Introduction
Numerous previous studies have confirmed that
patients who suffer from inflammatory diseases of the colon,
including Crohn's disease and ulcerative colitis, have a markedly
greater risk of colorectal cancer (CRC) (1–3). This
therefore indicates that inflammation has a critical role in the
pathogenesis of numerous types of cancers. The pro-inflammatory
enzyme cyclooxygenase (COX), alternatively named prostaglandin
endoperoxide synthases, converts arachidonic acid into
prostaglandins, which have an important role in the inflammatory
response (3).
There are two main isozymes of COX: COX-1, which is
continuously expressed and is involved in maintaining homeostasis;
and COX-2, which is primarily involved in the production of
prostaglandins throughout the inflammatory response, the
overexpression of which enhances proliferation, attenuates
apoptosis and promotes the invasion of cancer cells, leading to
angiogenesis (4–7). Previous studies have provided evidence
for an association between COX-2 and certain types of cancer,
including CRC, gastric cancer and esophageal carcinoma (8–10).
Single-nucleotide polymorphisms (SNP) have been
detected in the COX-2 gene promoter region, including rs20417 G/C
and rs2745557 G/A (11). In
comparison to other SNPs, these polymorphisms have received much
attention in regards to their association with the risk of cancer.
It has been suggested that they may disrupt gene transcription
and/or mRNA stability as well as modulate the inflammatory
response, which may result in varied individual susceptibility to
cancer (12,13). In addition, numerous molecular
epidemiological studies have evaluated the association between
these COX-2 promoter polymorphisms and varied susceptibility to
types of cancer among different populations; however, the results
of these studies remain controversial (14,15).
One study reported that the COX-2 rs2745557 G/A SNP
promoted COX-2 transcription through producing a transcriptional
factor c-Myb-binding site, which in turn enhanced the risk of
esophageal squamous cell carcinoma in a Chinese population
(16). In addition, the COX-2 rs20417
G/C polymorphism was reported to result in an increased risk of
esophageal cancer (16). However, the
functional implications of these COX-2 SNPs remain controversial
(11,17).
It was proposed that COX-2 genetic polymorphisms
that alter protein expression and/or activity may affect the
inflammatory response and in turn influence the risk of CRC. The
present case-control study was performed using a population of Han
Chinese CRC patients and healthy volunteers in Shaanxi, China. The
study aimed to evaluate the associations of the COX-2 promoter
region variants, rs20417 G/C and rs2745557 G/A, with the
susceptibility for CRC. In addition, the present study aimed to
analyze the association between these genetic variants and
metastatic CRC.
Subjects and methods
Study subjects and clinical
characteristics
A case-control study was performed in order to
evaluate the risk factors for CRC. A total of 300 CRC patients and
300 healthy controls were enrolled in the present study between
October 2009 and October 2013. All subjects were of Han Chinese
ethnicity and from Shaanxi, China. In addition, all CRC patients
enrolled in the study were cases which had been newly diagnosed and
were confirmed as colorectal adenocarcinoma through
histopathological analysis; these patients were enrolled at the
First Affiliated Hospital of Xi'an Jiaotong University (Xi'an,
Shaanxi, China). Patients were excluded from the present study if
they had a history of previous cancer diagnosis, chemotherapy or
radiotherapy. At the time of diagnosis, the pathological stage of
CRC was classified according to the 1987 Union for International
Cancer Control Tumor-Node-Metastasis (TNM) grade classification
(TNM I–IV) (18). In addition, tumor
grades were classified according to the World Health Organization
grade classification (19): Well
differentiated, low; moderately differentiated, intermediate; and
poorly differentiated, high. The control group consisted of
healthy, cancer-free individuals who had attended a community
cancer screening program for the early detection of cancer based,
which was conducted in Shaanxi province during the time period when
the cases were collected and involved physical examinations,
including chest radiography, endoscopy and abdominal
ultrasonography. Controls had no individual history of cancer and
were frequency matched to patients based on gender and age. CRC
patients and controls were asked a series of questions in order to
determine demographic characteristics and potential risk factors
for CRC. Written informed consent was obtained from all
participants; this included their agreement to participate in the
study and to allow their biological samples to be genetically
analyzed. The study was approved by the Ethics Committee of Xi'an
Jiao Tong University.
DNA extraction
A total of 4 ml fasting peripheral blood was
collected from all subjects and added to blood collection tubes
with k2-EDTA anticoagulant, then stored at −80°C. The DNA from
whole blood was extracted using a standard proteinase K digestion
and the phenol/chloroform method, as previously described (16).
COX-2 rs20417 G/C and rs2745557 G/A
polymorphism genotype
Genomic DNA was isolated from the peripheral blood
lymphocytes of each participant using the Puregene DNA Isolation
Kit, according to the manufacturer's recommendation (Gentra
Systems, Inc., Big Lake, MN, USA). Genotypes of the COX-2 rs20417
G/C and rs2745557 G/A polymorphisms were examined using polymerase
chain reaction-based restriction fragment length polymorphism
(PCR-RFLP) as previously described (16). All chemicals used were of analytical
grade and were purchased from Sigma-Aldrich (St. Louis, MO, USA)
unless otherwise stated. All PCRs were conducted in total volume of
25 µl, comprising 19.8 µl H2O, 2.5 µl 10X reaction
buffer, 0.5 µl dNTP (10 mM), 0.2 µl Taq DNA polymerase and 0.5 µl
of each forward and reverse primers (20 µM). The primers were
obtained from Applied Biosystems Life Technologies (Foster City,
CA, USA) and the sequences were as follows: Forward,
5′-TTTAGCGTTTGTCCATCAGAAG-3′ and reverse,
5′-GGGGCGAGTAAGGTTAAGAAA-3′ for COX-2 rs20417 G/C; and forward,
5′-TCAGCCATACAGGTGAGTACC-3′ and reverse, 5′-CTGGGAGCAGGAAAGAACTG-3′
for COX-2 rs2745557 G/A. The PCR commenced with 10 min at 96̊C,
followed by 45 cycles of 30 sec at 96̊C, 30 sec at Tm
(60–63°C) and 30 sec at 72̊C, and finally 10 min at 72̊C. The
genotypes determined using PCR-RFLP were confirmed through direct
DNA sequencing of the PCR products from all samples using Big Dye
Terminator Cycle Sequencing kit (Applied Biosystems Life
Technologies) on an ABI 3500xl DNA Analyzer (Applied Biosystems
Life Technologies). Genotyping was performed blind to the samples
patient or control status; all samples were evaluated three times
by different investigators and the reproducibility was 100%.
Statistical analysis
Associations between COX-2 genotypes and
susceptibility to CRC were calculated using odds ratios (ORs) and
95% confidence intervals (CIs) through logistic regression. ORs
were adjusted for age and gender. Differences between genotype
distributions among subgroups were evaluated using χ2
tests. P<0.05 was considered to indicate a statistically
significant difference between values and all statistical tests
were two-sided. All statistical analyses were performed using SPSS
19.0 software (International Business Machines, Armonk, NY,
USA).
Results
Characteristics of the study
population
The mean age of CRC patients was 60±10.21 years and
the mean age of healthy control group was 58±12.35 years. No
significant differences were identified between age and gender
between the case and control groups. As shown in Fig. 1, the frequency distribution of CRC
patients with a history of smoking or drinking was significantly
increased compared with the healthy controls; in addition, a
significant difference was detected between those with a family
history of CRC in the CRC and healthy control groups
(P<0.05).
Genotype frequencies of COX-2 rs20417
G/C and rs2745557 G/A and their association with CRC risk
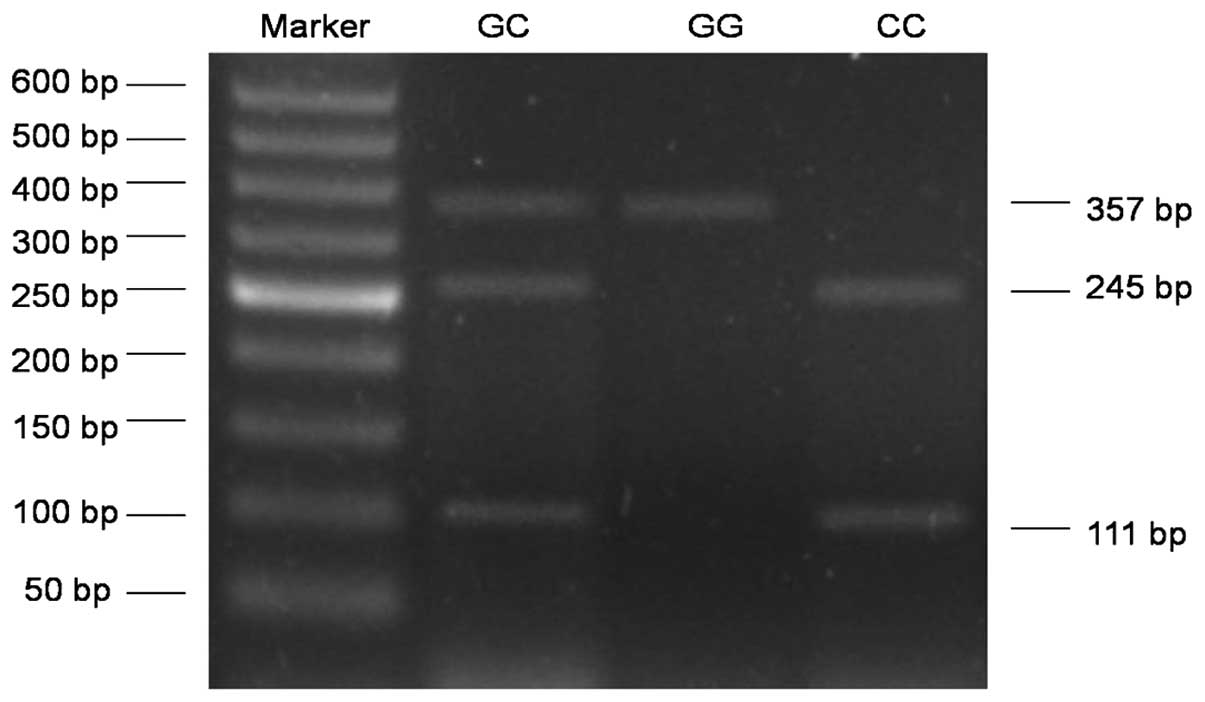
The genotype of COX-2 rs20417 G/C polymorphism
included GG, GC and CC. The fragments with 245 and 111 bp
demonstrated a GG wild-type homozygous genotype, a single 357 bp
fragment stood for the CC homozygous genotype and three fragments
of 357, 245 and 111 bp indicated the GC heterozygous genotype
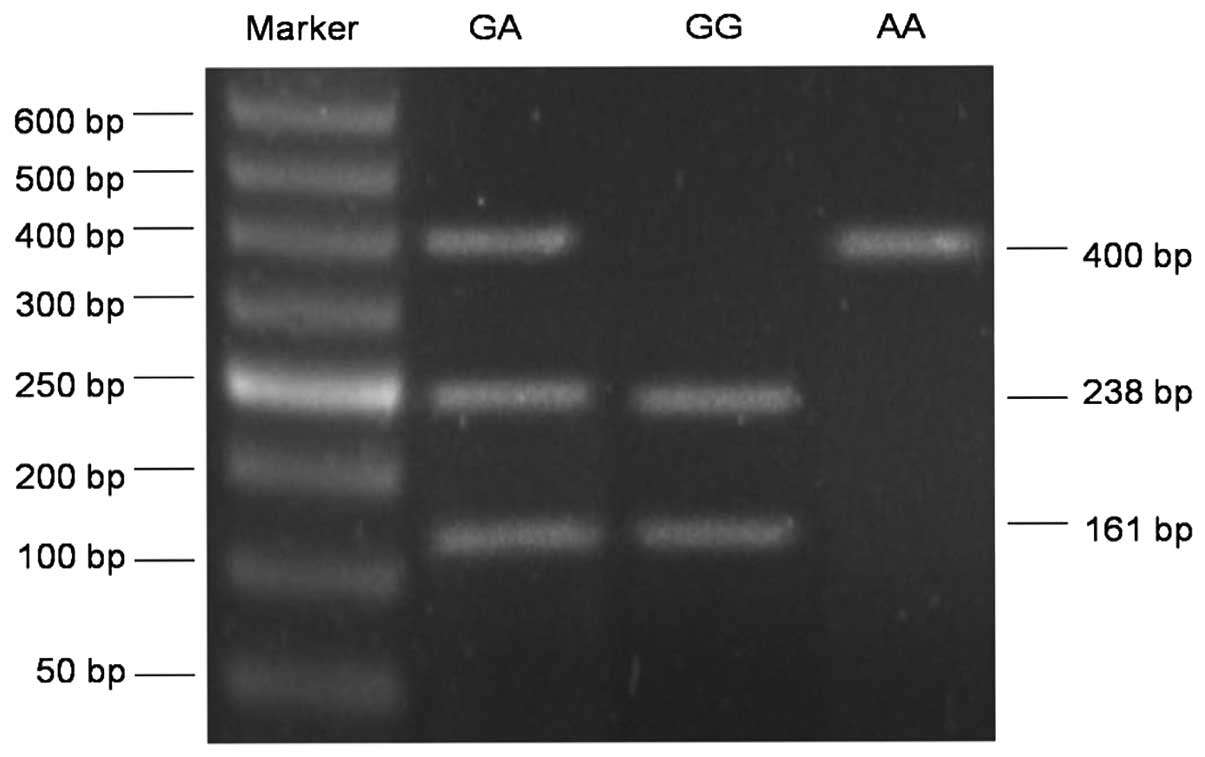
(Fig. 1). The COX-2 rs2745557 G/A
polymorphism also consisted of three genotypes: GG, GA and AA. As
shown in Fig. 2, the fragments with
238 and 161 bp represented the GG homozygous wild-type genotype,
the single fragment of 400 bp depicted the AA homozygous genotype
and the GA heterozygous genotype was indicated by three fragments
with 400, 238 and 161 bp.
As show in Table II,
GG, GC and CC allele frequencies of the COX-2 rs20417 G/C
polymorphism were 29, 34.7 and 36.3%, respectively, in the healthy
control group and 20, 39 and 41%, respectively, in the CRC group.
This therefore indicated that the observed genotype frequencies of
SNPs in the two groups were in accordance with the Hardy-Weinberg
equilibrium. The distribution of these genotypes and their
association with CRC risk was then analyzed between the CRC and
healthy control groups. For the rs20417 G/C polymorphism, the GC+CC
allele frequency was 80% in CRC patients and 71% in healthy
controls (OR=1.63; 95% CI, 1.12–2.38; P=0.01).
 | Table II.Frequency distribution of
cyclooxygenase-2 rs20417G/C in the CRC and control groups. |
Table II.
Frequency distribution of
cyclooxygenase-2 rs20417G/C in the CRC and control groups.
| rs20417 | Control (%) | CRC (%) | χ2 | P-value | OR (95% CI) |
|---|
| GG | 87 (29) | 60
(20) |
|
|
|
| GC | 104 (34.7) | 117 (39) | 5.20 | 0.02 | 1.63 (1.07–2.49) |
| CC | 109 (36.3) | 123 (41) | 5.36 | 0.02 | 1.64 (1.08–2.49) |
| GC+CC | 213 (71) | 240 (80) | 6.57 | 0.01 | 1.63 (1.12–2.38) |
As show in Table
III, the allele frequencies of GG, GA and AA of the COX-2
rs2745557 G/A polymorphism were 27, 35 and 38%, respectively, in
healthy controls and 16, 41 and 43%, respectively, in patients with
CRC. Therefore, the observed genotype frequencies of SNPs in the
two groups were in accordance with the Hardy-Weinberg equilibrium.
In addition, there was a significant difference in genotype
distribution of the COX-2 rs2745557 G/A polymorphism between the
two groups (P<0.01). The GA+AA allele frequency was 84% in CRC
patients and 73% in healthy controls (OR=1.94; 95% CI, 1.30–2.90;
P<0.01). Based on these results, it was determined that COX-2
rs20417 C allele (GC+CC) carriers and rs2745557 A allele (GA+AA)
carriers have an increased risk of CRC compared with GG genotype
carriers.
 | Table III.Frequency distribution of
cyclooxygenase-2 rs2745557 G/A in the CRC and control groups. |
Table III.
Frequency distribution of
cyclooxygenase-2 rs2745557 G/A in the CRC and control groups.
| rs2745557 | Control (%) | CRC (%) | χ2 | P-value | OR (95% CI) |
|---|
| GG | 81
(27) | 48 (16) |
|
|
|
| GA | 105 (35) | 123 (41) | 9.25 | <0.01 | 1.98 (1.27–3.08) |
| AA | 114 (38) | 129 (43) | 8.52 | <0.01 | 1.91 (1.23–2.96) |
| GA+AA | 219 (73) | 252 (84) | 10.75 | <0.01 | 1.94
(1.30–2.90) |
Association of COX-2 rs20417 G/C and
rs2745557 G/A polymorphism with age, gender, drinking history,
smoking history and family history of CRC
The potential interactions between COX-2 rs20417 G/C
and rs2745557 G/A polymorphisms and characteristics including age,
gender, smoking history, drinking history and family history of CRC
were analyzed using SPSS software. As shown in Tables IV and V, there was no statistically difference in
age and gender between the CRC and healthy control groups. It was
demonstrated that among subjects who had a history of smoking,
those who were COX-2 rs20417 (GC+CC) carriers had a significantly
greater risk of developing CRC in comparison with GG genotype
carriers in the healthy controls (OR=2.30; 95% CI 1.33–4.03;
P<0.05). In addition, COX-2 rs2745557 (GA+AA) carriers with a
smoking history had an increased risk of CRC relative to that of GG
genotype carriers in the healthy controls (OR=2.31; 95% CI,
1.09–4.86; P<0.05). For subjects with a history of drinking,
COX-2 rs20417 (GC+CC) carriers exhibited a markedly increased risk
of CRC compared with healthy control GG genotype carriers (OR=3.01;
95% CI 1.71–5.29; P<0.05); while COX-2 rs2745557 (GA+AA)
carriers also demonstrated a higher risk for CRC in comparison with
GG genotype carriers in the healthy control group (OR=2.75; 95% CI,
1.59–4.76; P<0.05). Furthermore, in subjects with a family
history of CRC, COX-2 rs20417 (GC+CC) carriers had an increased
risk of CRC compared with GG genotype carriers in healthy controls
(OR=2.78; 95% CI, 1.10–7.06; P<0.05); while COX-2 rs2745557
(GA+AA) carriers also exhibited an elevated risk for CRC compared
with GG genotype carriers in healthy controls (OR=3.28; 95% CI,
1.24–8.70; P<0.05).
 | Table IV.Frequency distribution of
cyclooxygenase-2 rs20417 G/C with age, gender, smoking history,
drinking history and family history of CRC in the CRC and control
groups. |
Table IV.
Frequency distribution of
cyclooxygenase-2 rs20417 G/C with age, gender, smoking history,
drinking history and family history of CRC in the CRC and control
groups.
|
| Control | CRC |
|
|---|
|
|
|
|
|
|---|
| Classified
variable | GG (%) | GC+CC (%) | GG (%) | GC+CC (%) | OR (95% CI) |
|---|
| Age, years |
|
|
|
|
|
|
<65 | 134 (44.7) | 36 (12.0) | 117 (39.0) | 45 (15.0) | 1.43
(0.87–2.37) |
|
≥65 | 106 (35.3) | 24 (8.0) | 101 (33.7) | 37 (12.3) | 1.70
(0.96–3.04) |
| Gender |
|
|
|
|
|
|
Male | 168 (56.0) | 34 (11.3) | 165 (55.0) | 44 (14.7) | 1.32
(0.80–2.17) |
|
Female | 70
(23.3) | 28 (9.4) | 59 (19.7) | 32 (10.6) | 1.36
(0.73–2.51) |
| Smoking
history |
|
|
|
|
|
| No | 136 (45.3) | 47 (15.7) | 110 (36.7) | 45 (15.0) | 1.18
(0.73–1.91) |
|
Yes | 92
(30.7) | 25 (8.3) | 89 (29.7) | 56 (18.6) | 2.30
(1.33–4.03)a |
| Drinking
history |
|
|
|
|
|
| No | 95
(31.7) | 22 (7.3) | 69 (23.0) | 18 (6.0) | 1.13
(0.56–2.26) |
|
Yes | 164 (54.7) | 19 (6.3) | 158 (52.7) | 55 (18.3) | 3.01
(1.71–5.29)b |
| Family history |
|
|
|
|
|
| No | 246 (82.0) | 25 (8.3) | 214 (71.3) | 32 (10.7) | 1.47
(0.85–2.56) |
|
Yes | 18 (6.0) | 11 (3.7) | 20 (6.7) | 34 (11.3) | 2.78
(1.10–7.06)c |
 | Table V.Frequency distribution of
cyclooxygenase-2 rs2745557 G/A with age, gender, smoking history,
drinking history and family history of CRC in the CRC and control
groups. |
Table V.
Frequency distribution of
cyclooxygenase-2 rs2745557 G/A with age, gender, smoking history,
drinking history and family history of CRC in the CRC and control
groups.
|
| Control | CRC |
|---|
|
|
|
|
|
|---|
| Classified
variable | GG (%) | GC+CC (%) | GG (%) | GC+CC (%) | OR (95% CI) |
|---|
| Age |
|
|
|
|
|
| <65
years | 139 (46.3) | 31 (10.4) | 126 (42.0) | 36 (12.0) | 1.28
(0.75–2.19) |
| ≥65
years | 108 (36.0) | 22 (7.3) | 105 (35.0) | 33 (11.0) | 1.54
(0.85–2.82) |
| Gender |
|
|
|
|
|
|
Male | 169 (56.3) | 33 (11.0) | 168 (56.0) | 41 (13.7) | 1.25
(0.75–2.08) |
|
Female | 81 (27.0) | 17 (5.7) | 66 (22.0) | 25 (8.3) | 1.81
(0.90–3.62) |
| Smoking
history |
|
|
|
|
|
| No | 146 (48.7) | 37 (12.3) | 115 (38.3) | 40 (13.3) | 1.37
(0.83–2.28) |
|
Yes | 106 (35.3) | 11 (3.7) | 117 (39.0) | 28 (9.4) | 2.31
(1.09–4.86)a |
| Drinking
history |
|
|
|
|
|
| No | 105 (35.0) | 12 (4.0) | 72 (24.0) | 15 (5.0) | 1.82
(0.80–4.12) |
|
Yes | 162 (54.0) | 21 (7.0) | 157 (52.3) | 56 (18.7) | 2.75
(1.59–4.76)b |
| Family history |
|
|
|
|
|
| No | 236 (78.7) | 35 (11.6) | 204 (68.0) | 42 (14.0) | 1.39
(0.85–2.26) |
|
Yes | 21 (7.0) | 8 (2.7) | 24 (8.0) | 30 (10.0) | 3.28
(1.24–8.70)c |
Association of COX-2 rs20417 G/C and
rs2745557 G/A polymorphism with CRC disease status
The associations between COX-2 genotypes and tumor
stage or grade, as determined at the time of diagnosis, were
further evaluated. Significant associations were not observed
between COX-2 genotypes and disease location of CRC; however, the
results revealed that TNM stage of CRC at diagnosis was associated
with the COX-2 rs20417 G/C and rs2745557 G/A polymorphisms. As
shown in Tables VI and VII, the allelic frequencies of COX-2
rs20417 G/C (GG, 18.8%; GC, 43.7%; CC, 37.5%) and rs2745557 G/A
(GG, 9.7%; GA, 22.9%; AA, 67.4%) in stage III/IV CRC were
significantly different from COX-2 rs20417 G/C (GG, 28.2%; GC,
45.5%; CC, 26.3%) and rs2745557 G/A (GG, 35.3%; GA, 25.6%; AA,
39.1%) in stage I/II CRC (P<0.05). In addition, the COX-2
rs20417 G/C and rs2745557 G/A genotype was demonstrated to be
significantly associated with lymph node metastasis. The allelic
frequencies of the COX-2 rs20417 G/C (GG, 9.8%; GC, 31.1%; CC,
59.1%) and rs2745557 G/A (GG, 15.9%; GA, 22.7%; AA, 61.4%) in
patients with lymph node metastasis were significantly different
from COX-2 rs20417 G/C (GG, 28.0%; GC, 34.5%; CC, 37.5%) and
rs2745557 G/A (GG, 34.5%; GA, 20.8%; AA, 44.7%) in patients without
lymph node metastasis. These results indicated that patients with
CRC, who express a COX-2 rs20417 C allele (GC or CC genotype)
and/or a COX-2 rs2745557 A allele (GA or AA genotype) had a
markedly increased risk of developing higher stages of CRC,
including high-grade CRC and lymph node metastasis.
 | Table VI.Associations between COX-2 rs20417
G/C single-nucleotide polymorphism and biological behaviors in
colorectal cancer. |
Table VI.
Associations between COX-2 rs20417
G/C single-nucleotide polymorphism and biological behaviors in
colorectal cancer.
|
|
| COX-2
rs20417G/C |
|---|
|
|
|
|
|---|
| Pathological
features | n | GG (%) | GC (%) | CC (%) | GC+CC (%) |
|---|
| Location |
|
|
|
|
|
|
Colon | 144 | 27 (18.8) | 63 (43.7) | 54 (37.5) | 117 (81.2) |
|
Rectum | 156 | 33 (21.2) | 54 (34.6) | 69 (44.2) | 123 (78.8) |
| OR (95%
CI) |
| 0.70
(0.38–1.31) | 1.05
(0.56–1.95) | 0.86
(0.49–1.31) |
| Lymphatic
metastasis |
|
|
|
|
|
| No | 168 | 47 (28.0) | 58 (34.5) | 63 (37.5) | 121 (72.0) |
|
Yes | 132 | 13 (9.8) | 41 (31.1) | 78 (59.1) | 119 (90.2) |
| OR (95%
CI) |
| 2.56
(1.23–5.32)a | 4.48
(2.23–9.00)a | 3.56
(1.83–6.91)a |
| TNM stage |
|
|
|
|
|
|
I/II | 156 | 54 (28.2) | 61 (45.5) | 41 (26.3) | 102 (71.8) |
|
III/IV | 144 | 27 (18.8) | 63 (43.7) | 54 (37.5) | 117 (81.2) |
| OR (95%
CI) |
| 2.07
(1.16–3.69)b | 2.63
(1.42–4.87)b | 2.30
(1.35–3.91)b |
|
 | Table VII.Associations between COX-2 rs2745557
G/A single-nucleotide polymorphism and biological behaviors in
colorectal cancer. |
Table VII.
Associations between COX-2 rs2745557
G/A single-nucleotide polymorphism and biological behaviors in
colorectal cancer.
|
|
| COX-2
rs20417G/C |
|---|
|
|
|
|
|---|
| Pathological
features | n | GG (%) | GC (%) | CC (%) | GC+CC (%) |
|---|
| Part |
|
|
|
|
|
|
Colon | 144 | 26 (18.1) | 60 (41.7) | 58 (40.2) | 118 (81.9) |
|
Rectum | 156 | 23 (14.7) | 63 (40.4) | 70 (44.9) | 133 (85.3) |
| OR (95%
CI) |
|
| 1.19
(0.61–2.30) | 1.36
(0.71–2.64) | 1.27
(0.69–2.35) |
| Lymphatic
metastasis |
|
|
|
|
|
| No | 168 | 58 (34.5) | 35 (20.8) | 75 (44.7) | 110 (65.5) |
|
Yes | 132 | 21 (15.9) | 30 (22.7) | 81 (61.4) | 111 (84.1) |
| OR (95%
CI) |
| 2.36
(1.18–4.76)a | 2.98
(1.65–5.38)a | 2.79
(1.59–4.90)a |
|
| TNM stage |
|
|
|
|
|
|
I/II | 156 | 55 (35.3) | 40 (25.6) | 61 (39.1) | 101 (64.7) |
|
III/IV | 144 | 14 (9.7) | 33 (22.9) | 97 (67.4) | 130 (90.3) |
| OR (95%
CI) |
| 3.24
(1.54–6.84)b | 6.25
(1.87–4.91)b | 5.06
(2.66–9.61)b |
|
Discussion
The present study aimed to investigate the
correlation between polymorphisms in the COX-2 promoter region
(rs20417 G/C and rs2745557 G/A) and the risk of CRC. Genotyping of
300 CRC patients and 300 healthy controls was performed in a Han
Chinese population in Shaanxi, China. The results revealed that
COX-2 rs20417 G/C and rs2745557 G/A polymorphisms were associated
with a greater risk of developing CRC. In addition, these results
demonstrated that the COX-2 rs20417 G/C and rs2745557 G/A
polymorphisms were associated with metastatic CRC and that patients
with these variant alleles had a greater susceptibility to the
development of advanced CRC. Based on these results, it was
suggested that the functional variants of COX-2 may result in
susceptibility to cancer and therefore have an important role in
colorectal carcinogenesis.
Case-control studies have been performed in several
countries and different ethnic populations (20–23). In
line with the results of the present study, a previous study in a
Singapore Chinese population indicated that the COX-2 rs20417 C
allele was associated with an elevated susceptibility for colon
cancer (20). In addition, a previous
study in a Spanish population revealed that a COX-2 polymorphism in
the untranslated region of exon 10 was associated with a greater
risk of CRC (24). By contrast, a
certain study reported that the COX-2 rs20417 C allele was
associated with a reduced risk of colorectal adenoma in non-users
of NSAIDs in a Caucasian American population (21). Furthermore, COX-2 polymorphism of an
amino acid substitution was correlated with a decreased risk of
colorectal neoplasia in an African-American population (22,23).
Therefore, there is difference between COX-2 polymorphisms and CRC
risk among various ethnic populations; however, the biological
function of the COX-2 polymorphism remains elusive (24,25).
Although the invasion and metastasis of CRC are a
prominent cause of cancer-associated mortality worldwide, the
initial pathogenesis of CRC remains to be fully elucidated. Several
studies have demonstrated that COX-2 overexpression was associated
with the invasive capacity of colon cancer cells and advanced
stages of CRC (26,27). In addition, COX-2 and its downstream
prostaglandins are strongly correlated with cell proliferation,
angiogenesis and immunosuppression, all of which may be involved in
the development of advanced stages of CRC (28–30). In
the present study, the COX-2 rs20417 C allele and rs2745557 A
allele were revealed to be associated with an enhanced
susceptibility to high-grade CRC. Based on these findings, it was
speculated that COX-2 may be involved in the tumorigenesis and
progression of CRC, as the COX-2 rs20417 C allele and rs2745557 A
allele were found to be significantly correlated with the risk of
CRC progression.
In conclusion, the results of the present study
demonstrated that the COX-2 rs20417 G/C and rs2745557 G/A
polymorphisms were factors which were associated with genetic
susceptibility for developing CRC in a Han Chinese population. In
addition, the present study revealed that the COX-2 rs20417 G/C and
rs2745557 G/A alleles were associated with an enhanced
susceptibility for the advanced stages of CRC. Furthermore, these
findings were in concurrence with the biological functions
associated with these polymorphisms and confirmed the hypothesis
that genetic polymorphisms in the COX-2 promotor region, which
result in altered protein expression and/or activity, may mediate
the inflammatory response, resulting in altered susceptibility to
CRC.
Acknowledgements
The present study was funded by programs from the
National Natural Science Foundation of China (General Program no.
30771895).
Glossary
Abbreviations
Abbreviations:
|
CRC
|
colorectal cancer
|
|
PCR-RFLP
|
polymerase chain reaction and
restriction fragment length polymorphism
|
|
COX
|
cyclooxygenase
|
|
SNP
|
Single-nucleotide polymorphism
|
|
OR
|
odds ratio
|
|
CI
|
confidence interval
|
References
|
1
|
Lashner BA, Silverstein MD and Hanauer SB:
Hazard rates for dysplasia and cancer in ulcerative colitis.
Results from a surveillance program. Dig Dis Sci. 34:1536–1541.
1989.
|
|
2
|
Lennard-Jones JE, Morson BC, Ritchie JK,
et al: Cancer in colitis: Assessment of the individual risk by
clinical and histological criteria. Gastroenterology. 73:1280–1289.
1977.PubMed/NCBI
|
|
3
|
Rhodes JM and Campbell BJ: Inflammation
and colorectal cancer: IBD associated and sporadic cancer compared.
Trends Mol Med. 8:10–16. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Romano M and Claria J: Cyclooxygenase-2
and 5-lipoxygenase converging functions on cell proliferation and
tumor angiogenesis: Implications for cancer therapy. FASEB J.
17:1986–1995. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Simmons DL, Botting RM and Hla T:
Cyclooxygenase isozymes: The biology of prostaglandin synthesis and
inhibition. Pharmacol Rev. 56:387–437. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tsujii M, Kawano S, Tsuji S, et al:
Cyclooxygenase regulates angiogenesis induced by colon cancer
cells. Cell. 93:705–716. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ranger GS, Thomas V, Jewell A and Mokbel
K: Elevated cyclooxygenase-2 expression correlates with distant
metastases in breast cancer. Anticancer Res. 24:2349–2351.
2004.PubMed/NCBI
|
|
8
|
Eberhart CE, Coffey RJ, Radhika A, et al:
Up-regulation of cyclooxygenase 2 gene expression in human
colorectal adenomas and adenocarcinomas. Gastroenterology.
107:1183–1188. 1994.PubMed/NCBI
|
|
9
|
Ristimäki A, Honkanen N, Jänkälä H, et al:
Expression of cyclooxygenase-2 in human gastric carcinoma. Cancer
Res. 57:1276–1280. 1997.PubMed/NCBI
|
|
10
|
Liu X, Li P, Zhang ST, et al: COX-2 mRNA
expression in esophageal squamous cell carcinoma (ESCC) and effect
by NSAID. Dis Esophagus. 21:9–14. 2008.PubMed/NCBI
|
|
11
|
Brosens LA, Donahue CA, Keller JJ, et al:
Increased cyclooxygenase-2 expression in duodenal compared with
colonic tissues in familial adenomatous polyposis and relationship
to the −765 G/C COX-2 polymorphism. Clin Cancer Res. 11:4090–4096.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fritsche E, Baek SJ, King LM, Zeldin DC,
Eling TE and Bell DA: Functional characterization of
cyclooxygenase-2 polymorphisms. J Pharmacol Exp Ther. 299:468–476.
2001.PubMed/NCBI
|
|
13
|
Papafili A, Hill MR, Brull DJ, et al:
Common promoter variant in cyclooxygenase-2 represses gene
expression: Evidence of role in acute-phase inflammatory response.
Arterioscler Thromb Vasc Biol. 22:1631–1636. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hamajima N, Takezaki T, Matsuo K, et al:
Genotype frequencies of cyclooxygenease 2 (COX2) rare polymorphisms
for Japanese with and without colorectal cancer. Asian Pac. Asian
Pac J Cancer Prev. 2:57–62. 2001.
|
|
15
|
Lee TS, Jeon YT, Kim JW, et al: Lack of
association of the cyclooxygenase-2 and inducible nitric oxide
synthase gene polymorphism with risk of cervical cancer in Korean
population. Ann N Y Acad Sci. 1095:134–142. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang X, Miao X, Tan W, et al:
Identification of functional genetic variants in cyclooxygenase-2
and their association with risk of esophageal cancer.
Gastroenterology. 129:565–576. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Szczeklik W, Sanak M, Szczeklik A, et al:
Functional effects and gender association of COX-2 gene
polymorphism G-765C in bronchial asthma. J Allergy Clin Immunol.
114:248–253. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hermanek P, Scheibe O, Spiessl B and
Wagner G: TNM classification of malignant tumors: The new 1987
edition. Rontgenblatter. 40:2001987.(In German). PubMed/NCBI
|
|
19
|
Hamilton SR, Bosman FT, Boffetta P, et al:
Tumours of the colon and rectumWHO Classification of Tumours of the
Digestive System. Bosman FT, Carneiro F, Hruban RH and Theise ND:
IARC Press; Lyon: pp. 131–181. 2010
|
|
20
|
Koh WP, Yuan JM, Van Den Berg D, et al:
Interaction between cyclooxygenase-2 gene polymorphism and dietary
n-6 polyunsaturated fatty acids on colon cancer risk: The Singapore
Chinese Health Study. Br J Cancer. 90:1760–1764. 2004.PubMed/NCBI
|
|
21
|
Ulrich CM, Whitton J, Yu JH, et al: PTGS2
(COX-2) −765G>C promoter variant reduces risk of colorectal
adenoma among nonusers of nonsteroidal anti-inflammatory drugs.
Cancer Epidemiol Biomarkers Prev. 14:616–619. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lin HJ, Keku TO, Reddy ST, et al:
Prostaglandin H synthase 2 variant (Val511Ala) in African Americans
may reduce the risk for colorectal neoplasia. Cancer Epidemiol
Biomarkers Prev. 11:1305–1315. 2002.PubMed/NCBI
|
|
23
|
Sansbury LB, Millikan RC, Schroeder JC, et
al: COX-2 polymorphism, use of nonsteroidal anti-inflammatory drugs
and risk of colon cancer in African Americans (United States).
Cancer Causes Control. 17:257–266. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Goodman JE, Bowman ED, Chanock SJ, et al:
Arachidonate lipoxygenase (ALOX) and cyclooxygenase (COX)
polymorphisms and colon cancer risk. Carcinogenesis. 25:2467–2472.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cox DG, Pontes C, Guino E, Navarro M,
Osorio A, Canzian F and Moreno V: Bellvitge Colorectal Cancer Study
Group: Polymorphisms in prostaglandin synthase 2/cyclooxygenase 2
(PTGS2/COX2) and risk of colorectal cancer. Br J Cancer.
91:339–343. 2004.PubMed/NCBI
|
|
26
|
Tomozawa S, Tsuno NH, Sunami E, et al:
Cyclooxygenase-2 overexpression correlates with tumour recurrence,
especially haematogenous metastasis, of colorectal cancer. Br J
Cancer. 83:324–328. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang H and Sun XF: Overexpression of
cyclooxygenase-2 correlates with advanced stages of colorectal
cancer. Am J Gastroenterol. 97:1037–1041. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Konno H, Baba M, Shoji T, Ohta M, Suzuki S
and Nakamura S: Cyclooxygenase-2 expression correlates with uPAR
levels and is responsible for poor prognosis of colorectal cancer.
Clin Exp Metastasis. 19:527–534. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yao M, Lam EC, Kelly CR, Zhou W and Wolfe
MM: Cyclooxygenase-2 selective inhibition with NS-398 suppresses
proliferation and invasiveness and delays liver metastasis in
colorectal cancer. Br J Cancer. 90:712–719. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tsujii M, Kawano S and DuBois RN:
Cyclooxygenase-2 expression in human colon cancer cells increase
metastatic potential. Proc Natl Acad Sci USA. 94:3336–3340. 1997.
View Article : Google Scholar : PubMed/NCBI
|