%,
respectively (Fig. 4M–O).
Overexpression of cyclin D1, one of the factors associated with
cell cycle control, was not evident in the epithelial-myoepithelial
or myoepithelial overgrowth components (Fig. 4P and Q); however, it was apparent in
the myoepithelial carcinoma component (Fig. 4R).
Considering all the results, a diagnosis of EMC,
demonstrating progression of a myoepithelial overgrowth to a
myoepithelial carcinoma with higher-grade status was rendered. The
surgical margins were tumor-free.
Discussion
Based on the morphological analysis, the present
case conformed to the well-established observation of HGT in
salivary gland carcinomas; in this case, the pulmonary EMC
demonstrated progression to a higher-grade myoepithelial carcinoma
via myoepithelial overgrowth (11).
In addition, the present study aimed to resolve the criteria for
HGT in terms of immunohistochemical characteristics. As a marker,
Ki-67 is frequently used in salivary gland tumors to distinguish
HGT lesions from pre-existing components (12). The previously reported Ki-67 labeling
index for salivary gland EMCs ranged from <1–12% (14). The Ki-67 labeling index in the
higher-grade component in the present case was 14.2%, a value that
exceeds the acceptable range for a low-grade lesion, as the
salivary EMC is low grade (5). There
have been a number of reports that attempted to digitize the
labeling index of Ki-67 in the HGT area of an EMC, and one case
documented that the Ki-67 labeling index of the HGT area in a
salivary EMC was 40% (for a review, see reference 15). A detailed
image demonstrating Ki-67 immunostaining was presented in another
study on salivary EMC; although the value of the labeling index was
not mentioned (10), it was estimated
to be higher compared with the index in the present case.
Therefore, the higher-grade component in the present case cannot be
categorized as an HGT; another term, HGT-like, is better applied to
the present case. This designation is well-defined in terms of the
malignant range of salivary EMCs. It falls within the range of
intermediate- to high-grade malignancy (10). Thus, for the present study,
progression from a low-grade to an intermediate-grade malignancy is
defined as HGT-like.
Other markers used to define HGT are p53 and cyclin
D1. In the majority of salivary gland tumor types, the p53 staining
is stronger in the HGT component compared with the pre-existing
component (12). Unlike conventional
HGT, in the present case, p53+ cells were scattered
throughout the tumor, with no recognizable variability. However,
previous studies have also described negative staining in acinic
cell carcinomas (16,17). These inconsistencies indicate that p53
alteration is not the primary mechanism for HGT.
Cyclin D1 is highly expressed in HGT of salivary
gland tumors (12) and was
overexpressed at the HGT-like site in the present case. This
indicates the stepwise progression from the pre-existing tumor, in
a pathway similar to that in HGT in salivary gland tumors. Cyclin
D1 is established to induce chromosomal instability (18); it is recruited to DNA via
sequence-specific binding proteins and leads to differential gene
expression of chromatin reorganizing proteins (19). This abnormal mitotic regulation can
result in increased aneuploidy, in addition to structural
chromosomal aberrations, including translocation and duplications
(19). This molecular basis provides
a plausible explanation to the hypothesis that increased cyclin D1
expression resulted in the HGT-like histological features of the
tumor in the present case.
A significant correlation between the size of the
EMC and the occurrence of HGT in salivary glands has been
documented (20). A range of 2–11 cm
(mean, 6.3 cm) was reported in 17 cases of salivary EMC
demonstrating HGT. Pulmonary salivary gland-type tumors are
detected earlier since patients experience discomfort due to the
tumor's proximity with the bronchial tree (3); thus, the tumor size was smaller in these
cases, ranging between 1.3 and 4.0 cm (mean, 2.5 cm) in 7 cases of
pulmonary EMC (2). In addition,
several previous studies have described the size of pulmonary EMCs:
The size in the majority of these cases was smaller than the mean
size for EMCs with HGT, as described above (3). This observation may explain why definite
HGTs have not been detected in pulmonary EMCs.
Pathological features corresponding to HGT were
traditionally termed ‘dedifferentiation’; this term applied solely
to high-grade neoplasms, which had progressed from low-grade
neoplasms and had lost all the histological characteristics of
their original lineage (10). More
recently, it has been identified that neoplasms maintaining their
original lineage also demonstrate significant malignancy. Previous
studies have identified EMCs with two types of HGT as follows: i)
Those that lack myoepithelial features (20,21); and
ii) those that maintain myoepithelial characteristics with a
certain degree of nuclear atypia (11,21). The
latter are also termed EMCs with myoepithelial anaplasia. The
high-grade area in the latter often develops from a gradual
transition from myoepithelial overgrowth in the low-grade EMC area
(11,15,20). The
tumor in the present case exhibited weak, but evidently positive
staining of αSMA in the HGT-like component, similar to the staining
observed in EMCs with myoepithelial anaplasia (11,21).
HGT occurs as three forms in EMCs of salivary
glands. Of the 22 cases investigated by Baker et al
(13) HGT was demonstrated in the
epithelial component in 10 cases (45.5%), in the myoepithelial
component in 2 cases (9.1%), and in both of these components in 3
cases (13.6%), with the remaining 7 cases not clearly defined. The
present case possessed an HGT-like component originating from the
myoepithelial part, corresponding to the second most frequent form
of the three.
In the present case, metastasis to nodes and distal
organs was not observed and the clinical outcome of the patient is
good at present. Since this outcome contradicts the highly
malignant character of HGT, the use of the term ‘HGT-like’ is
verified from the clinical point of view (21). However, venous invasion and
myoepithelial anaplasia, which are 2 of the 4 significant
predictors of reduced disease-free survival in EMCs of the salivary
glands (positive margin status, necrosis, angiolymphatic invasion
and myoepithelial anaplasia), were present in the current case
(11). EMCs of the salivary gland
have a local recurrence rate of 23–80% and a rate of metastasis of
14–25% (11), with long intervals
between recurrence (mean, 5 years) and metastasis (mean, 15 years)
(22,23). These data indicate the possibility of
a poor outcome in the form of recurrence and/or metastasis to
distant organs in the future. A thorough follow-up, including the
examination of distant organs for signs of metastasis, is required
for this patient.
The present case identified a pulmonary EMC
demonstrating progression from a myoepithelial overgrowth to a
myoepithelial carcinoma (with an HGT-like component). Morphological
and immunohistochemical status, particularly overexpression of
cyclin D1 and its possible molecular functions, indicated a
stepwise progression towards a higher grade of malignancy; the
present case appeared to follow a similar pathway towards HGT as is
observed in salivary gland-type tumors. Although the Ki-67 labeling
index in the HGT-like component did not reach the value reported
previously in other salivary EMCs, cyclin D1 was overexpressed
exclusively in the HGT-like component of the tumor. To the best of
our knowledge, this is the first report of pulmonary EMC described
with a thorough investigation of the potential HGT-associated
molecular mechanisms. Additional studies with more subjects
enrolled are required to elucidate the nature of pulmonary EMC and
the clinicopathological importance of an HGT and/or HGT-like
status.
References
|
1
|
Kang DY, Yoon YS, Kim HK, et al: Primary
salivary gland-type lung cancer: surgical outcomes. Lung Cancer.
72:250–254. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhu F, Liu Z, Hou Y, et al: Primary
salivary gland-type lung cancer: clinicopathological analysis of 88
cases from China. J Thorac Oncol. 8:1578–1584. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Song DH, Choi IH, Ha SY, et al:
Epithelial-myoepthelial carcinoma of the tracheobronchial tree: the
prognostic role of myoepithelial cells. Lung Cancer. 83:416–419.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Donath K, Seifert G and Schmitz R:
Diagnosis and ultrastructure of the tubular carcinoma of salivary
gland ducts. Epithelial-myoepithelial carcinoma of the intercalated
ducts. Virchows Arch A Pathol Pathol Anat. 356:16–31. 1972.(In
German). View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fonseca I and Soares J:
Epithelial-myoepithelial carcinomaWorld Health Organization
Classification of Tumours: Pathology and Genetics of Head and Neck
Tumours. Barnes L, Eveson JW, Reichart P and Sidransky D: 3rd. IARC
Press; Lyon, France: pp. 225–226. 2005
|
|
6
|
Corio RL, Sciubba JJ, Brannon RB and
Batsakis JG: Epithelial-myoepithelial carcinoma of intercalated
duct origin. A clinicopathologic and ultrastructural assessment of
sixteen cases. Oral Surg Oral Med Oral Pathol. 53:280–287. 1982.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Moran CA: Primary salivary gland-type
tumors of the lung. Semin Diagn Pathol. 12:106–122. 1995.PubMed/NCBI
|
|
8
|
Pelosi G, Fraggetta F, Maffini F, Solli P,
Cavallon A and Viale G: Pulmonary epithelial-myoepithelial tumor of
unproven malignant potential: report of a case and review of the
literature. Mod Pathol. 14:521–526. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nguyen CV, Suster S and Moran CA:
Pulmonary epithelial-myoepithelial carcinoma: a clinicopathologic
and immunohistochemical study of 5 cases. Hum Pathol. 40:366–373.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nagao T: “Dedifferentiation” and
high-grade transformation in salivary gland carcinomas. Head Neck
Pathol. 7:(Suppl 1). S37–S47. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Seethala RR, Barnes EL and Hunt JL:
Epithelial-myoepithelial carcinoma: a review of the
clinicopathologic spectrum and immunophenotypic characteristics in
61 tumors of the salivary glands and upper aerodigestive tract. Am
J Surg Pathol. 31:44–57. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Costa AF, Altemani A and Hermsen M:
Current concepts on dedifferentiation/high-grade transformation in
salivary gland tumors. Patholog Res Int. 2011:3259652011.PubMed/NCBI
|
|
13
|
Baker AR, Ohanessian SE, Adil E, Crist HS,
Goldenberg D and Mani H: Dedifferentiated epithelial-myoepithelial
carcinoma: analysis of a rare entity based on a case report and
literature review. Int J Surg Pathol. 21:514–519. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Arif F, Wu S, Andaz S and Fox S: Primary
epithelial myoepithelial carcinoma of lung, reporting of a rare
entity, its molecular histogenesis and review of the literature.
Rep pathol. 2012:3194342012.
|
|
15
|
Yang S and Chen X:
Epithelial-myoepithelial carcinoma with high grade transformation.
Int J Oral Maxillofac Surg. 41:810–813. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Henley JD, Geary WA, Jackson CL, Wu CD and
Gnepp DR: Dedifferentiated acinic cell carcinoma of the parotid
gland: A distinct rarely described entity. Hum Pathol. 28:869–873.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Di Palma S, Corletto V, Lavarino C,
Birindelli S and Pilotti S: Unilateral aneuploid dedifferentiated
acinic cell carcinoma associated with bilateral-low grade diploid
acinic cell carcinoma of the parotid gland. Virchows Arch.
434:361–365. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Casimiro MC and Pestell RG: Cyclin d1
induces chromosomal instability. Oncotarget. 3:224–225.
2012.PubMed/NCBI
|
|
19
|
Casimiro MC, Crosariol M, Loro E, et al:
ChIP sequencing of cyclin D1 reveals a transcriptional role in
chromosomal instability in mice. J Clin Invest. 122:833–843. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Roy P, Bullock MJ, Perez-Ordoñez B,
Dardick I and Weinreb I: Epithelial-myoepithelial carcinoma with
high grade transformation. Am J Surg Pathol. 34:1258–1265. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cheuk W and Chan JK: Advances in salivary
gland pathology. Histopathology. 51:1–20. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cho KJ, el-Naggar AK, Ordonez NG, Luna MA,
Austin J and Batsakis JG: Epithelial-myoepithelial carcinoma of
salivary glands. A clinicopathologic, DNA flow cytometric, and
immunohistochemical study of Ki-67 and HER-2/neu oncogene. Am J
Clin Pathol. 103:432–437. 1995.PubMed/NCBI
|
|
23
|
Luna MA, Ordonez NG, Mackay B, Batsakis JG
and Guillamondegui O: Salivary epithelial-myoepithelial carcinomas
of intercalated ducts: a clinical, electron microscopic, and
immunocytochemical study. Oral Surg Oral Med Oral Pathol.
59:482–490. 1985. View Article : Google Scholar : PubMed/NCBI
|