Introduction
Gastric cancer (GC) is an aggressively invasive
tumor, and one of the most common lethal cancers worldwide
(1–2).
Surgical resection may successfully treat GC in the early stage of
disease (3). However, a considerable
number of patients with GC are diagnosed at an advanced stage of
disease, resulting in a poor prognosis (4). Thus, exploring the potential novel
markers of GC is critical to make an early diagnosis, guide the
treatment regimens and predict the prognosis of patients with
GC.
T-box 2 (TBX2) is a member of the T-box family of
transcription factors, which are involved in embryonic development
(5). In addition to playing a role in
development, it has been suggested that TBX2 is involved in cell
cycle regulation and cancer (6).
Deregulated TBX2 gene expression is found in breast cancer
(7), melanoma (8) and pancreatic cancer (9). However, the expression and role of TBX2
in GC have yet to be fully elucidated. In the present study, the
expression of TBX2 in GC was investigated using reverse
transcription-quantitative polymerase chain reaction (RT-qPCR),
western blotting and immunohistochemistry. The correlation between
the expression of TBX2 and the clinicopathological features of
patients with GC was then analyzed.
Patients and methods
Patients
In total, 25 fresh samples of GC and paired adjacent
non-cancerous tissues were obtained between May 1, 2013 and August
1, 2013 at Shandong Provincial Qianfoshan Hospital (Jinan, China),
and used for RT-qPCR and western blot analysis to determine the
TBX2 expression levels. In addition, tissue samples obtained from
266 patients that underwent surgery for the treatment of GC between
January 1, 2004 and December 1, 2008 at Shandong Provincial
Qianfoshan Hospital were used for immunohistochemical analysis. The
group included 371 men and 245 women, with a mean age of 62.6±17.4
years. The present study was approved by the Ethics Committee of
Shandong Provincial Qianfoshan Hospital and was performed in
accordance with the 2000 Declaration of Helsinki (10). Written informed consent was obtained
from all patients prior to surgery.
Follow-up
All tissue samples were used for immunohistochemical
analysis. All patients underwent gastrectomy, and were monitored by
chest, abdominal and pelvic computed tomography (CT) and blood
testing at three-month intervals and yearly gastroscopy. The
overall survival time was defined as the time between surgery and
clinically or radiologically confirmed recurrence or metastasis, or
mortality.
RT-qPCR
Total RNA was extracted using TRIzol reagent
(Invitrogen, Carlsbad, CA, USA) and reverse transcribed into cDNA
using the Reverse Transcription kit (Invitrogen). The cDNA was then
amplified by RT-qPCR using the SYBR Green PCR kit (Invitrogen). The
primers used for PCR were as follows: TBX2 forward,
5′-TGCGGAGAACTCCAGGTT-3′ and reverse, 5′-AGCTGGTGAGTGGAGCCA-3′; and
GAPDH forward, 5′-ACAGTCAGCCGCATCTTCTTTTG-3′ and reverse,
5′-CCCACTTGATTTTGGAGGGATCT-3′. The data were analyzed using the
ΔΔCt method and the expression of TBX2 was normalized against the
GAPDH transcripts.
Western blot analysis
Fresh tumor sections obtained from 25 patients
recently diagnosed with GC and the corresponding adjacent
non-cancerous tissues were lysed in cell lysis buffer
(Sigma-Aldrich, St. Louis, MO, USA). The samples were homogenized
and then maintained at 4°C for 30 min. Cell extracts were
centrifuged at 12,000 × g for 30 min at 4°C and the protein
concentration was determined using a bicinchoninic acid kit (Pierce
Biotechnology, Inc., Rockford, IL, USA). Total protein (50 µg) from
each sample was heated at 95°C for 5 min subsequent to mixing with
an equal volume of 2X SDS loading buffer. The samples were
separated on 12.5% SDS-PAGE gels and transferred to polyvinylidene
difluoride membranes (EMD Millipore, Billerica, MA, USA). The
membrane was blocked in Tris-buffered saline with Tween 20 (TBS-T)
containing 5% skim milk at room temperature for 2 h. The membranes
were incubated with TBX2 antibody (dilution, 1:2,000) in TBS-T
overnight at 4°C. Subsequent to washing with TBS-T, the membrane
was incubated in 5% skim milk in TBS-T buffer containing a
secondary antibody (dilution, 1:5,000) for 60 min at room
temperature. Proteins of interest were detected using a
Chemiluminescence Reagent Plus kit (Perkin Elmer, Beaconsfield,
Buckinghamshire, UK). For normalization of protein loading, the
monoclonal antibody against GAPDH (dilution, 1:5,000) was used.
Immunohistochemistry
Immunohistochemical analysis was performed on the
formalin-fixed paraffin-embedded sections of GC tumor tissues and
the corresponding adjacent benign tissue specimens. The slides were
microwaved in citrate buffer antigen retrieval solution (Vector
Laboratories, Inc., Burlingame, CA, USA) and washed with
phosphate-buffered saline (PBS) for 5 min. The slides were then
blocked with 10% normal goat serum in PBS for 30 min and incubated
overnight in the presence of rabbit polyclonal IgG anti-TBX2
antibody (dilution, 1:50; cat no. sc-48780; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA). The slides were then washed
three times using PBS and incubated with goat anti-rabbit secondary
antibody (dilution, 1:500) for 30 min. The slides were
counterstained with light green (Beijing Bitab Biotechnology Co.,
Ltd., Beijing, China) and evaluated semi-quantitatively based on
the intensity and percentage of staining. The TBX2 staining was
classified as strong (3+), moderate (2+), weak (1+) or absent (−)
compared with the adjacent non-tumor tissues. Scores of 0 or 1+
were considered to indicate a lack of TBX2 expression, and scores
of 2+ or 3+ were considered to indicate the presence of TBX2
expression. No staining was observed using the isotype control
immunoglobulin G, indicating stain specificity.
Statistical analysis
The χ2 and Fisher's exact tests were used
to compare categorical variables. Kaplan-Meier plot and log-rank
tests were used for survival analysis. Univariate and multivariate
analyses were based on the Cox proportional hazards regression
model. Statistical analyses were performed using SPSS 15.0 software
(SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
Increased TBX2 mRNA and protein
expression in human GC tissues
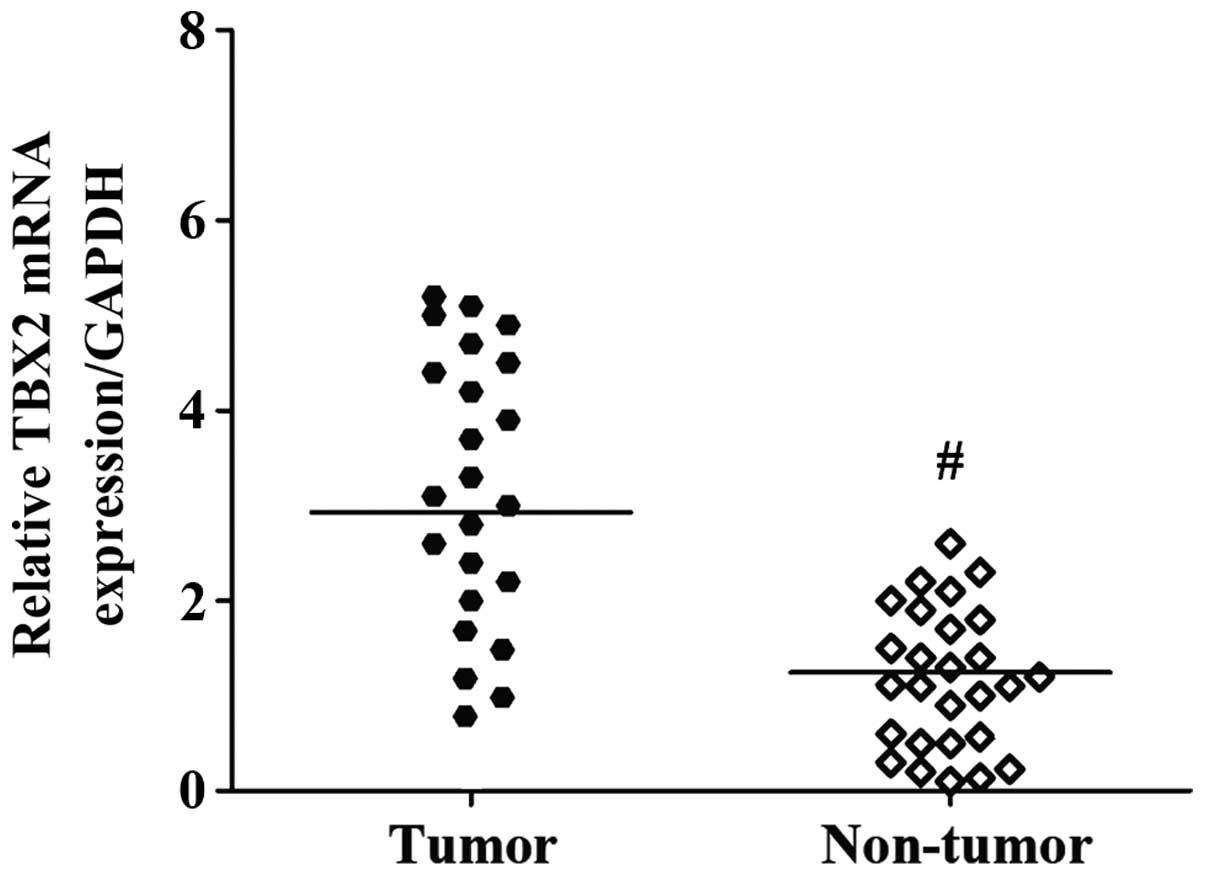
The mRNA level of TBX2 was determined by RT-qPCR in
25 fresh GC specimens and paired corresponding adjacent
non-cancerous gastric tissues. The TBX2 gene expression level was
significantly increased in the cancerous tissues compared with the
adjacent non-cancerous tissues (P<0.001; Fig. 1).
Furthermore, western blot analysis was performed on
the aforementioned specimens. Consistently, the results revealed
that the expression of the TBX2 protein was significantly increased
in the GC tissues compared with the matched adjacent non-cancerous
tissues, as semi-quantified by densitometry (P<0.001; Fig. 2).
Immunohistochemical analysis of TBX2
expression and the association with clinicopathological
parameters
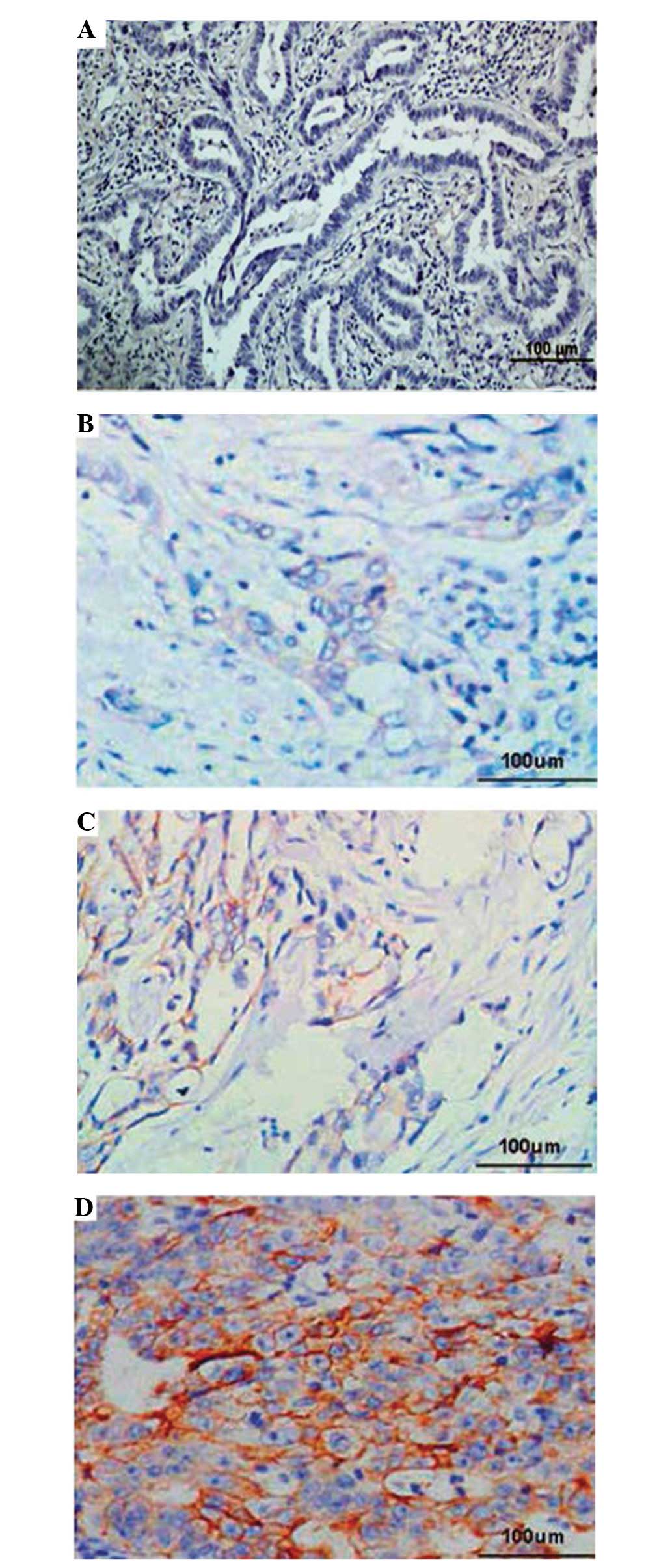
To investigate the clinicopathological significance
of TBX2 expression, immunohistochemical analysis was performed
using 266 paraffin-embedded GC tissue blocks. The results of the
immunohistochemical analysis were graded for the expression of TBX2
as without TBX2 expression or positive for TBX2 expression. As
revealed in Fig. 3, the expression
rate of TBX2 in GC samples was 66.2% (176/266). By contrast, only
8.27% (22/266) of paired adjacent non-cancerous tissues exhibited
TBX2 expression. The association between the expression of TBX2 and
various clinicopathological parameters is listed in Table I. The results revealed that the
increased expression of TBX2 was significantly associated with the
clinical stage of disease (P=0.022), incidence of vascular invasion
(P=0.031) and presence of metastasis (P=0.005).
 | Table I.Association between TBX2 expression
and clinicopathological parameters in 266 patients with gastric
cancer. |
Table I.
Association between TBX2 expression
and clinicopathological parameters in 266 patients with gastric
cancer.
|
|
| TBX2 expression |
|
|---|
|
|
|
|
|
|---|
| Parameters | Total, n | Present, n | Absent, n | P-value |
|---|
| Total | 266 | 176 | 90 |
|
| Age |
|
|
| NS |
|
<48 | 100 | 62 | 38 |
|
| ≥48 | 166 | 114 | 52 |
|
| Gender |
|
|
| NS |
| Male | 161 | 102 | 59 |
|
|
Female | 105 | 74 | 31 |
|
| Clinical stage |
|
|
| 0.022 |
| I–II | 68 | 32 | 36 |
|
|
III–IV | 198 | 144 | 54 |
|
| T-stage |
|
|
| NS |
|
T1-T2 | 90 | 56 | 34 |
|
|
T3-T4 | 176 | 120 | 56 |
|
| N-stage |
|
|
| NS |
| N0 | 78 | 50 | 28 |
|
|
N1-N3 | 188 | 126 | 62 |
|
| Vascular
invasion |
|
|
| 0.031 |
| No | 185 | 148 | 37 |
|
| Yes | 81 | 28 | 53 |
|
| Metastasis |
|
|
| 0.005 |
| No | 200 | 121 | 79 |
|
| Yes | 66 | 55 | 11 |
|
Association between TBX2 expression
and prognosis, as determined using the Cox proportional hazards
model
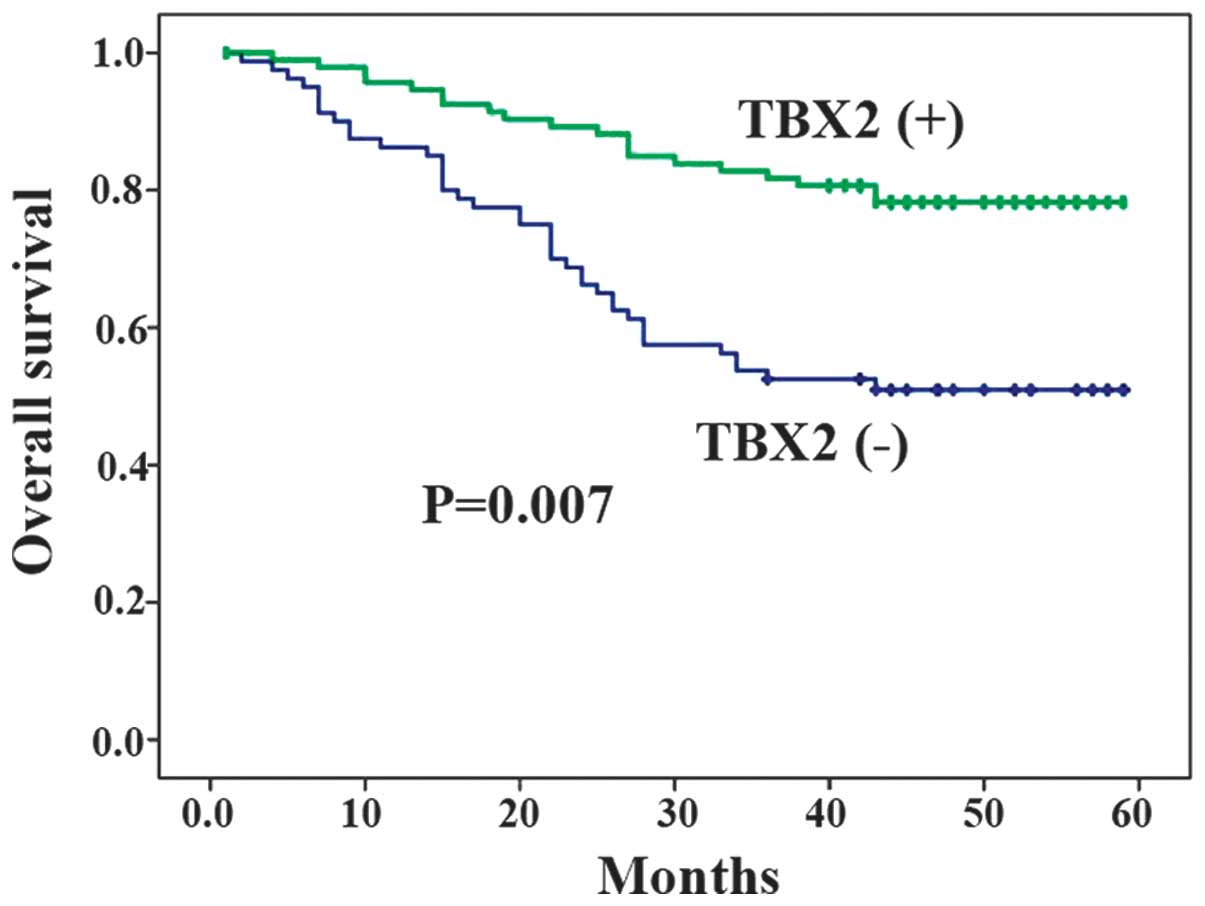
The prognostic effect of TBX2 expression on the
survival of patients with GC was analyzed. The results revealed
that the overall survival time of the patients with GC that
demonstrated TBX2 expression was shorter compared with the survival
time of patients without TBX2 expression (P=0.007; Fig. 4).
Univariate analysis revealed that the overall
survival time of patients was significantly associated with the
tumor stage and presence of vascular invasion, metastasis and
expression of TBX2 (Table II). In
addition, the association between the presence of TBX2 expression
and survival time was found to remain significant subsequent to
controlling other prognostic factors in multivariate analysis
[hazard ratio (HR), 3.930; 95% confidence interval (CI),
2.041–7.917; P=0.009; Table III].
Multivariate analysis also revealed metastasis and vascular
invasion to be independent prognostic factors.
 | Table II.Univariate analysis of the prognosis
in 266 patients with gastric cancer. |
Table II.
Univariate analysis of the prognosis
in 266 patients with gastric cancer.
| Variables | HR (95% CI) | P-value |
|---|
| Depth of
invasion |
|
|
| T1+T2 vs.
T3+T4 | 0.984
(0.301–1.996) |
0.086 |
| Nodal status |
|
|
| N0 vs.
N1+N2+N3 | 0.712
(0.165–1.523) |
0.125 |
| Metastasis |
|
|
| Absent
vs. present | 5.292
(2.009–12.327) | <0.001 |
| Stage |
|
|
| I+II vs.
III+IV | 2.573
(1.410–5.694) |
0.019 |
| Vascular
invasion |
|
|
| Absent
vs. present | 3.112
(1.271–6.044) | <0.001 |
| TBX2 expression |
|
|
| Present
vs. absent | 3.018
(1.979–7.208) |
0.002 |
 | Table III.Multivariate analysis of the prognosis
in patients with gastric cancer. |
Table III.
Multivariate analysis of the prognosis
in patients with gastric cancer.
| Variables | HR (95% CI) | P-value |
|---|
| Depth of
invasion |
|
|
| T1+T2 vs.
T3+T4 | 0.921
(0.132–2.413) |
0.093 |
| Nodal status |
|
|
| N0 vs.
N1+N2+N3 | 1.099
(0.264–3.073) |
0.098 |
| Metastasis |
|
|
| Absent
vs. present | 9.320
(3.117–19.344) | <0.001 |
| Stage |
|
|
| I+II vs.
III+IV | 0.902
(0.305–2.098) |
0.076 |
| Vascular
invasion |
|
|
| Absent
vs. present | 1.874
(1.003–4.615) |
0.020 |
| TBX2 expression |
|
|
| Present
vs. absent | 3.930
(2.041–7.917) |
0.009 |
Discussion
Evolutionarily conserved genes perform critical and
well-established roles in embryonic development. T-box factors have
gained increasing prominence in the field of cancer biology, as a
wide range of cancers exhibit deregulated expression of T-box
factors that possess tumor suppressor or tumor promoter functions.
TBX2 is one of the best characterized members of the T-box family
of transcription factors and contributes directly to tumor
progression, and particularly to the suppression of senescence and
control of invasiveness (11). TBX2,
located at 17q23, has been revealed to be highly amplified and
overexpressed in breast cancer cell lines through fluorescence
in situ hybridization (FISH) (12). Jacobs et al (7) revealed that TBX2 was amplified in a
subset of primary human breast cancers, indicating that this gene
may contribute to breast cancer development. In addition, Vance
et al (13) found that TBX2
was poorly expressed in the melanocyte cell line, while it was
overexpressed in all melanoma cell lines tested. This study also
found that TBX2 played an important role in maintaining cell
proliferation and suppression of senescence in melanomas.
Furthermore, Mahlamaki et al (9) detected that TBX2 was amplified in 50% of
pancreatic cancer cell lines tested by FISH. TBX2 was also
overexpressed in several cancers, including breast and pancreatic
cancers and melanoma, where TBX2 was demonstrated to function as a
tumor-associated gene. Furthermore, Duo et al (14) reported previously in pancreatic cancer
that TBX2 was closely associated with the degree of tumor
differentiation, increased TNM stage and presence of distant
metastasis. However, extremely little is known on the deregulation
of TBX2 in GC.
In the present study, TBX2 expression was measured
in fresh frozen specimens of GC and it was found that the
expression levels of TBX2 mRNA and protein in GC tumor tissues were
increased compared with the corresponding non-cancerous mucosa. It
was suggested that TBX2 was upregulated at the transcriptional and
post-transcriptional levels. Additional validation by
immunohistochemistry revealed that 66.2% (176/266) of GC tissues
exhibited positive staining, while only 8.27% (22/266) of the
matched non-cancerous tissues were immunoreactive for TBX2. These
data indicated that TBX2 may be involved in the progression of
gastric carcinogenesis. In the present study, the association
between TBX2 expression and the clinicopathological features of GC
was also analyzed. The current results revealed a correlation
between the expression of TBX2 in tumors and the clinical tumor
stage, and a correlation between vascular invasion and distant
metastasis. These data indicate that upregulated expression of TBX2
may contribute to the invasion and metastasis of tumors. Therefore,
TBX2 may be a possible biomarker for the identification of subsets
of colon cancer with a more aggressive phenotype. Consistent with
the present results, Dimova et al (15) identified TBX2 to be a specific genomic
marker for late-stage ovarian cancers. Similarly, Kandimalla et
al (16) recognized TBX2
expression as a pTa stage-specific prognostic marker in bladder
cancer.
Overall, the present study revealed that patients
with tumors expressing TBX2 demonstrated a worse survival rate
compared with patients without TBX2 expression. In addition, TBX2
expression was strongly associated with an increased risk of tumor
invasion and metastasis compared with the absence of TBX2
expression. Using the univariate and multivariate Cox model
analysis, it was confirmed that TBX2 may act as a significant
independent prognostic factor for patients with GC.
References
|
1
|
Liang JW, Gao P, Wang ZN, Song YX, Xu YY,
Wang MX, Dong YL and Xu HM: The integration of macroscopic tumor
invasion of adjacent organs into TNM staging system for colorectal
cancer. PLoS One. 7:e522692012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sun J, Jiang T, Qiu Z, Cen G, Cao J, Huang
K, Pu Y, Liang H, Huang R and Chen S: Short-term and medium-term
clinical outcomes of laparoscopic-assisted and open surgery for
colorectal cancer: A single center retrospective case-control
study. BMC Gastroenterol. 11:852011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Popa F, Bratucu M and Radu P: Present and
future tense in operable rectal cancer. Chirurgia (Bucur).
106:11–16. 2011.PubMed/NCBI
|
|
5
|
Rowley M, Grothey E and Couch FJ: The role
of Tbx2 and Tbx3 in mammary development and tumorigenesis. J
Mammary Gland Biol Neoplasia. 9:109–118. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Abrahams A, Parker MI and Prince S: The
T-box transcription factor Tbx2: Its role in development and
possible implication in cancer. IUBMB Life. 62:92–102.
2010.PubMed/NCBI
|
|
7
|
Jacobs JJ, Keblusek P, Robanus-Maandag E,
Kristel P, Lingbeek M, Nederlof PM, van Welsem T, van de Vijver MJ,
Koh EY, Daley GQ and van Lohuizen M: Senescence bypass screen
identifies TBX2, which represses Cdkn2a (p19(ARF)) and is amplified
in a subset of human breast cancers. Nat Genet. 26:291–299. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Vance KW, Carreira S, Brosch G and Goding
CR: Tbx2 is overexpressed and plays an important role in
maintaining proliferation and suppression of senescence in
melanomas. Cancer Res. 65:2260–2268. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mahlamäki EH, Bärlund M, Tanner M,
Gorunova L, Höglund M, Karhu R and Kallioniemi A: Frequent
amplification of 8q24, 11q, 17q and 20q-specific genes in
pancreatic cancer. Genes Chromosomes Cancer. 35:353–358. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Salako SE: The declaration of Helsinki
2000: Ethical principles and the dignity of difference. Med Law.
25:341–354. 2006.PubMed/NCBI
|
|
11
|
Wansleben S, Peres J, Hare S, Goding CR
and Prince S: T-box transcription factors in cancer biology.
Biochim Biophys Acta. 1846:380–391. 2014.PubMed/NCBI
|
|
12
|
Bärlund M, Monni O, Kononen J, Cornelison
R, Torhorst J, Sauter G, Kallioniemi OLLI-P and Kallioniemi A:
Multiple genes at 17q23 undergo amplification and overexpression in
breast cancer. Cancer Res. 60:5340–5344. 2000.PubMed/NCBI
|
|
13
|
Vance KW, Carreira S, Brosch G and Goding
CR: Tbx2 is overexpressed and plays an important role in
maintaining proliferation and suppression of senescence in
melanomas. Cancer Res. 65:2260–2268. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Duo S, Tiao-Dong T, Lei Z, Wei W, Hong-Li
S and Xian-Wei D: Expression and clinical significance of tbx2 in
pancreatic cancer. Asian Pac J Cancer Prev. 10:118–122.
2009.PubMed/NCBI
|
|
15
|
Dimova I, Orsetti B, Negre V, Rouge C,
Ursule L, Lasorsa L, Dimitrov R, Doganov N, Toncheva D and Theillet
C: Genomic markers for ovarian cancer at chromosomes 1, 8 and 17
revealed by array CGH analysis. Tumori. 95:357–366. 2009.PubMed/NCBI
|
|
16
|
Kandimalla R, van Tilborg AA, Kompier LC,
Stumpel DJ, Stam RW, Bangma CH and Zwarthoff EC: Genome-wide
analysis of CpG island methylation in bladder cancer identified
TBX2, TBX3, GATA2 and ZIC4 as pTa-specific prognostic markers. Eur
Urol. 61:1245–1256. 2012. View Article : Google Scholar : PubMed/NCBI
|