Introduction
Differentiated thyroid carcinoma (DTC) is among the
most common metabolic disorders, and accounts for 98% of all cases
of thyroid cancer. Annually, 20,000 new cases of DTC are diagnosed
in USA, and 200,000 patients undergo monitoring for recurrence or
progression of the disease (1,2). There are
three main types of thyroid carcinoma: Well-differentiated thyroid
carcinoma (WDTC), poorly differentiated thyroid carcinoma (PDTC)
and undifferentiated thyroid carcinoma (UDTC). WDTC has a better
prognosis and decreased mortality and morbidity rates compared with
PDTC and UDTC. WDTC neoplasms may arise from follicular cells
[papillary (PTC), follicular (FTC) or Hürthle cell (HTC)carcinomas]
or from parafollicular cells (medullary thyroid carcinoma)
(3,4).
Although WDTC accounts for <1% of all cancers, it results in
>70% of mortalities from thyroid carcinomas (5).
A number of studies have attempted to determine the
prognostic factors associated with WDTC. However, these studies
yielded conflicting results (6–8), which may
be due variations in the study settings and in the participants
enrolled in each study. Furthermore, the use of different
pathological classifications of WDTC and of different treatment
approaches may have contributed to the heterogeneity among the
studies. In order to overcome these limitations, the present study
used a sample of WDTC patients undergoing the same treatment, to
test the prognostic significance of clinical and pathological
factors of WDTC. The identification of clinicopathological factors
in cancer is crucial to improve the accuracy of recurrence rate
estimates, and to facilitate the calculation of patient-specific
disease mortality rates. Furthermore, it aids in the selection of
therapeutic modalities and in determining the frequency of
follow-up examinations (9).
Materials and methods
Subjects
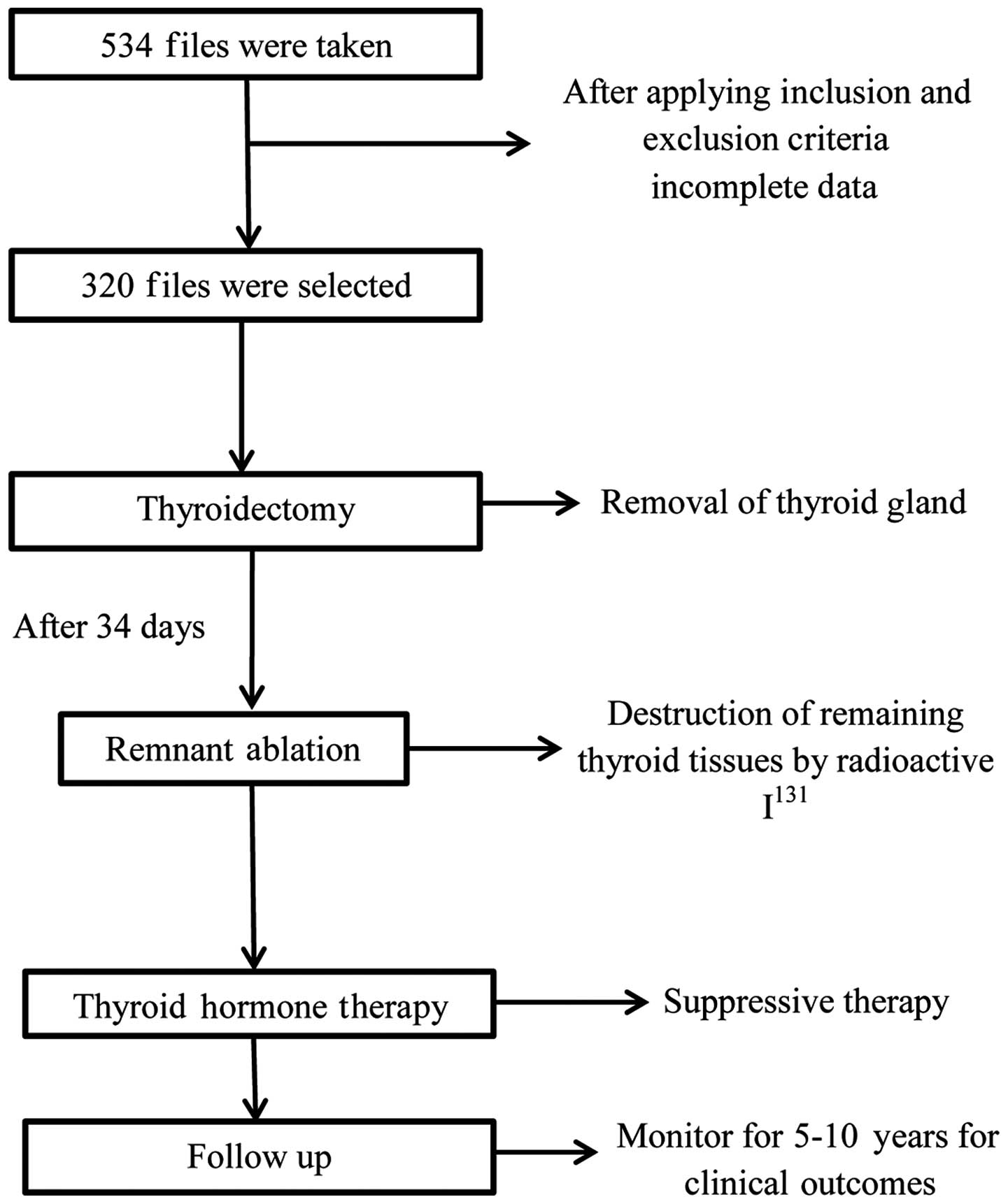
The current study utilized a retrospective study
design to collect medical documentation of patients suffering from
WDTC, who were treated at the same institute with a minimum
follow-up period of 10 years. A single center was used for data
collection to avoid variation in treatment modalities and
facilitation of follow-up. A number of classification systems may
be used to classify WDTC: De Groot (stage 1, intrathyroidal; stage
2, cervical node metastases; stage 3, extrathyroidal system; stage
4, distant metastases) (10); AMES
(age, metastases, extra thyroidal invasion, size of tumor)
(11); or TNM (tumor size, nodal
status, metastases) (12). For the
current study, the De Groot classification system was selected, as
it utilizes the combination of clinical, surgical and pathological
data (13,14).
Inclusion criteria
Patients with a complete medical record of ≥10 years
with the selected treatment regimen were included. The study was
approved by the ethical review board of Nanfang Hospital
(Guangzhou, China; reference no. HG/2012/6776891).
Exclusion criteria
Patients with incomplete medical records, with a
follow-up period of <10 years, or who succumbed to medical
conditions other than thyroid carcinoma were excluded from the
study.
Treatment
All patients were given I131 treatment 34
days following thyroidectomy. The dosage of I131 used
varied depending on the clinical condition of the patients.
Following whole body scan, the presence of serum thyroglobulin was
considered a ‘positive result’, and an absence was considered a
‘negative result’. Thyroglobulin levels were measured using RIA
kits (#KR6270; Kronus, Inc., Star, ID, USA) that were able to
detect <1µg/l, while a radioimmunoassay was used to detect
anti-thyroglobulin antibodies. Follow-up examinations were
conducted every three months for the first two years, and
biannually thereafter, and clinical factors were recorded for each
patient. Unfavorable clinical factors included the presence or
persistence of increased thyroglobulin levels, recurrence or
mortality due to thyroid carcinoma. The following were considered
to be favorable clinical outcomes, indicating effectiveness of the
treatment regimen: Undetectable serum thyroglobulin, very low level
of serum thyroglobulin or complete absence of thyroglobulin
(negative result at follow-up scan). A brief overview of the
methodology adopted for the current study is shown in Fig. 1.
All subjects underwent the following treatment: i)
After performing radical surgery (i.e. thyroidectomy) all patients
underwent a radioiodine scan and evaluation of serum thyroglobulin
level; ii) all subjects with hypothyroidism were treated with
radioactive iodine I131 thyroid ablation (dose, 25–30
mCi) 34 days after the first surgery; iii) subjects with traceable
amounts of serum thyroglobulin without the presence of
anti-thyroglobulin antibodies were treated with an increased dose
of I131 (100–150 mCi) to achieve normal thyroglobulin
levels; iv) all subjects were treated with thyroid stimulating
hormone suppressive therapy.
Statistical analysis
Continuous data are presented as the mean ± standard
deviation, while categorical data are presented by numbers or
frequencies. All the data was analyzed using SPSS software, version
20.0 (IBM SPSS, Armonk, NY, USA). Univariate and multivariate
analyses were conducted in stepwise manner to evaluate prognostic
factors associated with WDTC. P<0.05 was considered to indicate
a statistically significant difference.
Results
In total, 320 patients with WDTC were selected to
participate in current study. The demographic profile of the
subjects is summarized in Table
I.
 | Table I.Demographic and clinical profile of
study participants. |
Table I.
Demographic and clinical profile of
study participants.
| Demographic
factor | Cases, n (%) |
|---|
| Gender |
|
| Male | 80
(25%) |
|
Female | 240 (75%) |
| Age, years (mean ±
SD) |
45.3±17.9 |
| Follow-up, months
(mean) | 87.9 |
| Pathological
type |
|
| Papillary
carcinoma | 240 (75%) |
|
Follicular carcinoma | 67
(21%) |
| Hurtle
cell carcinoma | 13
(4%) |
| Clinical outcome |
|
|
Favorable | 198 (62%) |
|
Unfavorable | 122 (38%) |
The mean age of the participants was calculated to
be 45.3±17.9 years. The mean follow-up period was 87.9 months. Of
the 320 patients, 253 (79%) had undergone a single thyroidectomy,
while the remaining 67 (21%) had undergone a second surgery 14 days
after the initial surgery (i.e. partial resection). The following
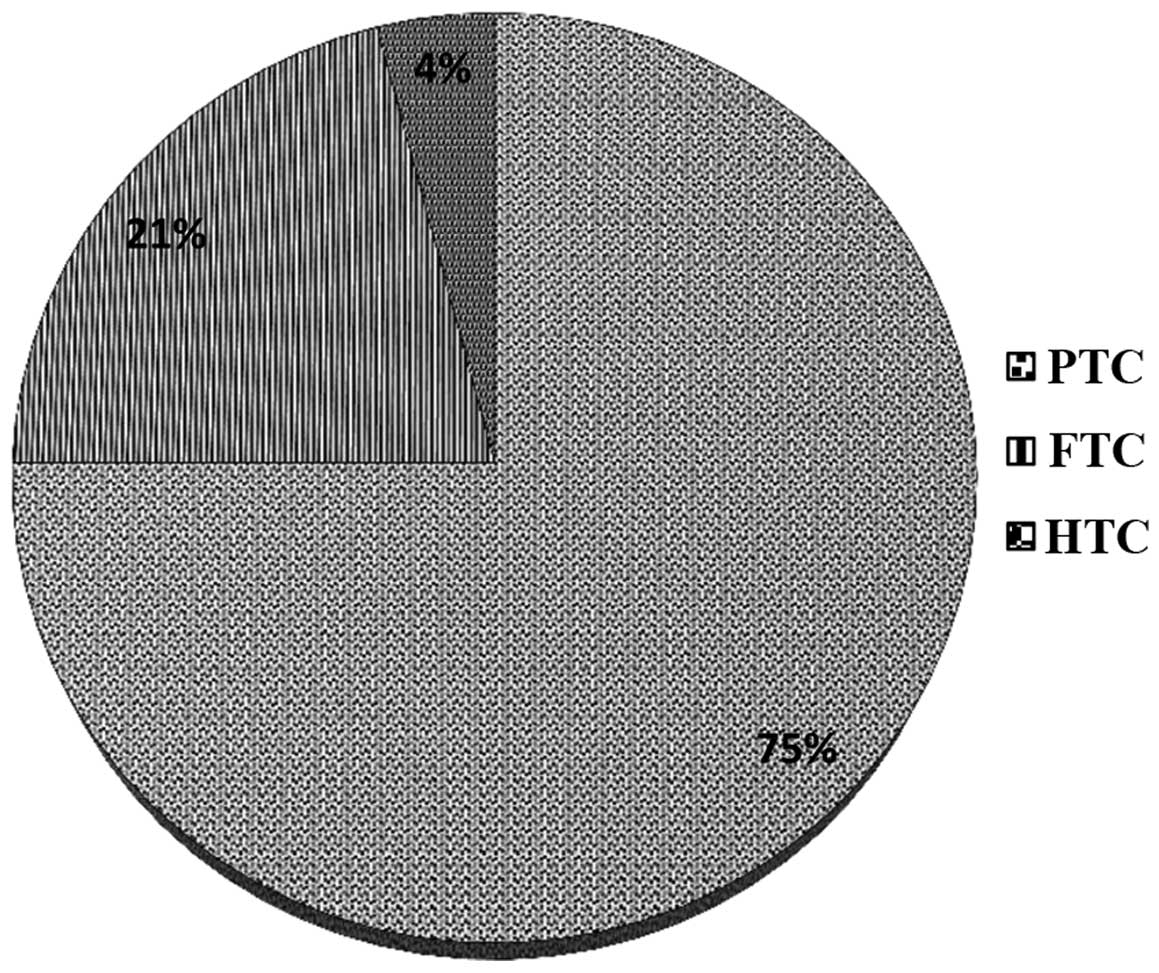
pathological subtypes of WDTC were identified among the
participants: 240 (75%) PTCs, 67 (21%) FTCs and 13 (4%) HTCs. At
the end of follow-up, the following unfavorable outcomes were
observed: 109 (34%) patients presented with persistent or recurrent
carcinoma and 13 (4%) patients succumbed to carcinoma. Table II summarizes the prognostic factors
along with their statistical significance as determined by
multivariate analysis.
 | Table II.Prognostic factors associated with
well-differentiated thyroid carcinoma at the end of the follow-up
period. |
Table II.
Prognostic factors associated with
well-differentiated thyroid carcinoma at the end of the follow-up
period.
| Prognostic
factors | P-value |
|---|
| Gender | 0.435 |
| Advanced age (>48
years) | 0.001 |
| Type of
carcinoma | 0.325 |
| Tumor size | 0.03 |
| Lymph node
involvement | 0.126 |
| Thyroglobulin | 0.001 |
| De Groot staging | 0.005 |
| Tumor extension | 0.387 |
| Multi-focality | 0.425 |
Gender
The rate of unfavorable clinical outcomes did not
differ significantly between males and females; unfavorable
clinical outcomes were observed in 62 (51%) females versus 60 (49%)
males at the end of study (P=0.435).
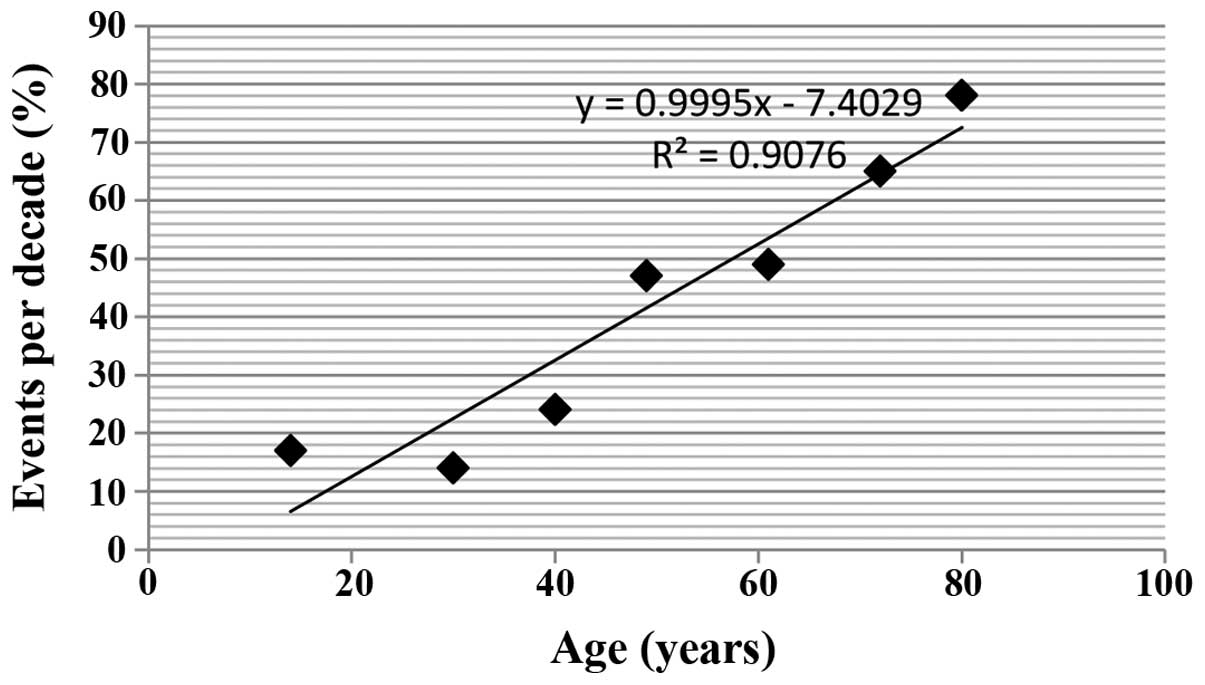
Age
In the present study, 48 years was observed to be
the age above which the majority (66%; 81 individuals) of the
unfavorable outcome population were. By contrast, only 73 (37%)
disease-free subjects were above this age (P<0.001).
Additionally, the presence of distal metastases during the
follow-up period was more common among the subjects >48 years of
age, compared with the younger subjects (P<0.001). Young age
(<20 years) was associated with better clinical outcome than the
older age group (>48 years) (71.7% vs. 28.3%; P<0.001,
respectively). The association between age and unfavorable clinical
outcomes is shown in Fig. 2.
Pathological subtype of carcinoma
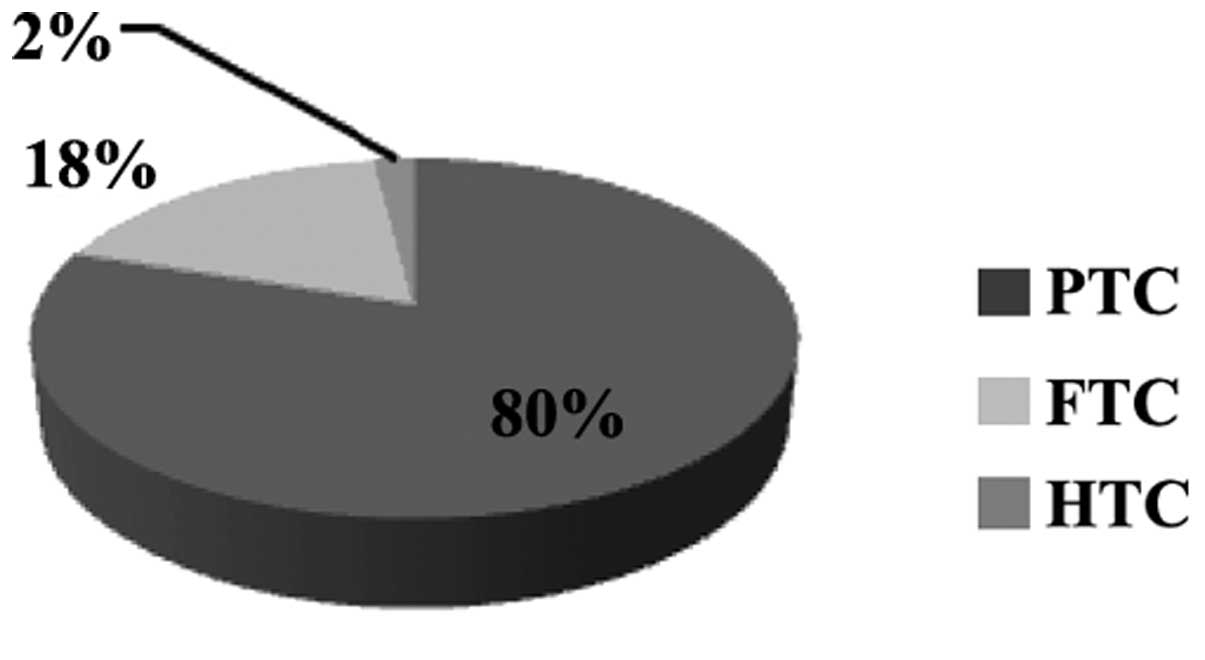
Histological examination at the time of diagnosis,
revealed that PTC was observed in the greatest number of subjects
(240 cases; 75%), followed by FTC (67 cases; 21%) and HTC (13
cases; 4%) (Fig. 3). During diagnosis
and follow-up, PTC was associated with the highest rate of lymph
node involvement (P=0.002 vs. 0.004) compared with FTC and HTC. In
subjects with unfavorable clinical outcomes, HTC was observed in
the highest number of subjects (69 cases; 57%) and was associated
with poorer clinical outcomes (P=0.005) on univariate analysis.
However, multivariate analysis demonstrated no significant
difference (P=0.325).
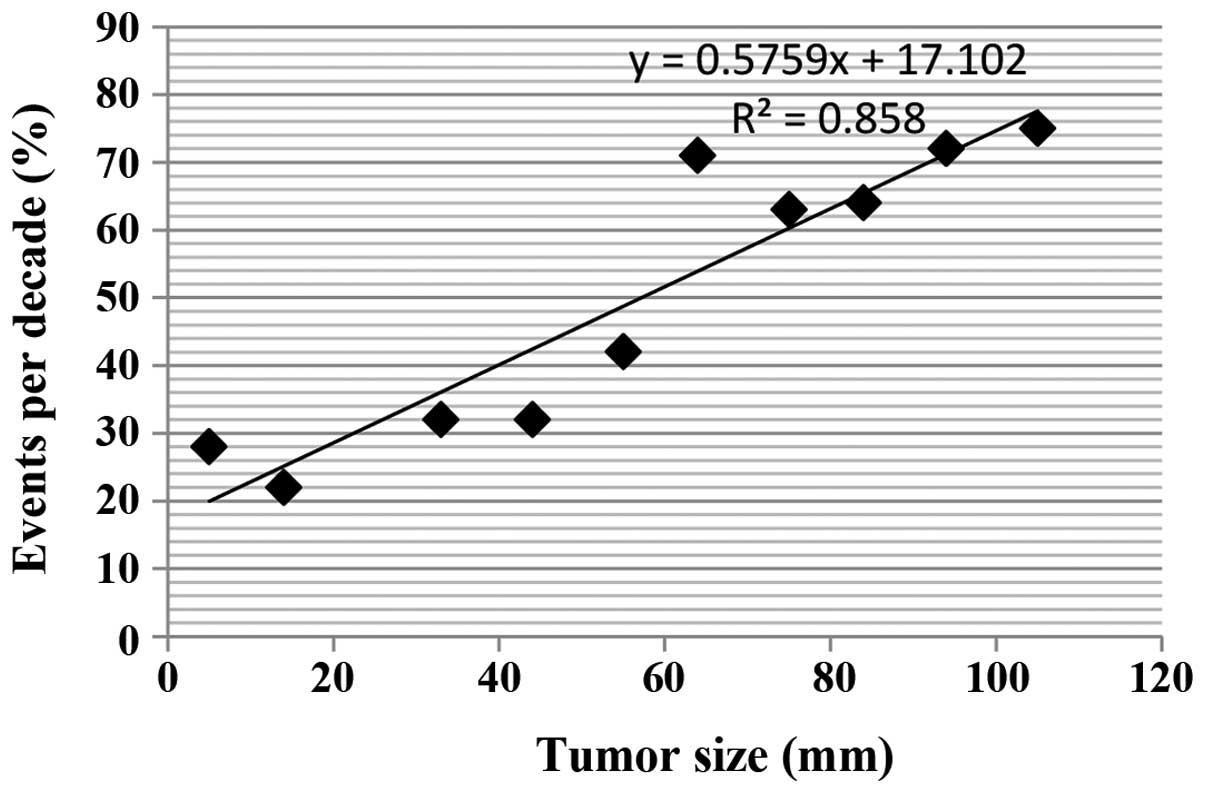
Tumor size
Examination of tumor size revealed that FTCs had the
greatest diameter (mean ± SD, 44.7±16.8 mm] followed by HTC (mean ±
SD, 29.8±9.8 mm). The mean diameter of PTC was the smallest (mean ±
SD, 26.3±15 mm). The size of the tumor was strongly correlated with
clinical outcome (Fig. 4); regardless
of tumor type, a tumor size of >50 mm was significantly
associated (P=0.03) with unfavorable clinical outcomes, including
recurrence of disease or mortality.
Involvement of nodes
The involvement of nodes, at the time of surgery or
follow-up examination, was not significantly associated with
clinical outcomes. At the time of surgery, 121 (38%) subjects had
involvement of nodes; 47 (39%) of these subjects showed unfavorable
clinical outcome at end of the follow-up period (P=0.126). During
follow-up, 80 patients (25%) exhibited nodal involvement, and 12
(15%) had unfavorable outcomes (P=0.354; Fig. 5).
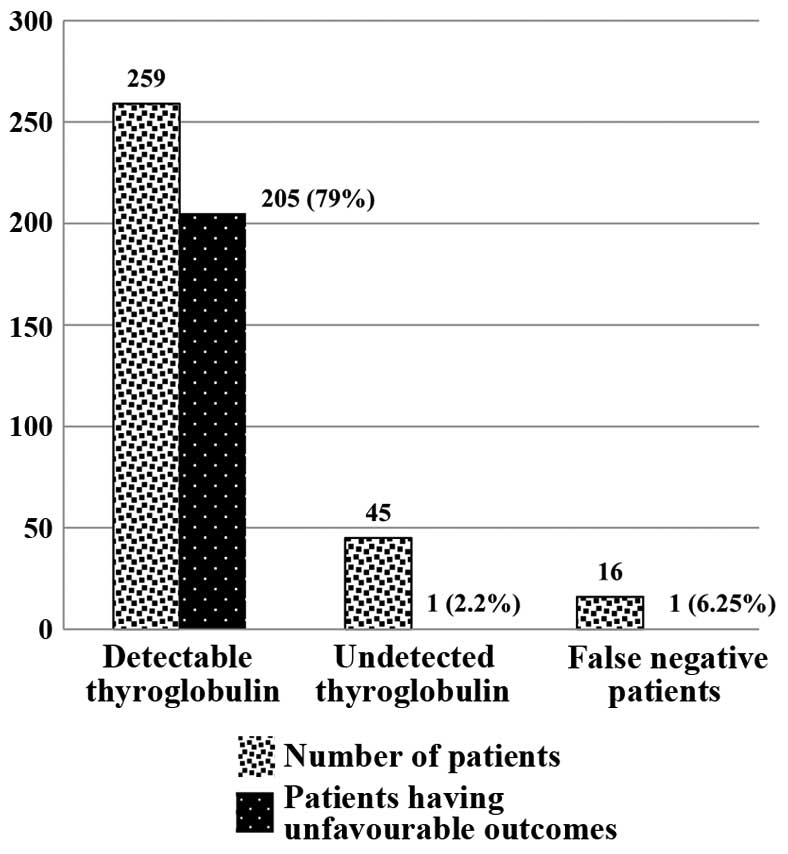
Thyroglobulin
At 6 months following thyroid surgery, 259 (80%)
subjects had detectable serum thyroglobulin levels, while 45 (14%)
had no detectable serum thyroglobulin; 16 (6%) had positive
thyroglobulin antibodies with no detectable serum thyroglobulin
(false negative patients). At the end of the 10-year follow-up
period, unfavorable outcomes were observed in 205 (79%) patients
with detectable thyroglobulin levels six months following thyroid
ablation (P=0.001), while unfavorable outcomes were observed in
only 1 (2.2%) patient without detectable serum thyroglobulin levels
and 1 (6.25%) false negative patient (Fig. 6).
De Groot staging system
The De Groot classification system was used to
categorize subjects into stages according to their level of risk.
At the time of diagnosis, subjects were categorized as follows:
Stage 1, 166 (52%); stage 2, 77 (24%); stage 3, 61 (19.2%); and
stage 4, 16 (5%; Table III). At the
end of the follow-up period, the highest percentage of unfavorable
clinical outcomes was observed in subjects categorized as stage 4
(12 cases; 77%) while the lowest was identified in stage 1 (16
cases; 31%). These results indicated that the De Groot
classification system is significantly associated with clinical
outcome (P=0.005).
 | Table III.Association between De Groot staging
and clinical outcome. |
Table III.
Association between De Groot staging
and clinical outcome.
| De Groot stage | Cases at time of
diagnosis, n (%) | Unfavorable outcome
at end of follow-up, n (%) | P-value |
|---|
| Stage 1 | 166 (52%) | 16 (31%) |
|
| Stage 2 | 77
(24%) | 26 (34%) |
|
| Stage 3 | 61
(19.2%) | 27 (44%) |
|
| Stage 4 | 16
(5%) | 12 (77%) |
|
|
|
|
| 0.005 |
Tumor extension
Extrathyroidal tumor extension was observed in 64
(20%) patients at the time of diagnosis. Table IV shows the prevalence of tumor
extension with respect to type of WDTC. This was independent of
patient age and tumor size (P=0.561 and P=0.328, respectively). At
the end of follow-up, 25 (39%) patients with extra-thyroidal
extension showed favorable clinical outcomes (i.e. disease-free
status). The results clearly showed that extension of tumors to
recurrent sites is not a statistically significant prognostic
factor of WDTC (P=0.387).
 | Table IV.Prevalence of extrathyroidal tumor
extension in different types of WDTC. |
Table IV.
Prevalence of extrathyroidal tumor
extension in different types of WDTC.
| Type of WDTC | Extrathyroidal tumor
extension, n (%) |
|---|
| Papillary thyroid
carcinoma | 47 (74%) |
| Follicular thyroid
carcinoma | 12 (19%) |
| Hurtle cell thyroid
carcinoma | 5 (7%) |
Tumor focality
At the time of diagnosis, multiple tumor foci in one
or both lobes of the thyroid, were present in 70 (22%) study
subjects. The prevalence of multifocality in different pathological
types of WDTC is shown in Fig. 7; no
statistically significant association was observed (P=0.234).
At the end of follow-up, 43 (61%) of the 70 patients
that had exhibited multifocality showed favorable clinical outcomes
(P=0.425). The prevalence of multifocality associated risk factors
among patients at the time of surgery was also assessed (Fig. 8), demonstrating that multifocality is
significantly associated with the risk of node development during
follow-up (P=0.003).
Discussion
Although WDTC is the most common type of thyroid
carcinoma, it is the only type of this carcinoma with a low
mortality rate (15–17) and increased survival rate; the current
study found a rate of mortality of 4% for this condition. However,
a number of studies have reported a mortality rate of ≤10%
(5,18). This difference may be attributable to
variability in the use of treatment approaches and classification
systems between studies. Despite the low mortality rate observed in
the current study, the rate of recurrence is high. Therefore, the
rate of persistence or recurrence of WDTC may be a better indicator
of the mortality rate associated with this disease. The main
limitation associated with the current study is its retrospective
nature, with participants recruited from a single centre. The
current study can serve as a basis for future prospective studies.
Future studies should be conducted prospectively with a large
number of patients from different centres, to enable effective
generalization.
A number of studies have observed that males have
poorer clinical outcomes than females, however, the current study
found no significant difference between genders with regard to
clinical outcome or prognosis (6,18–20). At the beginning of the study,
involvement of nodes was more common among males compared with
females (0.045), however, this difference was not significant based
on multivariate analysis. Patient age was observed to be
significantly associated with outcome in WDTC. As age increases,
particularly after 48 years, the likelihood of unfavorable outcome
increases. Similarly, young age was associated with favorable
prognosis, consistent with a number of other studies (16,18,21).
Previous studies demonstrated that patients with HTC or FTC were
more likely to show unfavorable clinical outcomes compared with
those suffering from PTC (22–24).
Consistently, the present study found that patients suffering from
HTC or FTC upon diagnosis were more susceptible to bone metastases.
However, multivariate analysis showed that tumor size, and not
tumor type, is a predictor of unfavorable clinical outcomes
(P=0.03). Other factors associated with prognosis were the presence
of thyroglobulin and De Groot stage. Thyroglobulin is a
well-established marker of WDTC, and may be used to detect
persistence or recurrence of the disease. The present study
observed the highest rate of unfavorable outcomes in patients who
had detectable serum thyroglobulin six months following thyroid
ablation. The De Groot staging system, which classifies thyroid
carcinomas based on extent of disease, was also found to be
associated with prognosis.
The present 10-year follow-up study, conducted among
patients undergoing same treatment, confirmed previous findings
that WDTC is associated with a high survival rate and a low
mortality rate. Unfavorable clinical outcomes were significantly
associated with advanced age, tumor size, presence of thyroglobulin
and extent of disease as classified by De Groot staging system.
Acknowledgements
The present study was supported by the Guangdong
Provincial Natural Scientific Fund (S2013010016565) and the Natural
Science Foundation of Southern Medical University (PY2013N034).
References
|
1
|
Kebebew E and Clark OH: Differentiated
thyroid cancer: “complete” rational approach. World J Surg.
24:942–951. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ringel MD and Ladenson PW: Controversies
in the follow-up and management of well-differentiated thyroid
cancer. Endocr Relat Cancer. 11:97–116. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Caron NR and Clark OH: Well differentiated
thyroid cancer. Scand J Surg. 93:261–271. 2004.PubMed/NCBI
|
|
4
|
Patel KN and Shaha AR: Poorly
differentiated and anaplastic thyroid cancer. Cancer Control.
13:119–128. 2006.PubMed/NCBI
|
|
5
|
Cushing SL, Palm CE, Audet N, Eski S,
Walfish PG and Freeman JL: Prognostic factors in
well-differentiated thyroid carcinoma. Laryngoscope. 114:2110–2115.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mazzaferri EL: An overview of the
management of papillary and follicular thyroid carcinoma. Thyroid.
9:421–427. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Akslen LA and LiVolsi VA: Prognostic
significance of histologic grading compared with subclassification
of papillary thyroid carcinoma. Cancer. 88:1902–1908. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sugg SL, Zheng L, Rosen IB, Freeman JL,
Ezzat S and Asa SL: ret/PTC-1, -2 and -3 oncogene rearrangements in
human thyroid carcinomas: implications for metastatic potential? J
Clin Endocrinol Metab. 81:3360–3365. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tuttle RM: Differentiated thyroid cancer:
Clinicopathologic staging. http://www.uptodate.com/contents/differentiated-thyroid-cancer-clinicopathologic-stagingOctober
6th–2013
|
|
10
|
DeGroot LJ, Kaplan EL, McCormick M and
Straus FH: Natural history, treatment, and course of papillary
thyroid carcinoma. J Clin Endocrinol Metab. 71:414–424. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kebebew E and Clark OH: Differentiated
thyroid cancer: “Complete” rational approach. World J Surg.
24:942–951. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rami-Porta R, Crowley JJ and Goldstraw P:
The revised TNM staging system for lung cancer. Ann Thorac
Cardiovasc Surg. 15:4–9. 2009.PubMed/NCBI
|
|
13
|
Weetman AP: Expert Consult:
ThyroiditisConn's Current Therapy 2012. Bope ET and Kellerman RD:
Elsevier Saunders; Philadelphia, USA: 2012
|
|
14
|
Elisei R, Molinaro E, Agate L, et al: Are
the clinical and pathological features of differentiated thyroid
carcinoma really changed over the last 35 years? Study on 4187
patients from a single Italian institution to answer this question.
J Clin Endocrinol Metab. 95:1516–1527. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tanaka K, Sonoo H, Saito W, Ohta Y, Shimo
T, Sohda M, Yamamoto Y and Kurebayashi J: Analysis of clinical
outcome of patients with poorly differentiated thyroid carcinoma.
ISRN Endocrinol. 2011:3080292011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Holler T, Theriault J, Payne RJ, Clark J,
Eski S and Freeman JL: Prognostic factors in patients with multiple
recurrences of well-differentiated thyroid carcinoma. J Oncol.
2009:6503402009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Palme CE, Waseem Z, Raza SN, Eski S,
Walfish P and Freeman JL: Management and outcome of recurrent
well-differentiated thyroid carcinoma. Arch Otolaryngol Head Neck
Surg. 130:819–824. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fonseca E, Soares P, Rossi S and
Sobrinho-Simões M: Prognostic factors in differentiated thyroid
gland carcinoma. Pathologe. 18:275–285. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Akslen LA, Myking AO, Salvesen H and
Varhaug JE: Prognostic importance of various clinicopathological
features in papillary thyroid carcinoma. Eur J Cancer. 29A:44–51.
1992.PubMed/NCBI
|
|
20
|
Mazzaferri EL and Jhiang SM: Long-term
impact of initial surgical and medical therapy on papillary and
follicular thyroid cancer. Am J Med. 97:418–428. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Baloch ZW and LiVolsi VA: Prognostic
factors in well-differentiated follicular-derived carcinoma and
medullary thyroid carcinoma. Thyroid. 11:637–645. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Schlumberger M and Pacini F: Thyroid
tumors. 2nd. Nucléon Editions; Paris, France: pp. 18–24. 2003
|
|
23
|
Lin JD, Chao TC and Hsueh C: Clinical
characteristics of poorly differentiated thyroid carcinomas
compared with those of classical papillary thyroid carcinomas. Clin
Endocrinol (Oxf). 66:224–228. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nishida T, Katayama M, Tsujimoto M,
Nakamura J and Matsuda H: Clinicopathological significance of
poorly differentiated thyroid carcinoma. Am J Surg Pathol.
23:205–211. 1999. View Article : Google Scholar : PubMed/NCBI
|