Introduction
Glioblastoma (GBM) is the most malignant central
nervous system tumor. GBM is typically treated with a combination
of surgery, radiotherapy and chemotherapy; however, the effect of
this treatment is not satisfactory with regard to prognosis. GBM is
characterized by a strong resistance to postoperative radiotherapy
and chemotherapy. The cause of this resistance may be related to
GBM stem-like cells (GSCs).
Phosphatase and tensin homolog deleted on chromosome
10 (PTEN) is a tumor suppressor that inhibits the PI3K/Akt pathway.
PTEN dephosphorylates the inositol ring D3 position of the
substrate, PIP3, and transfers it to the cell membrane,
which inhibits the activation of cell proliferation-inducing Akt
and induces cell apoptosis (1,2). The
PI3K/Akt pathway was previously shown to be abnormally enhanced and
inhibited apoptosis in malignant tumors in which PTEN was deleted
and mutated, and this promoted cell proliferation and the process
of cells becoming malignant and resistant to irradiation and
chemotherapy (3–6).
Nuclear division and necrosis have frequently been
detected in GBM tissue of patients predicted to have a survival
time of shorter than one year, and tumors were found to rapidly
infiltrate the surrounding tissue and disseminate to the meninx
(7). A previous study reported that
microRNAs (miRNAs/miR) were complementarily bound to a specific
target nucleotide of the 3′-untranslated region (UTR) of mRNA and
inhibited the expression of mRNA and protein (8). Secretory miRNAs (circulating miRNAs) may
be applied clinically, as they are embedded in the granular
vesicles of exosomes and are then secreted so that they are stable
in body fluids, such as blood and cerebrospinal fluid, and
analyzable in histopathological preparations.
The presence of GSCs scattered in GBM tissue has
recently been identified as a factor involved in the development of
resistance to GBM treatments (9–11). GSCs
have been detected among cells that are positive for the surface
antigen cluster of differentiation (CD)133, as well as side
population cells. GSC were previously shown to be resistant to
irradiation and chemotherapy, as they are in the G0
phase of the cell cycle, which leads to the recurrence of tumors
(10,12). Molecular targeted therapies that
target GCSs and PTEN are now being investigated (13–15);
however, the radiation doses investigated in these studies are
lower than those used for cancer therapy in a clinical setting.
The present study analyzed miRNAs in GSCs irradiated
at a dose (total 60 Gy) applied in clinical practice to induce
apoptosis in GSCs, which are resistant to irradiation and cause
recurrence. The objective of this study was to identify miRNAs that
targeted PTEN, which induces apoptosis in irradiation-resistant
GSCs, using A172 cells.
Materials and methods
Cell line and culture conditions
The human glioblastoma A172 cell line, which was
derived from the Japanese Cancer Resources Bank (Japanese Cancer
Resources Bank, Osaka, Japan) was used in the present study. The
selected culture medium contained 10% fetal bovine serum and 100
mg/ml streptomycin (Gibco penicillin-streptomycin liquid; Gibco
Life Technologies, Carlsbad, CA, USA) in Dulbecco's modified Eagle's
medium Ham/F12 (Sigma-Aldrich, Oberhaching, Germany). The cells
were incubated at 37°C with 5% carbon dioxide.
Radiation instrument and radiation
conditions
The cells were irradiated with 4-MV X-rays at a dose
of 2.00 Gy/min over a 25×25-cm field at a radiation depth of 4.0 cm
using a Mevatron KD2/50 (Toshiba, Tokyo, Japan) at room
temperature. The cells were cultured for 7 h prior to roentgen
irradiation. A total of 2×105 cells were irradiated in a
10-cm dish. The radiation conditions used were 2 Gy/day for 5 days
over 1 week. The cells were irradiated for six weeks, up to a total
dose of 60 Gy.
Isolation of
CD133+/CD133− cells
Magnetic cell sorting (MiniMACS™ Separator and
Starting Kit; cat. no. 130-090-312; Miltenyi Biotec GmbH, Begisch
Gladbach, Germany) was used to separate CD133+ cells
from CD133− cells. Cells were counted using a cell
sorter (Z1™ Coulter Counter, Beckman Coulter, Brea, CA, USA). The
culture became turbid in buffer at 350 µl/108 cells. The
cells were labeled using the CD133 MicroBead Kit (MACS®; cat. no.
130-097-049; Miltenyi Biotec GmbH) containing
CD133/1(AC133)-Biotin, and the CD133 MicroBead Kit (human; cat. no.
130-050-801; Miltenyi Biotec GmbH), containing an Fc
receptor-blocking reagent, and anti-biotin MicroBeads in buffer.
The solution was loaded onto the column and set in the magnetic
field of the separator and the unlabeled (CD133−) cells
were extracted. The CD133-labeled (CD133+) cells were
eluted from the column with 1 ml autoMACS Rinsing Solution (cat.
no. 130-091-222; Miltenyi Biotec GmbH). The separated
CD133− and CD133+ cells were counted.
miRNA extraction and polymerase chain
reaction (PCR)
Small RNA-enriched RNA (500 ng/µl) was isolated from
the cells using the miRNeasy micro kit (Qiagen Inc., Valencia, CA,
USA). Reverse transcription and quantitative PCR were performed
using the RT2 miRNA PCR Array human brain cancer
(catalogue no. MIHS-108ZA-12; Qiagen Inc.), and an ABI PRISM 7000
sequence detection system (Applied Biosystems, Tokyo, Japan) was
used under the following cycling conditions: 1 cycle at 95°C for 15
min, followed by 40 cycles at 94°C for 15 sec, 55°C for 30 sec and
70°C for 30 sec. Comparative Ct analysis (2−ΔΔCT) was
used to identify a set of 86 mRNAs that were differentially
expressed between irradiated and non-irradiated CD133+
cells. Measurements were recorded in triplicate.
Fluorescent immunohistochemistry
CD133+ cells were fixed in 10%
formaldehyde for 1 h. These cells were incubated with an anti-PTEN
antibody (1:100; Abcam, Cambridge, MA, USA) for 1 h at room
temperature, followed by a secondary, TRITC-conjugated anti-rabbit
immunoglobulin G antibody (A21428; Life Technologies, Carlsbad, CA,
USA) and bisbenzimide H33342 (Dojindo Molecular Technologies, Inc.,
Kumamoto, Japan) in a humid chamber at 37°C for 30 min. Images were
captured on a microscope with MetaXpress Image Analysis software
(Molecular Devices, Sunnyvale, CA, USA). Staining for PTEN
expression by AlexaFluor 594 was scored using a 0–3 scale.
Specimens with scores of 0 or 1 (no or negligible nuclear staining
in <0–30% of GSC) were considered immunonegative. Specimens that
showed an intermediate (borderline) score of 2 (weak to moderate
nuclear staining in <30–60% of GSCs) were considered equivocal.
Specimens with a score 3 (strong complete nuclear staining in
>60% of GSCs) were considered immunopositive.
Statistical analysis
Data were tested for significance using analysis of
variance, performed using miScript miRNA PCR Array Data Analysis
software (Qiagen Inc.). P<0.05 was considered to indicate a
statistically significant difference.
Results
Analysis of miRNA expression in
60-Gy-irradiated CD133+ A172 cells
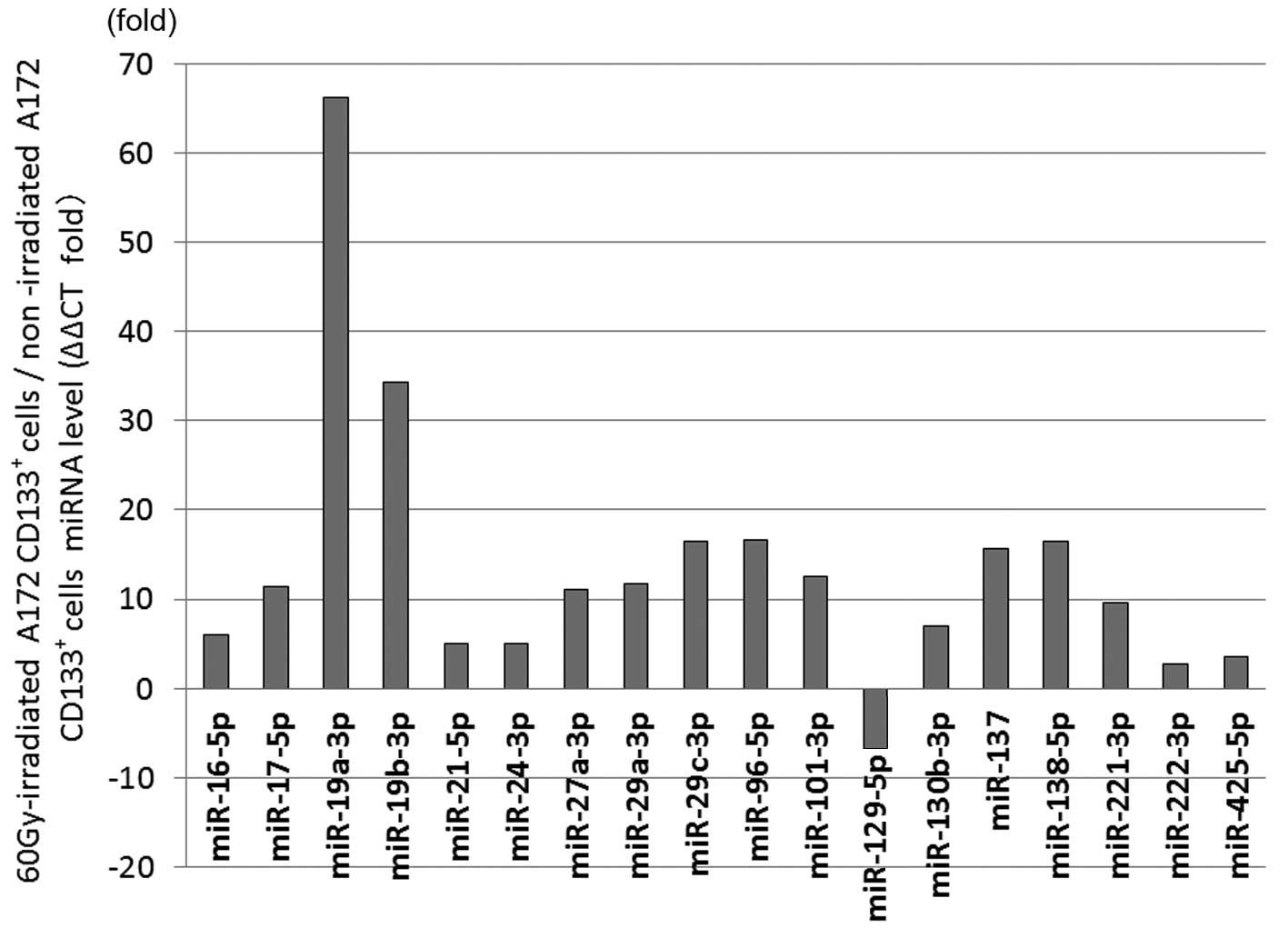
The 60-Gy-irradiated CD133+ A172 cells
showed significant differential expression in 18 miRNAs compared
with the non-irradiated cells. The expression was increased in the
60-Gy-irradiated CD133+ A172 cells in miR-16-5p by 5.97
times (P=0.0095), in miR-17-5p by 11.48 times (P=0.0054), in
miR-19a-3p by 66.18 times (P=0.000023), in miR-19b-3p by 34.25
times (P=0.0023), in miR-21-5p by 4.99 times (P=0.045), in
miR-24-3p by 4.97 times (P=0.024), in miR-27a-3p by 11.04 times
(P=0.002), in miR-29a-3p by 11.75 times (P=0.0012), in miR-29c-3p
by 16.46 times (P=0.014), in miR-96-5p by 16.68 times (P=0.028), in
miR-101-3p by 12.53 times (P=0.005), in miR-130b-3p by 6.92 times
(P=0.037), in miR-137 by 15.65 times (P=0.044), in miR-138-5p by
16.47 times (P=0.012), in miR-221-3p by 9.59 times (P=0.017), in
miR-222-3p by 2.72 times (P=0.045) and in miR-425-5p by 3.6 times
(P=0.039). The expression was decreased in the 60-Gy-irradiated
CD133+ A172 cells in miR-129-5p by 6.71 times (P=0.048)
(Fig. 1).
Immunohistochemistry
Immunostaining for PTEN was positive in the
non-irradiated A172 CD133+ cells (positive rate, 80%;
score, 3). The expression of PTEN was markedly decreased in the
A172 CD133+ cells following irradiation with 60 Gy
(positive rate, 10%; score, 1) (Fig.
2).
Discussion
Significant differences were noted in the expression
levels of 18 miRNAs between the 60-Gy-irradiated GSCs and the
non-irradiated cells in the present study. The expression levels of
17 miRNAs (miR-16-5p, -17-5p, -19a-3p, -19b-3p, -21-5p, -24-3p,
-27a-3p, -29a-3p, -29c-3p, -96-5p, -101-3p, -130b-3p, -137,
-138-5p, -221-3p, -222-3p and -425-5p) were significantly higher,
while that of miR-129-5p was significantly lower in the
60-Gy-irradiated GSCs compared with the non-irradiated cells. Of
these, miR-17-5p, -19a-3p, -19b-3p, -21-5p, -130b-3p, -221-3p and
-222-3p were previously shown to target PTEN, indicating that the
regulation of PTEN expression through these miRNAs induces
apoptosis in cultured brain tumor cells (16–18)
miRNA complementarily binds to a specific nucleotide
in the 3′-UTR of mRNA, and inhibits target mRNA translation and
protein expression. The inhibition of PTEN expression by the
upregulated expression of miR-17-5p, -19a-3p and -19b-3p in
malignant lymphomas, oligodendrocytes and oncogenic cells has been
reported previously (19,20), and an inverse correlation was found
between the expression of miR-130 and PTEN in osteosarcoma cell
lines (21). A previous study showed
that miR-21 acted together with miR-155 in a downstream signaling
pathway in natural killer cell tumors and cell lines, and inhibited
the expression of the target PTEN and PDCD4 (22). Furthermore, the increased expression
of miR-221-3p and -222-3p inhibited that of PTEN in glioma cells
(16,17). These findings suggest that the
expression of miR-17-5p, -19a-3p, -19b-3p, -21-5p, -130b-3p,
-221-3p and -222-3p enhanced and inhibited the PTEN expression in
the 60-Gy-irradiated GSCs in the present study. Immunostaining
confirmed the localization of PTEN expression in the nucleus, and
revealed that its expression was weaker in the 60-Gy-irradiated
GSCs than in the non-irradiated cells. The presence of PTEN in the
cytoplasm has been shown to inhibit the activation of Akt by
dephosphorylating PIP3, which induces cell apoptosis
(23,24). However, PTEN has also been found to be
strongly localized in the nuclei of undifferentiated cells in the
resting G0 phase, and to inhibit cell proliferation
through an Akt-independent molecular mechanism (18,25,26). PTEN
was expressed in the nuclei of the non-irradiated and
60-Gy-irradiated GSCs in the present study, suggesting that the
proliferation of the GSCs was inhibited through an Akt-independent
molecular mechanism, as were their self-differentiation and
replication abilities, which are characteristic of GSCs. In
addition, the expression of PTEN was weaker in the 60-Gy-irradiated
GSCs than in the non-irradiated cells, which indicated that the
inhibition of cell proliferation, self-differentiation and
self-replication was weaker in the 60-Gy-irradiated GSCs.
PTEN was previously shown to suppress tumors through
the Rb/E2F signaling pathway, and to promote Rb- and E2F-related
apoptotic reactions (27), suggesting
that the suppressed expression of PTEN in 60-Gy-irradiated GSCs
leads to the resistance to apoptosis.
Although PTEN has been identified as a protein that
induces apoptosis in cells, only a few treatments currently target
the PTEN protein. The PTEN protein may be applied to treatments in
order to induce apoptosis in irradiation-resistant GSCs.
References
|
1
|
BlancoAparicio C, Renner O, Leal JF and
Carnero A: PTEN, more than the AKT pathway. Carcinogenesis.
28:1379–1386. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Carnero A, BlancoAparicio C, Renner O,
Link W and Leal JF: The PTEN/PI3K/AKT signalling pathway in cancer,
therapeutic implications. Curr Cancer Drug Targets. 8:187–198.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hou W, Liu J, Chen P, Wang H, Ye BC and
Qiang F: Mutation analysis of key genes in RAS/RAF and PI3K/PTEN
pathways in Chinese patients with hepatocellular carcinoma. Oncol
Lett. 8:1249–1254. 2014.PubMed/NCBI
|
|
4
|
Kechagioglou P, Papi RM, Provatopoulou X,
Kalogera E, Papadimitriou E, Grigoropoulos P, Nonni A, Zografos G,
Kyriakidis DA and Gounaris A: Tumor suppressor PTEN in breast
cancer: Heterozygosity, mutations and protein expression.
Anticancer Res. 34:1387–1400. 2014.PubMed/NCBI
|
|
5
|
Appin CL, Gao J, Chisolm C, Torian M,
Alexis D, Vincentelli C, Schniederjan MJ, Hadjipanayis C, Olson JJ,
Hunter S, et al: Glioblastoma with oligodendroglioma component
(GBM-O): Molecular genetic and clinical characteristics. Brain
Pathol. 23:454–461. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Reifenberger G and Collins VP: Pathology
and molecular genetics of astrocytic gliomas. J Mol Med Berl.
82:656–670. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Thakkar JP, Dolecek TA, Horbinski C,
Ostrom QT, Lightner DD, BarnholtzSloan JS and Villano JL:
Epidemiologic and molecular prognostic review of glioblastoma.
Cancer Epidemiol Biomarkers Prev. 23:1985–1996. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Qin J and Xu Q: Functions and application
of exosomes. Acta Pol Pharm. 71:537–543. 2014.PubMed/NCBI
|
|
9
|
Yan K, Yang K and Rich JN: The evolving
landscape of glioblastoma stem cells. Curr Opin Neurol. 26:701–707.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dahlrot RH, Hansen S, Jensen SS, Schrøder
HD, Hjelmborg J and Kristensen BW: Clinical value of CD133 and
nestin in patients with glioma: A population-based study. Int J
Clin Exp Pathol. 7:3739–3751. 2014.PubMed/NCBI
|
|
11
|
Kim SH, Kwon CH and Nakano I:
Detoxification of oxidative stress in glioma stem cells: Mechanism,
clinical relevance, and therapeutic development. J Neurosci Res.
92:1419–1424. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hide T, Takezaki T, Nakamura H, Kuratsu J
and Kondo T: Brain tumor stem cells as research and treatment
targets. Brain Tumor Pathol. 25:67–72. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sasaki A, Nakajo T, Tsunoda Y, Yamamoti G,
Kobayashi Y, Tsuji M, Udaka Y, Mizutani T and Oguchi K: Gene
analysis and dynamics of tumor stem cells in human glioblastoma
cells after radiation. Hum Cell. 26:73–79. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lv S, Teugels E, Sadones J, De Brakeleer
S, Duerinck J, Du Four S, Michotte A, De Grève J and Neyns B:
Correlation of EGFR, IDH1 and PTEN status with the outcome of
patients with recurrent glioblastoma treated in a phase II clinical
trial with the EGFR-blocking monoclonal antibody cetuximab. Int J
Oncol. 41:1029–1035. 2012.PubMed/NCBI
|
|
15
|
Stechishin OD, Luchman HA, Ruan Y, Blough
MD, Nguyen SA, Kelly JJ, Caimcross JG and Weiss S: On-target
JAK2/STAT3 inhibition slows disease progression in orthotopic
xenografts of human glioblastoma brain tumor stem cells. Neuro
Oncol. 15:198–207. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xie Q, Yan Y, Huang Z, Zhong X and Huang
L: MicroRNA-221 targeting PI3-K/Akt signaling axis induces cell
proliferation and BCNU resistance in human glioblastoma.
Neuropathology. 34:455–464. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li W, Guo F, Wang P, Hong S and Zhang C:
miR-221/222 confers radioresistance in glioblastoma cells through
activating Akt independent of PTEN status. Curr Mol Med.
14:185–195. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chung JH and Eng C: Nuclear-cytoplasmic
partitioning of phosphatase and tensin homologue deleted on
chromosome 10 (PTEN) differentially regulates the cell cycle and
apoptosis. Cancer Res. 65:8096–8100. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tagawa H, Ikeda S and Sawada K: Role of
microRNA in the pathogenesis of malignant lymphoma. Cancer Sci.
104:801–809. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rao E, Jiang C, Ji M, Huang X, Iqbal J,
Lenz G, Wright G, Staudt LM, Zhao Y, McKeithan TW, et al: The
miRNA-17-92 cluster mediates chemoresistance and enhances tumor
growth in mantle cell lymphoma via PI3K/AKT pathway activation.
Leukemia. 26:1064–1072. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Namløs HM, Meza-Zepeda LA, Barøy T,
Østensen IH, Kresse SH, Kuijjer ML, Serra M, Bürger H,
Cleton-Jansen AM and Myklebost O: Modulation of the osteosarcoma
expression phenotype by microRNAs. PLoS One. 7:e480862012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yamanaka Y, Tagawa H, Takahashi N,
Watanabe A, Guo YM, Iwamoto K, Yamashita J, Saitoh H, Kameoka Y,
Shimizu N, et al: Aberrant overexpression of microRNAs activate AKT
signaling via down-regulation of tumor suppressors in natural
killer-cell lymphoma/leukemia. Blood. 114:3265–3275. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ristorcelli E, Beraud E, Verrando P,
Villard C, Lafitte D, Sbarra V, Lombardo D and Verine A: Human
tumor nanoparticles induce apoptosis of pancreatic cancer cells.
FASEB J. 22:3358–3369. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Asano T, Fujishiro M, Kushiyama A, Nakatsu
Y, Yoneda M, Kamata H and Sakoda H: Role of phosphatidylinositol
3-kinase activation on insulin action and its alteration in
diabetic conditions. Biol Pharm Bull. 30:1610–1616. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chung JH, GinnPease ME and Eng C:
Phosphatase and tensin homologue deleted on chromosome 10 (PTEN)
has nuclear localization signal-like sequences for nuclear import
mediated by major vault protein. Cancer Res. 65:4108–4116. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu JL, Sheng X, Hortobagyi ZK, Mao Z,
Gallick GE and Yung WK: Nuclear PTEN-mediated growth suppression is
independent of Akt down-regulation. Mol Cell Biol. 25:6211–6224.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Morales LD, Casillas Pavón EA, Shin JW,
Garcia A, Capetillo M, Kim DJ and Lieman JH: Protein tyrosine
phosphatases PTP-1B, SHP-2, and PTEN facilitate Rb/E2F-associated
apoptotic signaling. PLoS One. 9:e971042014. View Article : Google Scholar : PubMed/NCBI
|