Introduction
Head and neck squamous cell carcinoma (HNSCC)
comprises tumors of the oral cavity, pharynx and larynx, and is a
relatively common human cancer. When grouped together, oral and
pharyngeal cancer represent the sixth most common type of cancer
worldwide. In South America and the Caribbean, cancers of the mouth
and pharynx rank fifth amongst males and sixth in females (1). A high incidence rate for oral and
laryngeal cancer is observed in Brazil, with up to 20,000 novel
cases reported annually (2). The most
significant etiological factors in the development of HNSCC are
cigarette smoking and alcohol consumption (3); however, high-risk human papillomavirus
(HPV), particularly HPV-16, has also been recognized as an
independent factor for a subset of HNSCC, and there is a marked
association between HPV infection and tonsil carcinoma development
(4).
Surgery is the most well-established initial
treatment strategy for the majority of oral cancers, however,
radiotherapy may be employed in conjunction with surgery (5). In order to enhance organ preservation
and survival, a multidisciplinary approach is encouraged, and
concurrent chemo-radiotherapy has been recommended (6). Currently, due to the improvements in
locoregional control, chemo-radiotherapy with cisplatin or other
platinum compounds is considered to be a standard treatment regimen
for patients with locoregionally advanced HNSCC. However, treatment
with this combination is only successful in 50–60% of patients.
Thus, a novel and more effective management strategy with favorable
toxicity levels is required (7).
A number of trials have indicated that a three-drug
regimen consisting of a taxel (including docetaxel), cisplatin and
5-fluorouracil (TPF) improves the outcome of patients with HNSCC
(8–10). These trials demonstrated that patients
who underwent TPF induction chemotherapy combined with radiotherapy
had a significantly longer survival compared with that of patients
treated with cisplatin and fluorouracil (PF) plus radiotherapy
(8). Compared with the standard PF
regimen, induction chemotherapy with the addition of docetaxel
significantly improved progression-free and overall survival in
patients with unresectable HNSCC (10). Based on these data, in 2007, the Food
and Drug Association (FDA) approved the use of docetaxel in
combination with cisplatin and fluorouracil for the induction
treatment of patients with locally advanced HNSCC (11).
Despite the FDA-approval of TPF for HNSCC treatment,
little has been established with regard to the cellular mechanisms
of action underlying this drug association. Based on the
aforementioned findings and to further understand how cells react
to this novel HNSCC treatment, the present study examined the
cytotoxic effects of TPF in human HNSCC cell lines in association
with irradiation, analyzed its effect on cell cycle progression and
cell death, and evaluated its capacity to alter cell migration.
Materials and methods
Cell lines and culture conditions
Two human HNSCC cell lines were used in the present
study: The tongue carcinoma cell line SCC-9, and the hypopharyngeal
carcinoma cell line FaDu. A keratinocyte cell line (HaCaT) was used
as a control. All cells were provided by Dr. Décio dos Santos Pinto
Júnior (Faculty of Dentistry, University of São Paulo, São Paulo,
Brazil). FaDu and HaCaT cells were cultured in Dulbecco's modified
Eagle's medium (DMEM) supplemented with 10% fetal bovine serum and
1% antibiotics (penicillin-streptomycin). SCC-9 cells were cultured
in a combination of DMEM and Ham's F12 (1:1 ratio), supplemented
with hydrocortisone, 10% fetal bovine serum and 1%
penicillin-streptomycin. Cells were maintained at 37°C in an
atmosphere of 5% CO2. For all experiments, cells were
detached from the growth surface using trypsin (0.25%)/EDTA (1 mM)
solution. All cell culture reagents were purchased from
Sigma-Aldrich (St. Louis, MO, USA).
Drug preparation
Paclitaxel (6 mg/ml; Laboratório Químico
Farmacêutico Bergamo Ltda., São Paulo, Brazil), cisplatin
(Citoplax, 1 mg/ml; Laboratório Químico Farmacêutico Bergamo Ltda.)
and 5-fluorouracil (Fluoracila, 50 mg/ml; Accord Farmacêutica
Ltda., São Paulo, Brazil), designated TPF, were diluted in Milli-Q
water at a ratio of 1:20:40, respectively, to obtain a 500 mg/ml
stock solution (12). Fresh stock
solutions were produced for each experiment. Final dilutions of 25,
50, 100 and 200 µg/ml were used in the treatment of cells.
Cisplatin alone was used in all experiments as a control, and in
order to compare its cytotoxic effect with that of TPF.
Dose-response cytotoxicity of TPF
For the cytotoxicity experiment, FaDu, SCC-9 and
HaCaT cells were seeded at a density of 5×103 cells/well
into 96-well plates, and incubated overnight at 37°C in 5%
CO2. Cells were then treated with serial dilutions of
TPF or cisplatin alone in decreasing concentrations (200, 100, 50
and 25 µg/ml), or with a vehicle control (culture medium).
Following 24 h of treatment, 10 µl MTT (5 mg/ml; Sigma-Aldrich)
solution was added to each well, prior to incubation for 4 h at
37°C. Following incubation, the treatment media were discarded, and
100 µl isopropanol containing 5% 1 M HCl solution was added to
dissolve the formazan crystals. The absorbance was measured at 570
nm with a Beckman Coulter DTX 800 reader (#987920; Beckman Coulter,
Brea, CA, USA).
TPF and irradiation cytotoxicity
assay
In order to evaluate the cytotoxicity of concurrent
TPF or cisplatin and irradiation treatment, cells were seeded into
96-well culture plates at a density of 5×103 cells/well,
incubated overnight at 37°C in 5% CO2, and treated with
the drug solutions at a concentration of 50 µg/ml for 24 h.
Following 24-h treatment, the medium was removed from the cells and
100 µl PBS was added prior to irradiation. The irradiation was
conducted using a Siemens PRIMUS linear accelerator, with 6 MV
photon beams at a dose rate of 2.0 Gy/min. As controls, one plate
was treated only with radiation, and another was seeded and not
irradiated. Immediately following irradiation, phosphate-buffered
saline (PBS) was removed, and cells were maintained in culture
medium without drug treatment. At 24 and 48 h after irradiation,
cell death was assessed by MTT assay, and absorbance was measured
at 570 nm using the DTX 800 reader.
Flow cytometric analysis
Flow cytometric analysis was performed to define the
cell cycle distribution and induction of apoptosis in TPF- and
cisplatin-treated and untreated cells. To determine the cell cycle
distribution, cells were seeded into six-well plates at a density
of 106 cells/well and incubated overnight. Following 24
h of incubation, cells were treated with TPF or cisplatin at a
concentration of 50 µg/ml for 6 h, prior to the collection of
floating and adherent cells, which were fixed in cold 70% ethanol
for 30 min, centrifuged at 537.6 × g for 5 min, washed with 1 ml
cold PBS and centrifuged again at 537.6 × g for 5 min. The pellet
was subsequently resuspended in 100 µl of RNAse A (250 µg/ml) and
incubated for 30 min. Propidium iodide solution (50 µg/ml) was then
added, followed by incubation in a dark chamber for 10 min. Cells
were analyzed with a Cyflow Space-9 flow cytometer (excitation, 488
nm; Sysmex Partec GmbH, Görlitz, Germany), with fluorescence
measured at 620–640 nm. A minimum of 10,000 events were analyzed
and the distribution of cells in each phase of the cell cycle was
determined.
Induction of apoptosis was assessed using flow
cytometric analysis of outer membrane phosphatidylserine
translocation. For this assay, a fluorescein isothiocyanate
(FITC)-Annexin V/Dead Cell Apoptosis kit (Invitrogen Life
Technologies, Carlsbad, CA, USA) was used. Cells were plated and
treated with TPF and cisplatin as described for the cell cycle
assay above. Following 6 h of treatment, the supernatant and cells
were collected, centrifuged at 537.6 × g for 5 min and resuspended
in 1X Annexin-binding buffer. FITC-Annexin V (5 µl) and 1 µl
propidium iodide (100 µg/ml) were added to 100 µl cell suspension.
Following 15 min of incubation, stained cells were analyzed by flow
cytometry using the FL1, FL2 and FL3 channels, and the percentages
of apoptotic, late apoptotic and necrotic cells were
identified.
Transwell migration assay
The capacity of TPF and cisplatin to alter human
oral cancer cell migration was assessed using a Transwell migration
assay. The 6.5 mm Costar® Transwell chambers (Corning Life
Sciences, Cambridge, MA, USA), with polycarbonate membrane inserts
(8-µm pore size), were placed in 24-well plates containing 600 µl
DMEM per well. Cells (2×104 per chamber) were seeded
onto the upper compartment of each chamber and incubated at 37°C
for 24 h. Following this period, cells were treated with TPF or
cisplatin at a concentration of 50 µg/ml; PBS was used as a
negative control. At 72 h after treatment, the cells that had
migrated through the membrane to the lower compartment were fixed
in methanol for 20 min, incubated with 0.2% violet crystal dye for
5 min and washed with PBS 7–10 times. Following the final wash, the
stained cells were viewed under a light microscope (Primovert;
Zeiss, Göttingen, Germany) equipped with a digital camera (Axiocam
ERc 5s; Zeiss) and photomicrographs from three randomly selected
fields were captured at x4 magnification, in order to count the
number of migrated cells using the image analysis ZEN 2012
software, blue edition (Carl Zeiss Microscopy GmbH, Göttingen,
Germany).
Statistical analysis
Statistical analysis was performed using the mean
values obtained in triplicate, from three independent replications
of each experiment. The values obtained from the MTT assay were
transformed into percentages representing the inhibitory effect of
the treatments on cellular mitochondrial activity, compared with
the negative controls (considered to represent 100% cell metabolic
activity). For the MTT assay, statistical analyses were performed
using SPSS version 21 (IBM SPSS, Armonk, NY, USA) and applying the
Kruskal-Wallis and Mann-Whitney non-parametric tests. For flow
cytometric analysis, data were analyzed with GraphPad Prism
(GraphPad Software, Inc., La Jolla, CA, USA) using a one-way
analysis of variance with Dunnett's post hoc test. P<0.05 was
considered to indicate a statistically significant difference.
Results
TPF reduces viability of FaDu, SCC-9
and HaCaT cells
Cisplatin and TPF regimens were cytotoxic to all
cell lines, however, there was no statistically significant
difference between TPF and cisplatin (Table I). Cisplatin induced increasing
toxicity up to a concentration of 100 µg/ml, exerting a clear
dose-dependent response (Fig. 1A).
The higher concentration, 200 µg/ml, did not result in a
significant reduction in viability compared with that of 100 µg/ml
in the tested cells. Therefore 100 µg/ml was selected as the
maximum concentration to be used in these cell lines. TPF treatment
resulted in a regular dose-response curve and produced a
considerable reduction in cell viability at 200 µg/ml (P<0.001
compared with 0 µg/ml) in all cell lines, even though this
concentration was notably aggressive to the keratinocyte cell line,
causing loss of cellular integrity and sharpness. In SCC-9 cells,
TPF demonstrated greater cytotoxicity. At 50 µg/ml, TPF was able to
induce a viability reduction of ~42%, and at the maximum
concentration, this reduction reached 78% (Fig. 1B). Treatment with cisplatin alone
reduced cell viability by only 43% at a concentration of 100 µg/ml.
For HaCaT cells, the two treatment regimens were found to be
aggressive: TPF and cisplatin were able to reduce cell viability by
almost 54 and 43%, respectively, at a concentration of 50 µg/ml.
These results confirmed that the chemotherapy regimen currently
used in the treatment of head and neck cancer is not selective for
tumor cells.
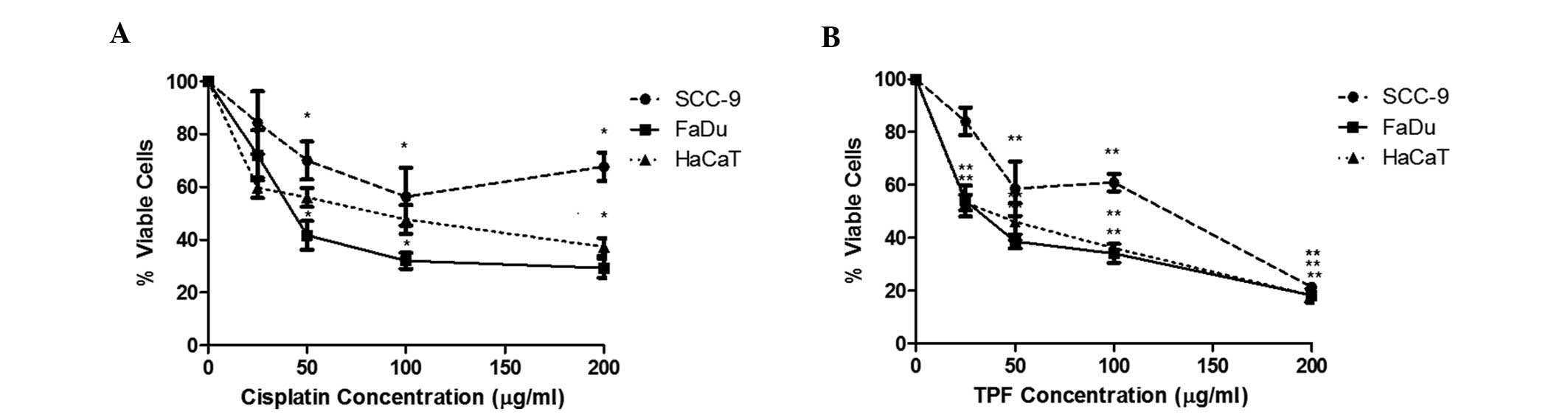 | Figure 1.TPF and cisplatin induce a
dose-dependent decrease in SCC-9, FaDu and HaCaT cell viability.
Dose-response curves of the cells (SCC-9, closed circles; FaDu,
closed squares; and HaCaT, closed triangles) treated for 24 h with
(A) cisplatin and (B) TPF, at concentrations of 0, 25, 50, 100 and
200 µg/ml. *P<0.05, **P<0.0001 vs. control group. These
results are representative of at least three independent
experiments and are presented as the mean ± standard deviation of
triplicate experiments. TPF, paclitaxel + cisplatin +
fluorouracil. |
 | Table I.Percentage of viable cells relative to
controls (determined by MTT assay) following 24 and 48 h treatments
with various combinations of cisplatin (50 µg/ml), TPF (50 µg/ml)
and irradiation, in head and neck squamous cell carcinoma or HaCaT
cells. |
Table I.
Percentage of viable cells relative to
controls (determined by MTT assay) following 24 and 48 h treatments
with various combinations of cisplatin (50 µg/ml), TPF (50 µg/ml)
and irradiation, in head and neck squamous cell carcinoma or HaCaT
cells.
| Treatment | Viable cells, % |
|---|
|
|---|
| FaDu | SCC-9 | HaCaT |
|---|
| Control | 100 | 100a | 100a |
| Cisplatin 24 h |
41.66±9.60a |
70.00±4.96ab |
56.00±7.27ab |
| TPF 24 h |
38.62±10.15a |
58.61±6.84ab |
46.07±8.91ab |
| Radiotherapy 24
h |
99.90±0.00 |
93.50±0.67 |
220.10±11.10 |
| Radiotherapy 48
h |
102.30±0.22 |
85.40±1.35 |
190.50±8.41 |
| Cisplatin +
radiotherapy 24 h |
15.15±15.07a |
20.74±14.08a |
27.78±12.83ac |
| Cisplatin +
radiotherapy 48 h |
11.03±15.80a |
28.31±12.74ab |
27.79±12.83ab |
| TPF + radiotherapy 24
h |
13.38±14.32a |
37.67±10.31a |
21.60±12.96a |
| TPF + radiotherapy 48
h |
13.34±14.33a |
19.24±13.35a |
1.41±11.34ac |
Table I shows that TPF
was more cytotoxic than cisplatin in the FaDu and SCC-9 cell lines,
however, this difference was not statistically significant. The two
chemotherapy regimens were significantly more cytotoxic in FaDu
cells, compared with SCC-9 cells (P<0.001 for cisplatin and TPF
at 100 µg/ml). These results demonstrated that, although all cells
have the same origin, cells from hypopharyngeal carcinoma are more
sensitive to chemotherapy.
A concentration of 50 µg/ml induced a cell viability
reduction of 50% for all cell lines with the two chemotherapy
regimens, and was therefore selected for use in the radiotherapy
experiment.
Treatment with TPF improves cellular
response to irradiation
Treatment with irradiation alone at 2 Gy/min, the
dose used for clinical application in patients, was not cytotoxic
for HNSCC cell lines and induced proliferation in keratinocytes
(Table I). Chemo-radiotherapy led to
higher cytotoxicity compared with that of each treatment method
alone. Radiotherapy following treatment with TPF (48 h
subsequently) and cisplatin (24 h subsequently) was significantly
less cytotoxic to keratinocytes than to cancer cells (P<0.05).
The results also revealed that irradiation following 48 h of
pretreatment with TPF produced enhanced cytotoxicity for SCC-9
cells (19.24% viable cells), and with cisplatin for FaDu cells
(11.03% viable cells), compared with control cells (100% viable
cells). Together, these results suggested that combined TPF and
radiotherapy may be an effective strategy for the treatment of oral
cancer with reduced toxicity in the HaCaT cells compared with
cancer cells.
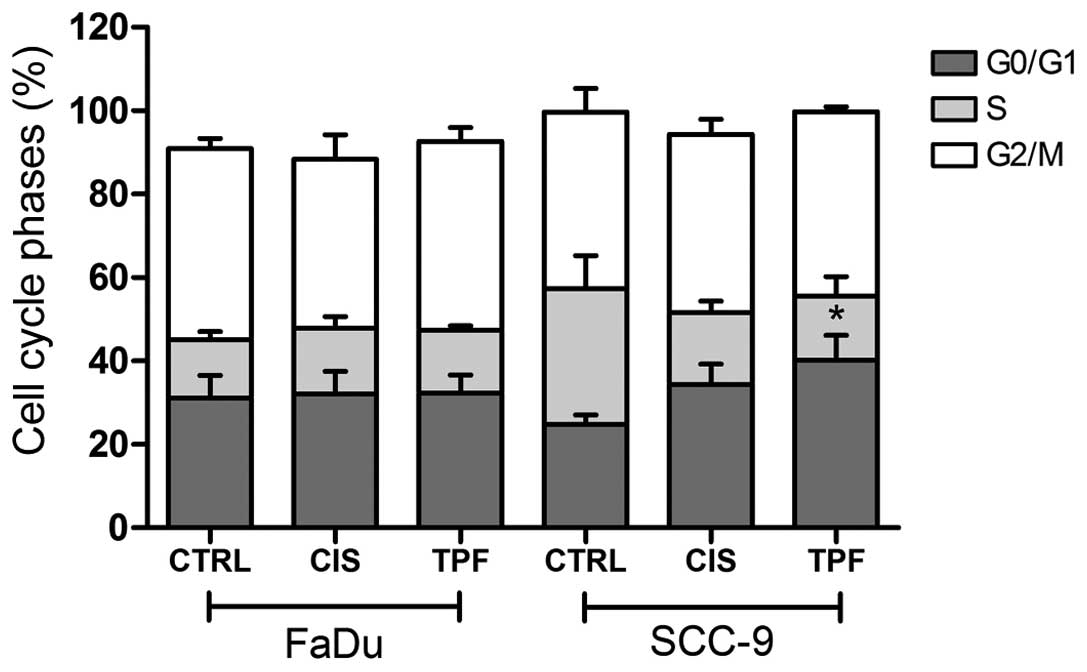
TPF induces G0/G1 cell cycle arrest
and enhances apoptosis
To further determine whether TPF and cisplatin
inhibited cell proliferation by induction of cell cycle arrest,
HNSCC cells were exposed to the two treatments at 50 µg/ml for 6 h
and cell-cycle distribution was evaluated by flow cytometric
analysis. Cisplatin and TPF treatments induced partial G0/G1 cell
cycle arrest in SCC-9 cells, however, only TPF treatment resulted
in a statistically significant effect (Fig. 2; P<0.05). For FaDu cells, no
difference was observed between treated and control cells, and the
distribution of cells in each phase of the cycle remained constant
following 6 h of treatment.
SCC-9 cells exhibited considerable changes in the
cell cycle when treated with one of the two regimens: Cisplatin and
TPF enhanced the percentage of cells in G0/G1 phase compared with
that of controls. A greater proportion of cells were found to be in
phase G0/G1 following TPF treatment (40%) compared with the control
group (22%), and this difference was statistically significant
(P<0.05). Additionally, a decrease in the percentage of cells in
S phase was observed, (15% of TPF-treated cells, compared with 33%
of control cells). The proportion of cells in phase G2/M remained
stable. The results of this experiment confirmed that the treatment
with TPF reduces the number of cells in the mitotic phase (Fig. 2).
Induction of apoptosis was also assessed using flow
cytometric analysis, which revealed that treatment with TPF
significantly increased cell death, as indicated by Annexin
staining (FL1 channel), while TPF and cisplatin markedly reduced
cell migration in SCC-9 cells (P<0.001). At 6 h following
treatment, the rate of apoptosis was observed to be 12.80% (TPF),
5.39% (cisplatin) and 4.48% (control) in SCC-9 cells (Fig. 3A).
In FaDu cells, cisplatin and TPF induced similar
rates of cell death. Annexin events occurred more frequently with
cisplatin, inducing a rate of apoptosis of 3.55% compared with
1.39% following TPF treatment (Fig.
3B). Staining with propidium iodide (FL2 channel) was observed
following TPF and cisplatin treatment in 7.89% and 3.63% of events,
respectively (Fig. 3B).
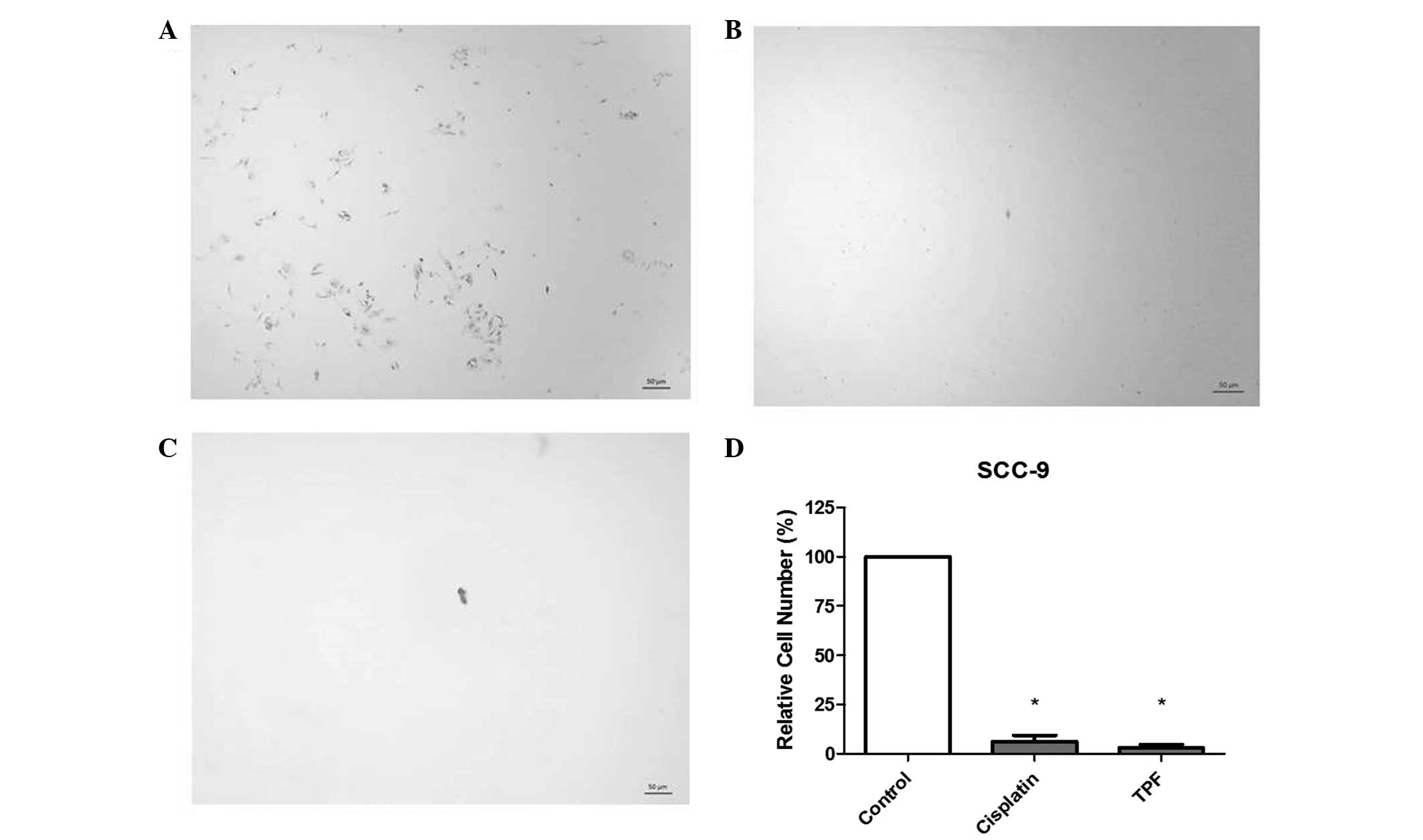
TPF reduces cell migration ability of
HNSCC cells
Data from the Transwell assay following TPF and
cisplatin treatment are presented in Fig.
4. Comparison between treated and control cells revealed that
TPF and cisplatin markedly decreased cell migration in SCC-9 cells.
A decrease in migration of 95.34 and 90.67% was observed following
TPF and cisplatin treatments, respectively.
Discussion
The role of concurrent chemo-radiation in the
treatment of HNSCC has previously been established and validated,
and cisplatin-based chemotherapy remains the current standard
treatment strategy (13). The
discovery of cisplatin as an anti-cancer drug in the 1960s marked a
novel era for cancer treatment. Cisplatin is able to induce
cytotoxicity, dependent upon on cell type and drug concentration;
this may occur via interference with transcription and/or DNA
replication mechanisms. Cisplatin may also act to induce apoptosis
of tumor cells, an effect which is mediated via the activation of
various signal transduction pathways, including calcium and death
receptor signaling, in addition to activation of mitochondrial
pathways. However, cytotoxicity and apoptosis are not induced
exclusively in cancer cells. Cisplatin also induces diverse
side-effects, for example neural and renal toxicity or bone
marrow-suppression. To minimize cisplatin resistance, combinatorial
therapies have been developed, which have been demonstrated to
exert greater efficacy in the treatment of cancer (14).
In September 2007, the FDA approved docetaxel for
use in combination with cisplatin and fluorouracil for the
induction treatment of patients with locally advanced HNSCC
(11). However, the cellular
mechanisms and the cytotoxic effect of this novel drug combination
have remained unclear. Thus, the objective of the present study was
to compare the effects of cisplatin monotherapy with a novel
combination regimen of paclitaxel, cisplatin and 5-fluorouracil in
head and neck cancer cells. In addition, the cellular mechanisms of
these drugs and their effect on the cell cycle and cell death were
analyzed.
The results revealed that FaDu cells, derived from
hypopharyngeal cancer, were more sensitive to all treatments than
SCC-9 cells, derived from tongue cancer. Although oral cancer and
hypopharyngeal cancer have identical epidermal origins, they behave
differently and, therefore exhibit differential reactions to the
treatments to which they are subjected. Clinical evidence indicates
that oral cancer is more aggressive and has a poorer response to
treatment compared with hypopharyngeal cancer (15). This evidence was confirmed by the
results of the present study.
TPF treatment produced greater cytotoxic effects in
FaDu and SCC-9 cells compared with that of cisplatin treatment,
however, this difference was not statistically significant. Based
on these findings, the two treatments provide viable treatment
options for consideration in HNSCC.
Cells treated with a single dose of radiation (2
Gy/min) exhibited no significant damage and cell viability was
unaffected; notably, proliferation was observed in keratinocytes.
Previous studies have identified similar responses when head and
neck cancer cells were subjected to a single dose of irradiation
(7,16). It has been reported that exposure of
cells to a single dose of irradiation may induce sublethal damage,
which is insufficient to induce apoptosis (16). In order to induce cell death, a
greater number of doses of irradiation, or a combination of
therapies is required. Chemotherapy regimens in combination with
radiation treatment enables enhanced cell cytotoxicity compared
with that of chemotherapy or radiation alone for head and neck
cancer cells, as demonstrated by the present cytotoxicity assays.
To the best of our knowledge, the results of the present study
demonstrate, for the first time in vitro, the supra-additive
effect of irradiation and TPF (in the respective ratio of 1:20:40)
for HNSCC. Although none of the treatments proposed were selective
for the cancer cell lines assessed, the combination of TPF 48 h
following irradiation, was significantly less cytotoxic to
keratinocyte cells (HaCaT) than to cancer cells (P<0.05), which
indicated that this therapy may result in fewer side effects for
patients undergoing cancer treatment. Thus the combined treatment
of TPF plus radiotherapy may present a more favorable option of
treatment for hypopharyngeal and tongue cancer, compared with the
48 h cisplatin with irradiation.
To further determine whether TPF and cisplatin
inhibited cell proliferation by induction of cell cycle arrest,
FaDu and SCC-9 cells were exposed to the two regimens for 6 h,
prior to the evaluation of cell cycle distribution by flow
cytometric analysis. TPF treatment resulted in partial G0/G1 cell
cycle arrest only in SCC-9 cells, and the number of cells in G0/G1
phase increased following each of the treatments, however, the
results were only statistically significant for TPF treatment
(Fig. 2). Cell cycle analysis
revealed that TPF induced G0/G1 cell cycle arrest in oral cancer
cells; further studies are necessary to identify which proteins
were modified by the treatment. Cyclin D1 is often amplified and
over-expressed in a variety of tumors, including HNSCC. Decreased
levels of cyclin D1 may be responsible for the G1 cell cycle arrest
and growth inhibition induced by TPF treatment. A recent phase III
trial evaluated standard treatment comprised of surgery and
postoperative radiotherapy, with and without prior induction TPF.
Subsequent immunohistochemical staining for cyclin D1 revealed that
the nodal stage cN2 patients (whose tumors were found to highly
express cyclin D1), had significantly greater overall survival and
distant metastasis-free survival when treated with TPF (17).
It has been established that apoptotic pathways are
deregulated in cancer (18),
therefore the induction of apoptotic and/or necrotic cell death in
HNSCC lines may represent a promising antineoplastic therapy. Using
flow cytometric analysis, the present study observed that TPF and
cisplatin induced apoptosis and necrosis in the two cell lines. It
was more significant, however, when SCC-9 cells were treated with
TPF. This indicated that TPF induces oral cancer cell death by
apoptosis. Bozec et al (19)
demonstrated that combined treatments of TPF/cetuximab or
TPF/cetuximab/bevacizumab significantly reduced tumor volume and
had a significant impact on the histological response in an
orthotopic head and neck cancer model. Ki67 is a nuclear protein
expressed in proliferating cells and is preferentially expressed
during late G1, S, M or G2 phases of the cell cycle, while cells in
the quiescent phase are negative for this protein. Thus, a
reduction in Ki67 labeling indicates a reduction in the number of
proliferating cells. Treatment with TPF and combinations decreased
Ki67 labeling and B cell lymphoma 2 (Bcl2) expression, indicating
that Bcl2 may be downregulated in oral cancer cells treated with
TPF.
An understanding of the process by which tumor cells
destroy the basement membrane of the surface epithelium, in
addition to invasion and metastasis, is required for the
development of novel treatments for HNSCC. The epithelial to
mesenchymal transition is a dynamic cellular process that is
fundamental to the development of metastatic disease (20,21).
Through the Transwell assay, it was demonstrated that the migratory
abilities of SCC-9 cells treated with 50 µg/ml of TPF or cisplatin
was decreased by 95.34 and 90.67%, respectively. To the best of our
knowledge, this is the first study to show that TPF inhibits
migration of oral squamous cell carcinoma (OSCC) cells in
vitro, suggesting that it is an important chemotherapic agent
for reducing the invasion and metastasis of OSCC.
In conclusion, these present findings highlight
certain cellular mechanisms induced by TPF in HNSCC cells,
including the inhibition of cell migration and the induction of
G0/G1 cell cycle arrest and apoptosis in oral cancer cell line.
Furthermore, TPF inhibits cell viability and enhances the effects
of ionizing radiation in head and neck cancer cell lines.
Acknowledgements
The authors would like to thank Dr André Ferreira
Leite (Dental Clinic, University Hospital of Brasília, Brasília,
Brazil) for his assistance with the statistical analysis.
References
|
1
|
Warnakulasuriya S: Global epidemiology of
oral and oropharyngeal cancer. Oral Oncol. 45:309–316. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Instituto Nacional de. Câncer, . Brazilian
cancer incidence. https://www.inca.gov.br/estimativa/2014Accessed.
February 15–2014
|
|
3
|
Warnakulasuriya S: Causes of oral cancer -
an appraisal of controversies. Br Dent J. 207:471–475. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Thavaraj S, Stokes A, Guerra E, Bible J,
Halligan E, Long A, Okpokam A, Sloan P, Odell E and Robinson M:
Evaluation of human papillomavirus testing for squamous cell
carcinoma of the tonsil in clinical practice. J Clin Pathol.
64:308–312. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shah JP and Gil Z: Current concepts in
management of oral cancer - surgery. Oral Oncol. 45:394–401. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Salama JK, Haddad RI, Kies MS, Busse PM,
Dong L, Brizel DM, Eisbruch A, Tishler RB, Trotti AM and Garden AS:
Clinical practice guidance for radiotherapy planning after
induction chemotherapy in locoregionally advanced head-and-neck
cancer. Int J Radiat Oncol Biol Phys. 75:725–733. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang N, Erjala K, Kulmala J, Qiu X,
Sundvall M, Elenius K and Grénman R: Concurrent cetuximab,
cisplatin, and radiation for squamous cell carcinoma of the head
and neck in vitro. Radiother Oncol. 92:388–392. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Posner MR, Hershock DM, Blajman CR, et al
TAX 324 Study Group: Cisplatin and fluorouracil alone or with
docetaxel in head and neck cancer. N Engl J Med. 357:1705–1715.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rapidis AD, Trichas M, Stavrinidis E,
Roupakia A, Ioannidou G, Kritselis G, Liossi P, Giannakouras G,
Douzinas EE and Katsilieris I: Induction chemotherapy followed by
concurrent chemoradiation in advanced squamous cell carcinoma of
the head and neck: Final results from a phase II study with
docetaxel, cisplatin and 5-fluorouracil with a four-year follow-up.
Oral Oncol. 42:675–684. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Vermorken JB, Remenar E, van Herpen C, et
al EORTC 24971/TAX 323 Study Group: Cisplatin, fluorouracil, and
docetaxel in unresectable head and neck cancer. N Engl J Med.
357:1695–1704. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
(NCI/NIH) NCI, . FDA approval for
docetaxel. simplewww.cancer.gov/cancertopics/druginfo/fda-docetaxelAccessed.
February 15–2014
|
|
12
|
Lim YC, Oh SY, Cha YY, Kim SH, Jin X and
Kim H: Cancer stem cell traits in squamospheres derived from
primary head and neck squamous cell carcinomas. Oral Oncol.
47:83–91. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Belcher R, Hayes K, Fedewa S and Chen AY:
Current treatment of head and neck squamous cell cancer. J Surg
Oncol. 110:551–574. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Florea AM and Büsselberg D: Cisplatin as
an anti-tumor drug: Cellular mechanisms of activity, drug
resistance and induced side effects. Cancers Basel. 3:1351–1371.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Leemans CR, Braakhuis BJ and Brakenhoff
RH: The molecular biology of head and neck cancer. Nat Rev Cancer.
11:9–22. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zheng XK, Chen LH, Wang WJ, Ye F, Liu JB,
Li QS and Sun HW: Impact of prolonged fraction delivery times
simulating IMRT on cultured nasopharyngeal carcinoma cell killing.
Int J Radiat Oncol Biol Phys. 78:1541–1547. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhong LP, Zhu DW, William WN Jr, et al:
Elevated cyclin D1 expression is predictive for a benefit from TPF
induction chemotherapy in oral squamous cell carcinoma patients
with advanced nodal disease. Mol Cancer Ther. 12:1112–1121. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hanahan D and Weinberg RA: The hallmarks
of cancer. Cell. 100:57–70. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bozec A, Sudaka A, Etienne-Grimaldi MC,
Brunstein MC, Fischel JL and Milano G: Antitumor activity of
cetuximab associated with the taxotere-cisplatin-fluorouracil (TPF)
combination on an orthotopic head and neck cancer model. Oral
Oncol. 47:940–945. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Scanlon CS, Van Tubergen EA, Inglehart RC
and D'Silva NJ: Biomarkers of epithelial-mesenchymal transition in
squamous cell carcinoma. J Dent Res. 92:114–121. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Smith A, Teknos TN and Pan Q: Epithelial
to mesenchymal transition in head and neck squamous cell carcinoma.
Oral Oncol. 49:287–292. 2013. View Article : Google Scholar : PubMed/NCBI
|