Introduction
The presence of two distinct tumor types in the
cranial cavity is rare; therefore, little is known regarding the
development and progression of the disease in these cases.
Pituitary adenomas comprise ≥90% of sellar masses and 10–15% of all
intracranial neoplasm (1). So the
coexistence of a pituitary adenoma and other intracranial tumors is
not an uncommon finding, but the exact incidence of pituitary
adenomas concomitant with other cranial tumors remains unclear.
However, numerous reports have described incidences of pituitary
adenoma concomitant with other types of cranial cavity tumor, such
as meningioma (2–4), medulloblastoma (5), neurinoma (6), craniopharyngioma (7), astrocytoma (8) and glioma (9). While the etiology and underlying
mechanisms for the development of these tumors remain unclear, it
has been proposed that genomic and endocrine disorders, as well as
radiation therapy and embryogenesis, may be important factors
(2–11). The majority of pituitary adenomas
concomitant with other cranial tumors are diagnosed by MRI or CT,
current treatment strategies include the simultaneous or staged
resection (12). At present,
prolactinoma associated with ependymoma remains a rare finding. The
current study describes a rare case of prolactinoma associated with
ependymoma in the fourth ventricle.
Case report
Symptoms
A 46-year-old male patient exhibiting impaired
vision and dizziness was admitted to the First Hospital of Jilin
University (Changchun, China) on March 5, 2010. In 1999, the
patient was diagnosed with prolactinoma in the sellar region and
underwent surgical resection. In 2001, tumor recurrence was
detected, with an hourglass-shaped lesion observed in the sellar
region as a low-density signal on T1-weighted magnetic resonance
imaging (MRI). Furthermore, upward displacement of the optic chiasm
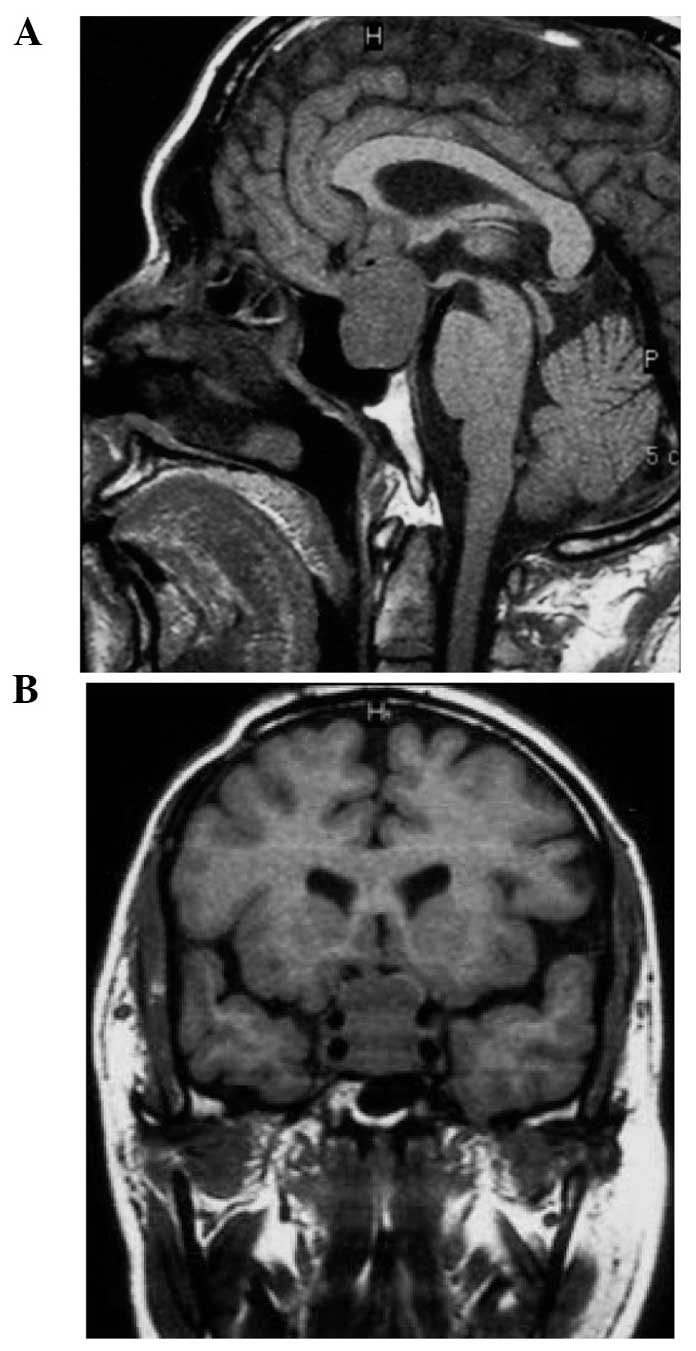
occurred (Fig. 1A) and the tumor was
observed to have invaded the cavernous sinus (Fig. 1B). For treatment, the patient
underwent transcranial surgery in combination with Gamma Knife
therapy (center dose, 45 Gy; peripheral dose, 20 Gy). The patient
recovered well; however, tumor recurrence was detected in 2010, as
described below.
Presenting clinical symptoms included dizziness with
progressive impaired vision. A nervous system examination detected
vision in the left and the right eyes of 4.3 and 4.7 diopters,
respectively (normal range, 5.0–5.3 diopters). Primary optic
atrophy at the fundus and binocular temporal hemianopsia were also
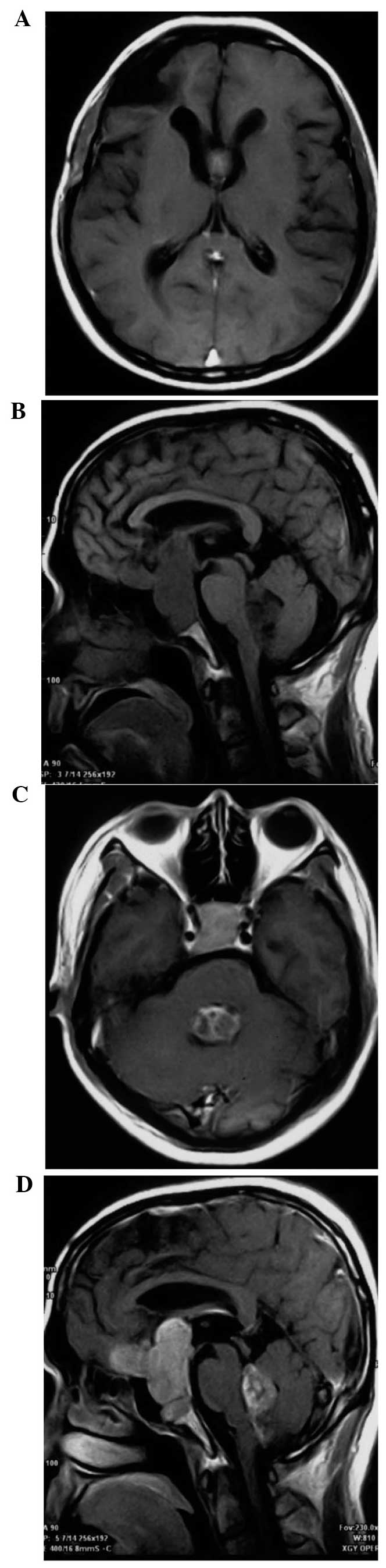
observed. Furthermore, an MRI scan detected lesions in the sellar
region and fourth ventricle that were associated with a low-density
signal on T1-weighted imaging (T1WI) and a relatively high-density
signal detected on T2WI, respectively. The lesion volumes were
4.6×4.7×2.3 cm and 3.5×3.5×3.0 cm, respectively. Additionally, the
fourth ventricle was observed to expand in response to compression,
but was not completely blocked (Fig
2A–D). Hormone levels were detected to be within the normal
ranges, with the exception of prolactin at 1,021.40 mIU/l (normal
range, 70.81–566.46 mIU/l) and growth hormone at 0.50 ng/ml (normal
range, 1.0–5.0 ng/ml). Considering the medical history, MRI and
laboratory examination results, the patient was diagnosed with
prolactinoma associated with ependymoma in the fourth
ventricle.
Treatment
Resection of the tumors in the fourth ventricle and
the sellar region was conducted in two phases. In phase I, surgical
resection of the ependymoma in the fourth ventricle was performed.
The tumor was observed to originate from, and to be adhered to, the
bottom of the fourth ventricle, with invasion towards the mesopore.
The tumor was faint red in color with a moderately tough texture
and an abundant blood supply. The tissue was fixed with formalin
for 12–48 hours, then following gross examination the tissue was
embedded in paraffin and cut to the slides using the cryostat
(Leica Biosystems Nussloch GmbH, Wetzlar, Germany).
Hematoxylin/eosin (H/E) staining (Leica Biosystems Nussloch GmbH)
was used to visualize the tissue architecture and a microscope
(CX22, Olympus Corporation, Tokyo, Japan) was used to capture
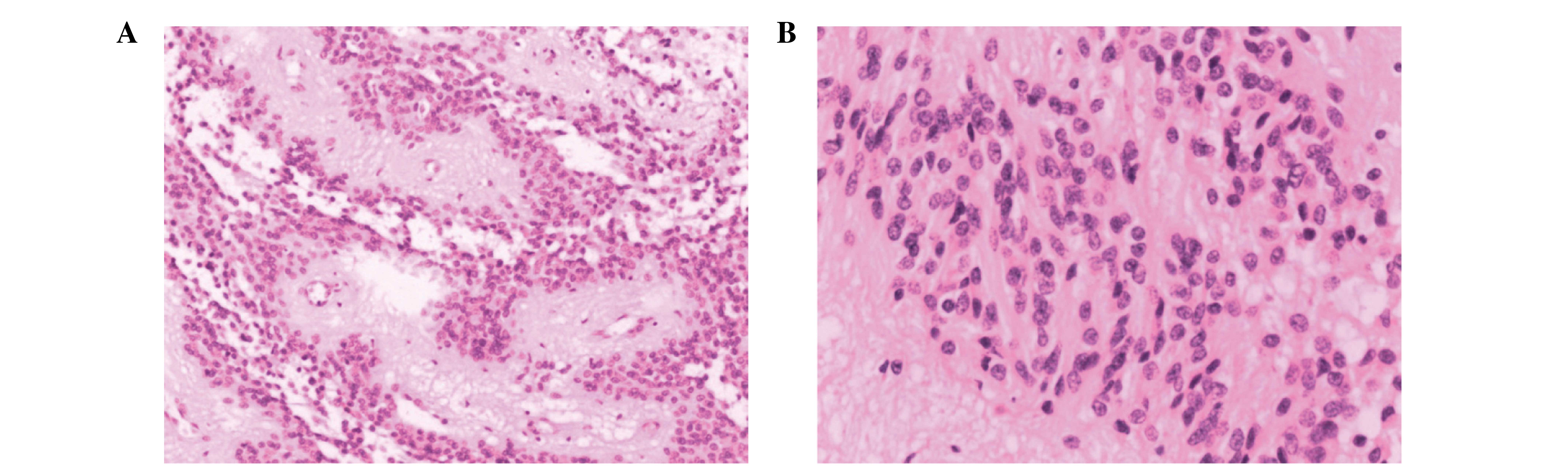
representative images. Pathological examination with H/E staining
detected densely arranged tumor cells with certain clusters forming
cell masses. The latter were characterized by round or ovoid
nuclei, dense chromatin and a slim nucleolus. Together, these
observations led to a diagnosis of ependymoma (World Health
Organization class II (13); Fig. 3A and B). Following the surgery, the
patient recovered well and no complications occurred. Furthermore,
postoperative MRI scans demonstrated that the tumor was completely
resected and the fourth ventricle was relieved from obstruction
(Fig. 4A and B).
The second surgical procedure (phase II) to remove a
pituitary adenoma in the sellar region was performed 10 days after
the phase I surgery. The tumor was observed to be dull red in
color, soft in texture and have a relatively abundant blood supply.
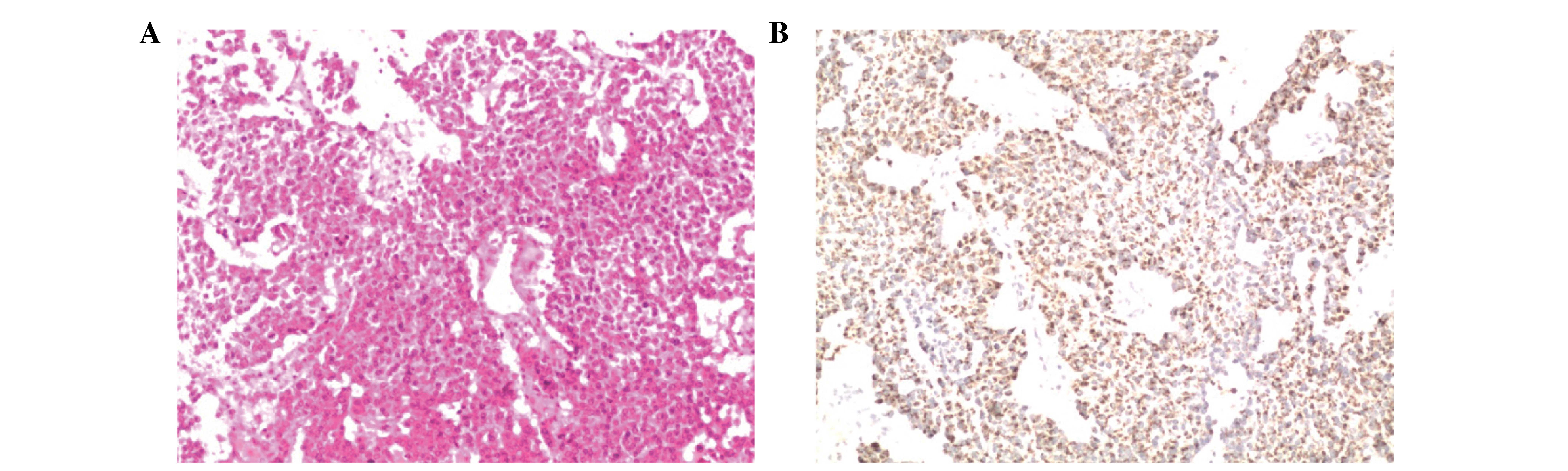
Based on H/E staining, the tumor cells were observed to be
diffusely distributed with minimal extracellular matrix. Additional
immunohistochemical assays detected expression of prolactin in the
tumor. Together, these characteristics led to a diagnosis of
prolactinoma (Fig. 5A and B).
Following the surgical procedure, the patient received Gamma Knife
therapy and recovered well; the symptoms derived from the mass
effects of the tumors improved.
Discussion
The presence of two different tumor types in the
cranial cavity of a single patient is rare (2–6). However,
of the cases reported thus far, meningioma associated with glioma
is the most frequently described, followed by meningioma associated
with pituitary adenoma and neurinoma (13,14).
Subsequent to a 1995 study by Love et al (15) that reported a case of pituitary
adenoma associated with meningioma, cases of pituitary adenoma
associated with medulloblastoma (5),
neurinoma (6), craniopharyngioma
(7), astrocytoma (8) and glioma (9) have been described. However, to the best
of our knowledge, no report has, thus far, described a case of
pituitary adenoma associated with an ependymoma in the fourth
ventricle. Therefore, the present study of prolactinoma associated
with ependymoma in the fourth ventricle represents a unique
case.
Similar genetic mutations have been identified in
pituitary adenomas and ependymomas, and these mutations appear to
be essential in the formation and progression of the two tumor
types (10,13). Furthermore, in neurofibroma (type 2),
a mutation in NF2, a tumor suppressor gene located at chromosome
position 22q12, has been associated with the formation of various
intracranial tumors, including meningiomas, gliomas and vestibular
schwannomas (16). In addition,
Furtado et al (11,15) reported three cases of pituitary
adenoma associated with two cases of glioma and one case of
meningioma, and demonstrated that these tumors resulted from gene
mutations common to both tumor types in each case. However, various
gene mutations have been identified in pituitary adenomas and other
intracranial tumors, such as gliomas and meningiomas, that have
different roles in each tumor type. For example, gene mutations
identified in pituitary adenomas include the deletion of segments
from chromosomes 1p, 2q, 4, 5, 6, 11q, 12q, 13q and 18q (10,17), and
the overexpression of genes on chromosomes 9q, 16p, 17p, 19 and 20q
(16,18). Furthermore, there have been a greater
number of gain-of-function than loss-of-function mutations
associated with pituitary adenomas (10,16,17). In
ependymomas, deletion of chromosome 22, and overexpression of genes
on chromosomes 1, 6, 7, 9–13, 16, 17, 19 and 20, have been detected
(19). Therefore, according to the
aforementioned studies, overexpression of genes on chromosomes 17
and 19 may be common genomic disorders associated with pituitary
adenomas and ependymomas. However, additional studies are required
to clarify this.
Hypotheses regarding the formation of intracranial
tumors induced by radiation therapy have been confirmed in specific
cases of glioblastoma, meningioma, sarcoma, astroma and ependymoma
(20–23). Additionally, there are reports
regarding the development of intracranial tumors, such as
meningiomas (24,25), primitive neuroectodermal tumors
(26), gliomas (20,27) and
ependymomas (28) in response to
radiation therapy for pituitary adenoma. Similarities between the
aforementioned cases have been reviewed (29,30) and
include the following: i) The development of secondary tumors
within regions exposed to radiation; ii) the formation of secondary
tumors following radiation therapy; iii) tissue origin of the
primary tumors distinct from the tissue origin of the secondary
tumors; iv) pathogenic genes identified in primary tumors differing
from those of the secondary tumors; and v) a latency period of
secondary tumor development of >5 years. In regard to the
present case, we hypothesize that the ependymoma present in the
fourth ventricle had a low probability of being induced by the
Gamma Knife radiation therapy used to treat the pituitary adenoma,
as the radiation was focused on the tumor in the sellar region,
which is a considerable distance from the ventricles. As a result,
the radiation dose that the fourth ventricle received would be
expected to be low. Therefore, it is proposed that induction of the
ependymoma in the present case was more likely to be the result of
gene mutations in chromosomes 17 and 19 that are common to
pituitary adenomas and ependymomas.
Amit et al (6)
and Furtado et al (11) have
previously speculated that different intracranial tumors may
originate from cellular remnants left following embryogenesis. This
hypothesis appears to be supported by a case of craniopharyngioma
associated with pituitary adenoma and notochordoma reported by
Shishkina et al (31).
However, the current authors propose that embryogenesis was not
involved in the present case, as these two tumors were different in
cell origin.
In the clinic, certain cases of pituitary adenoma
have been identified to be induced by factors secreted by the
pituitary adenoma itself. For example, Curto et al (10) reported a case of pituitary adenoma
associated with meningioma and an aneurysm, and demonstrated that
growth hormones may have had a role in tumor development.
Similarly, Fernandez et al (8)
reported a case of pituitary adenoma associated with astroma in
which insulin-like growth factor was involved. Somatostatin
receptors (32) and epidermal growth
factor receptors (33,34) are also expressed in pituitary adenomas
and ependymomas, and may have a role in inducing the formation of
pituitary adenomas and ependymomas.
In conclusion, the present study reports a rare case
of pituitary adenoma associated with ependymoma in the fourth
ventricle. Considering the characteristics of the current patient
in association with previous case reports involving two tumors
within the same cranial cavity, it is proposed that the basis of
these disorders includes a genomic and endocrine component.
References
|
1
|
Sautner D, Saegar W and Ludecke DJ: Tumors
of the sellar region mimicking pituary adenomas. Exp Clin
Endocrinol. 101:283–289. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Abs R, Parizel PM, Willems PJ, Van de
Kelft E, Verlooy J, Mahler C, Verhelst J, Van Marck E and Martin
JJ: The association of meningioma and pituitary adenoma: Report of
seven cases and review of the literature. Eur Neurol. 33:416–422.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
da Costa LB Jr, Riva-Cambrin J, Tandon A
and Tymianski M: Pituitary adenoma associated with intraventricular
meningioma: Case report. Skull Base. 17:347–351. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Prevedello DM, Thomas A, Gardner P,
Snyderman CH, Carrau RL and Kassam AB: Endoscopic endonasal
resection of a synchronous pituitary adenoma and a tuberculum
sellae meningioma: Technical case report. Neurosurgery. 60:(Suppl
2). E4012007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Samaras V, Samaras E, Stergiou I,
Konstantopoulou P, Arnaoutoglou C, Arnaoutoglou M, Varsos V and
Barbatis C: Simultaneous occurrence of cerebellar medulloblastoma
and pituitary adenoma: A case report. Cases J. 1:1752008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Amit A, Achawal S and Dorward N: Pituitary
macro adenoma and vestibular schwannoma: A case report of dual
intracranial pathologies. Br J Neurosurg. 22:695–696. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Moshkin O, Scheithauer BW, Syro LV,
Velasquez A, Horvath E and Kovacs K: Collision tumors of the sella:
craniopharyngioma and silent pituitary adenoma subtype 3: Case
report. Endocr Pathol. 20:50–55. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fernandez A, Karavitaki N, Ansorge O,
Fazal-Sanderson V and Wass JA: Acromegaly and anaplastic
astrocytoma: Coincidence or pathophysiological relation? Pituitary.
11:325–330. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mangiola A, De Bonis P, Guerriero M,
Pompucci A and Anile C: Gliomatosis cerebri and pituitary adenoma:
Case report and literature review. J Neurooncol. 74:321–324. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Curto L, Squadrito S, Almoto B, Longo M,
Granata F, Salpietro F, Torre ML, Marini F, Trimarchi F and Cannavo
S: MRI finding of simultaneous coexistence of growth
hormone-secreting pituitary adenoma with intracranial meningioma
and carotid artery aneurysms: Report of a case. Pituitary.
10:299–305. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Furtado SV, Venkatesh PK, Ghosal N and
Hegde AS: Coexisting intracranial tumors with pituitary adenomas:
Genetic association or coincidence? J Cancer Res Ther. 6:221–223.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jaiswal S, Vij M, Jaiswal AK, Chand G,
Behari S and Kumarjain V: Ossifying pituary adenoma co-existing
with astrocytoma and pituary adenoma associated with gangliocytoma:
Two unusual conditions. Turk Neurosurg. 22:127–133. 2012.PubMed/NCBI
|
|
13
|
Louis DN, Ohgaki H, Wiestler OD, et al:
The 2007 WHO classification of tumours of the central nervous
system. Acta Neuropathol. 114:97–109. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Love JG and Blackburn CM: Association of
intracranial meningioma with pituary adenoma; reported of
successfully treated case. Minn Med. 38:335–336. 1955.PubMed/NCBI
|
|
15
|
Furtado SV, Dadlani R, Ghosal N, Mahadevan
A, Shankar SK and Hegde AS: Co-existing thyrotropin secreting
pituitary adenoma and low grade glioma: Clinical considerations and
literature review. J Neurosurg Sci. 53:71–75. 2009.PubMed/NCBI
|
|
16
|
Asthagiri AR, Parry DM, Butman JA, Kim HJ,
Tsilou ET, Zhuang Z and Lonser RR: Neurofibromatosis type 2.
Lancet. 373:1974–1986. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chesnokova V, Zonis S, Ben-Shlomo A,
Wawrowsky K and Melmed S: Molecular mechanisms of pituitary adenoma
senescence. Front Horm Res. 38:7–14. 2010.PubMed/NCBI
|
|
18
|
Szymas J, Schluens K, Liebert W and
Petersen I: Genomic instability in pituitary adenomas. Pituitary.
5:211–219. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Collins VP: Brain tumours: Classification
and genes. J Neurol Neurosurg Psychiatry. 75:(Suppl 2). ii2–ii11.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Anderson JR and Treip CS:
Radiation-induced intracranial neoplasms. A report of three
possible cases. Cancer. 53:426–429. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim JH, Brown SL, Jenrow KA and Ryu S:
Mechanisms of radiation-induced brain toxicity and implications for
future clinical trials. J Neurooncol. 87:279–286. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pettorini BL, Park YS, Caldarelli M,
Massimi L, Tamburrini G and Di Rocco C: Radiation-induced brain
tumours after central nervous system irradiation in childhood: A
review. Childs Nerv Syst. 24:793–805. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Weinstein JL, Ayyanar K and Watral MA:
Secondary neoplasms following treatment for brain tumors. Cancer
Treat Res. 150:239–273. 2009.PubMed/NCBI
|
|
24
|
Honegger J, Buchfelder M, Schrell U, Adams
EF and Fahlbusch R: The coexistence of pituitary adenomas and
meningiomas: Three case reports and a review of the literature. Br
J Neurosurg. 3:59–69. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Partington MD and Davis DH:
Radiation-induced meningioma after treatment for pituitary adenoma:
Case report and literature review. Neurosurgery. 26:329–331. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bhansali A, Banerjee AK, Chanda A, Singh
P, Sharma SC, Mathuriya SN and Dash RJ: Radiation-induced brain
disorders in patients with pituitary tumours. Australas Radiol.
48:339–346. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tsang RW, Laperriere NJ, Simpson WJ,
Brierley J, Panzarella T and Smyth HS: Glioma arising after
radiation therapy for pituitary adenoma. A report of four patients
and estimation of risk. Cancer. 72:2227–2233. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Alexander MJ, DeSalles AA and Tomiyasu U:
Multiple radiation-induced intracranial lesions after treatment for
pituitary adenoma. Case report. J Neurosurg. 88:111–115. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Carlson ML, Babovic-Vuksanovic D, Messiaen
L, Scheithauer BW, Neff BA and Link MJ: Radiation-induced
rhabdomyosarcoma of the brainstem in a patient with
neurofibromatosis type 2. J Neurosurg. 112:81–87. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sheehan J, Yen CP and Steiner L: Gamma
knife surgery-induced meningioma. Report of two cases and review of
the literature. J Neurosurg. 105:325–329. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shishkina VL, Kasumova SIu, Snigireva RIa
and Miakota AE: Craniopharyngioma associated with pituitary adenoma
and chordoma of Blumenbach's clivus. Zh Vopr Neirokhir Im N N
Burdenko. (6):52–54. 1981.(In Russian). PubMed/NCBI
|
|
32
|
Guyotat J, Champier J, Jouvet A,
Signorelli F, Houzard C, Bret P, Saint Pierre G and Fevre Montange
M: Differential expression of somatostatin receptors in ependymoma:
Implications for diagnosis. Int J Cancer. 95:144–151. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mendrzyk F, Korshunov A, Benner A, Toedt
G, Pfister S, Radlwimmer B and Lichter P: Identification of gains
on 1q and epidermal growth factor receptor overexpression as
independent prognostic markers in intracranial ependymoma. Clin
Cancer Res. 12:2070–2079. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Vlotides G, Siegel E, Donangelo I, Gutman
S, Ren SG and Melmed S: Rat prolactinoma cell growth regulation by
epidermal growth factor receptor ligands. Cancer Res. 68:6377–6386.
2008. View Article : Google Scholar : PubMed/NCBI
|