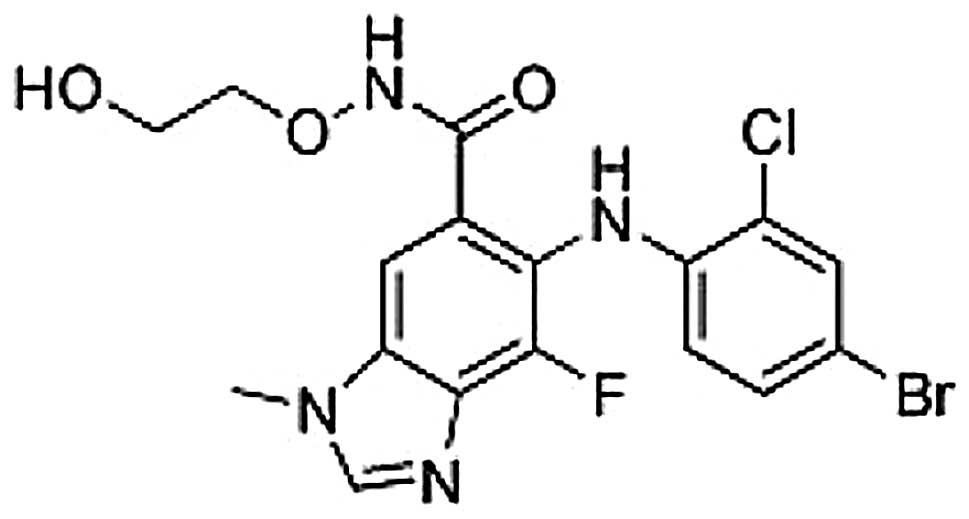
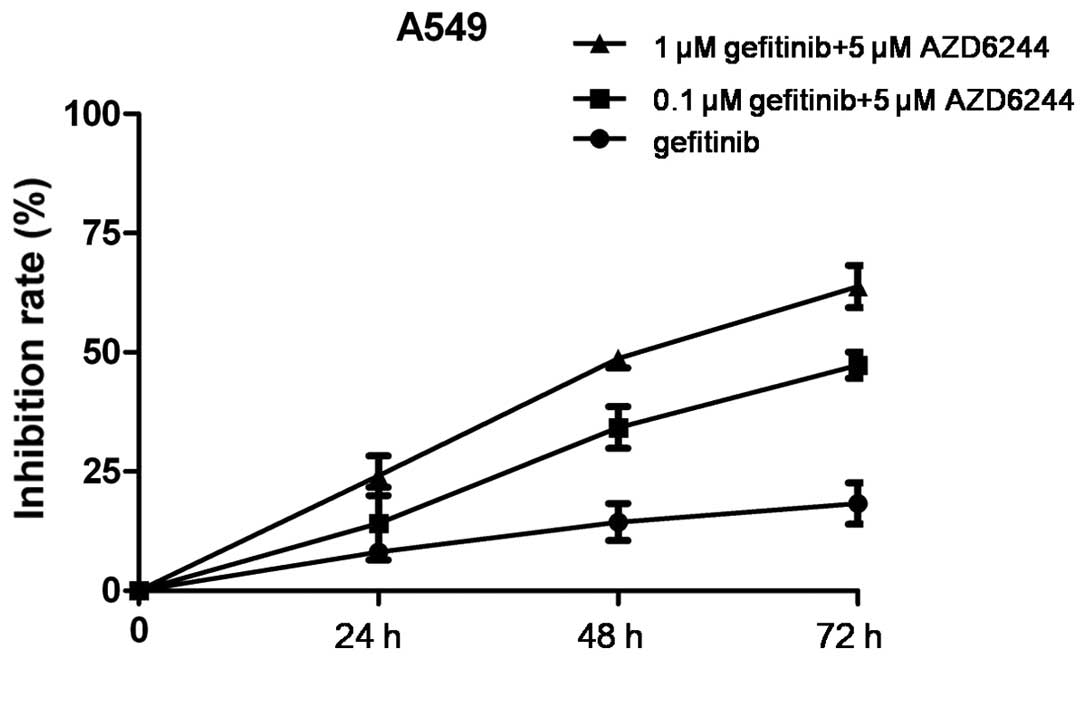
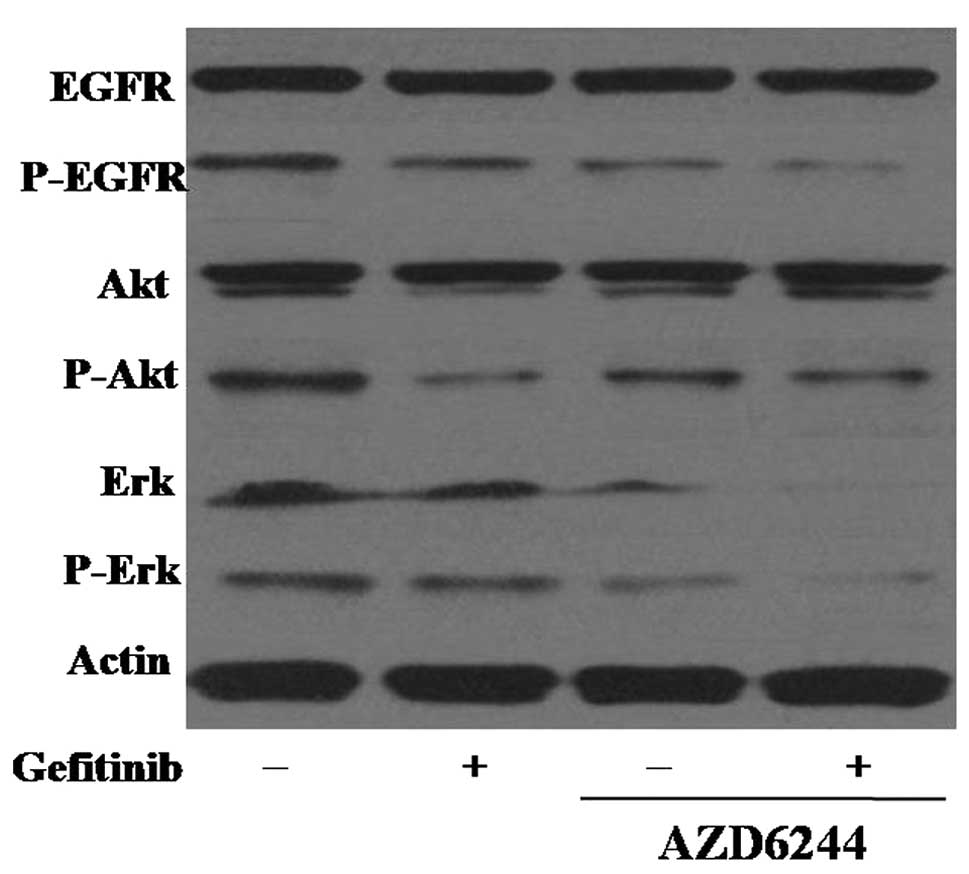
|
1
|
Mok TS, Wu YL, Thongprasert S, Yang CH,
Chu DT, Saijo N, Sunpaweravong P, Han B, Margono B, Ichinose Y, et
al: Gefitinib or carboplatin-paclitaxel in pulmonary
adenocarcinoma. N Engl J Med. 361:947–957. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Scagliotti GV, Parikh P, von Pawel J,
Biesma B, Vansteenkiste J, Manegold C, Serwatowski P, Gatzemeier U,
Digumarti R, Zukin M, et al: Phase III study comparing cisplatin
plus gemcitabine with cisplatin plus pemetrexed in
chemotherapy-naive patients with advanced-stage non-small-cell lung
cancer. J Clin Oncol. 26:3543–3551. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sandler A, Gray R, Perry MC, Brahmer J,
Schiller JH, Dowlati A, Lilenbaum R and Johnson DH:
Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell
lung cancer. N Engl J Med. 355:2542–2550. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhou C, Wu YL, Chen G, Feng J, Liu XQ,
Wang C, Zhang S, Wang J, Zhou S, Ren S, et al: Erlotinib versus
chemotherapy as first-line treatment for patients with advanced
EGFR mutation-positive non-small-cell lung cancer (OPTIMAL,
CTONG-0802): A multicentre, open-label, randomised, phase 3 study.
Lancet Oncol. 12:735–742. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ono M and Kuwano M: Molecular mechanisms
of epidermal growth factor receptor (EGFR) activation and response
to gefitinib and other EGFR-targeting drugs. Clin Cancer Res.
12:7242–7251. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Costa DB, Kobayashi S, Tenen DG and
Huberman MS: Pooled analysis of the prospective trials of gefitinib
monotherapy for EGFR-mutant non-small cell lung cancers. Lung
Cancer. 58:95–103. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pao W, Miller VA, Politi KA, Riely GJ,
Somwar R, Zakowski MF, Kris MG and Varmus H: Acquired resistance of
lung adenocarcinomas to gefitinib or erlotinib is associated with a
second mutation in the EGFR kinase domain. PLoS Med. 2:e732005.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sos ML, Rode HB, Heynck S, Peifer M,
Fischer F, Klüter S, Pawar VG, Reuter C, Heuckmann JM, Weiss J, et
al: Chemogenomic profiling provides insights into the limited
activity of irreversible EGFR Inhibitors in tumor cells expressing
the T790M EGFR resistance mutation. Cancer Res. 70:868–874. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Engelman JA, Zejnullahu K, Mitsudomi T,
Song Y, Hyland C, Park JO, Lindeman N, Gale CM, Zhao X, Christensen
J, et al: MET amplification leads to gefitinib resistance in lung
cancer by activating ERBB3 signaling. Science. 316:1039–1043. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang H, Xu Y, Filipovic A, Lit LC, Koo
CY, Stebbing J and Giamas G: SILAC-based phosphoproteomics reveals
an inhibitory role of KSR1 in p53 transcriptional activity via
modulation of DBC1. Br J Cancer. 109:2675–2684. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li J, Pan YY and Zhang Y: Synergistic
interaction between sorafenib and gemcitabine in EGFR-TKI-sensitive
and EGFR-TKI-resistant human lung cancer cell lines. Oncol Lett.
5:440–446. 2013.PubMed/NCBI
|
|
12
|
Kim KB, Kefford R, Pavlick AC, Infante JR,
Ribas A, Sosman JA, Fecher LA, Millward M, McArthur GA, Hwu P, et
al: Phase II study of the MEK1/MEK2 inhibitor Trametinib in
patients with metastatic BRAF-mutant cutaneous melanoma previously
treated with or without a BRAF inhibitor. J Clin Oncol. 31:482–489.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Metro G, Chiari R, Baldi A, De Angelis V,
Minotti V and Crinò L: Selumetinib: A promising pharmacologic
approach for KRAS-mutant advanced non-small-cell lung cancer.
Future Oncol. 9:167–177. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Meloche S and Pouysségur J: The ERK1/2
mitogen-activated protein kinase pathway as a master regulator of
the G1- to S-phase transition. Oncogene. 26:3227–3239. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Balmanno K and Cook SJ: Tumour cell
survival signalling by the ERK1/2 pathway. Cell Death Differ.
16:368–377. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bodoky G, Timcheva C, Spigel DR, La Stella
PJ, Ciuleanu TE, Pover G and Tebbutt NC: A phase II open-label
randomized study to assess the efficacy and safety of selumetinib
(AZD6244 [ARRY-142886]) versus capecitabine in patients with
advanced or metastatic pancreatic cancer who have failed first-line
gemcitabine therapy. Invest New Drugs. 30:1216–1223. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hainsworth JD, Cebotaru CL, Kanarev V,
Ciuleanu TE, Damyanov D, Stella P, Ganchev H, Pover G, Morris C and
Tzekova V: A phase II, open-label, randomized study to assess the
efficacy and safety of AZD6244 (ARRY-142886) versus pemetrexed in
patients with non-small cell lung cancer who have failed one or two
prior chemotherapeutic regimens. J Thorac Oncol. 5:1630–1636. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kim EY, Kim A, Kim SK and Chang YS:
AZD6244 inhibits cisplatin-induced ERK1/2 activation and
potentiates cisplatin-associated cytotoxicity in K-ras G12D
preclinical models. Cancer Lett. 358:85–91. 2015. View Article : Google Scholar : PubMed/NCBI
|