Introduction
Prostate cancer is the second most common urological
malignancy and the sixth leading cause of cancer-associated
mortality in men (1). Based on
GLOBOCAN 2012 estimates, ~1.1 million new cases occurred worldwide
in 2012, accounting for ~307,000 deaths (1). Previous studies indicated that miRNA
expression may be used as an optimal strategy for predicting the
therapeutic outcome of prostate cancer (2,3).
Therefore, the interference of cancer-specific miRNAs could be
exploited to produce a direct anticancer effect.
MicroRNAs (miRs) are short non-coding RNAs that bind
to the 3′ untranslated region (UTR) of target mRNAs and are
involved in post-transcriptional control of gene expression by
inhibiting translation (4). miRNAs
are also considered to target >50% of all human genes (5). Accumulating evidence indicate that
miRNAs are important regulatory molecules in various biological
processes, including cell differentiation, proliferation, apoptosis
and metabolism (6). miR-34a is
located on chromosome 1p-36.23 in humans and highly expressed in
multiple types of cancer such as colon cancer and lung cancer
(7). The tumor suppressor protein p53
induces transcription of miR-34a and the expression of miR-34a
correlates well with activation of p53 by genotoxic stress
activation (8). A previous study,
using non-transfected miR-34a mimics as the negative control,
demonstrated that increased expression levels of miR-34a were
associated with wild-type p53 tumors expressing reduced Bcl-2
levels compared with in tissues with reduced miR-34a expression
(9). Therefore, the role of miR-34a
as a tumor suppressive RNA may be p53 or p53-pathway dependent. The
ectopic expression of miR-34a induces arrest of the cell cycle in
G1 phase, apoptosis and senescence in tumors. Moreover, a number of
target mRNAs of miR-34a have been determined including CDK4/6,
cyclin D1, c-Met and E2F3 (10).
Sirtuin 1 (SIRT1), a NAD+-dependent class
III histone deacetylase, is involved in a wide range of cellular
processes including cell proliferation, senescence and apoptosis,
by affecting DNA repair, stress response and aging (11). It has been indicated that
SIRT1-defective or knockdown cells have an increased apoptotic
response to DNA damage or oxidative stress treatments (12). Although numerous studies have
indicated a role for SIRT1 in tumorigenesis, its role in cancer has
not been sufficiently studied. For example, SIRT1 demonstrates
anti-oncogene action in colon cancer and its expression level is
associated with prognosis, but it is considered to exhibit
oncogenic action in breast cancer (13,14). A
previous study indicated that tumors in SIRT1-deficient mice have
markedly increased numbers of cells undergoing apoptosis (15). Moreover, elevated expression levels of
SIRT1 have been observed in several types of human malignancies,
including ovarian, liver, stomach, ductal, and pancreatic cancers
(16). Additionally, previous studies
have demonstrated that SIRT1 regulated various molecules, including
p-53, FOXO1-4, NF-kB, hypermethylated in cancer 1 (HIC1) and E2F1
(17,18). However, the regulatory control of
SIRT1 in prostate cancer remains unclear.
The present study investigated the biological
function and molecular mechanisms of miR-34a regulation of SIRT1 in
human prostate cancer samples. In addition, the regulation of
miR-34a by SIRT1 and its potential molecular mechanisms in prostate
cancer were investigated by transfecting an miR-34a inhibitor into
human prostate cancer PC-3 cells.
Materials and methods
Tissue samples
Patient-matched prostate cancer and normal prostate
tissues (15 pairs) were obtained from patients who underwent
radical prostatectomy at the Department of Urology, the Third
People's Hospital of Zhengzhou (Zhengzhou, China). Prostate cancer
tissue specimens (n=15) were identified as prostatic
adenocarcinoma. None of the cases had received any previous
cancer-associated treatment, or had a history of any other types of
cancer. All patients underwent pre-treatment evaluation, including
bone scan, chest X-ray, and magnetic resonance imaging (MRI) of the
abdomen and pelvis to evaluate the tumor stage. Prostate cancer
stage was classified according to the seventh American Joint
Committee on Cancer (AJCC) system (19). All the samples were snap-frozen in
liquid nitrogen immediately and stored at −80°C following surgery,
until RNA extraction. The histological diagnosis was confirmed by
examining hematoxylin and eosin-stained original sections
simultaneously by two pathologists. The study protocol was approved
by the Local Ethics Committees of Zhengzhou University, and written
informed consent was obtained from all patients prior to tissue
collection.
Cell culture and transfection
The prostate cancer PC-3 cell line was purchased
from the Cell Resource Center of Shanghai Institutes for Biological
Sciences, Chinese Academy of Sciences (Shanghai, China). PC-3 cells
were cultured in RPMI-1640 medium containing 10% fetal bovine serum
(FBS) and 100 U/ml penicillin/streptomycin (Invitrogen Life
Technologies, Carlsbad, CA, USA) in a humidified atmosphere of 5%
CO2 maintained at 37°C. The PC-3 cells were seeded at a
density of 1×105 cells per well in 6-well plates and
transfected with 50 nM of miR-34a mimics or the negative control
(GenePharma, Co., Ltd., Shanghai, China) using Lipofectamine®
RNAiMAX (Invitrogen Life Technologies) according to the
manufacturer's instructions. The cells were then collected 48 h
after transfection.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA from fresh prostate tissues and cultured
PC-3 cells was isolated using TRIzol reagent (Omega Bio-Tek,
Norcross, GA, USA) according to the manufacturer's instructions.
RNA quantity and quality were determined using 1.0% agarose gel
electrophoresis and an optical density 260/280 absorption ratio of
>1.8 using the NanoDrop 2000 (Thermo Fisher Scientific, Waltham,
MA, USA). The High Capacity cDNA reverse transcription kit for
RT-PCR® (Takara Bio, Inc., Otsu, Japan) was used for the synthesis
of 20 µl complementary DNA (cDNA) from 1,000 ng of whole RNA.
mRNA expression levels were determined by RT-qPCR,
which were measured by the ABI PRISM 7300 Sequence Detection System
using the SYBR® Green PCR Master mix (Applied Biosystems, Foster
City, CA, USA). Cycling conditions were as follows: initial
activation at 50°C for 2 min, and denaturation at 95°C for 10 min,
followed by 40 cycles of 95°C for 15 sec and 60°C for 1 min. The
expression of the house-keeping gene, 18s rRNA, as an internal
control was examined under the same reaction conditions. The
experiment was conducted in triplicate. Gene expression was
quantified in relation to the values of the control group following
normalization against the internal control using the
2−ΔΔCt method. The following RT-qPCR oligonucleotide
primers were used: Forward, 5′-TGGCAGTGTCTTAGCT-3′ and reverse,
5′-TGGTGTCGTGGAGTCG-3′ for miR34a; forward,
5′-CCCAGAACATAGACACGCTGGA-3′ and reverse,
5′-ATCAGCTGGGCACCTAGGACA-3′ for SIRT1; and forward,
5′-TTCGGAACTGAGGCCATGAT-3′ and reverse, 5′-CGAACCTCCGACTTTCGTTT-3′
for 18S rRNA.
Cell proliferation analysis
The PC-3 cells were transferred to 96-well
microplates at 1,000 cells/well 24 h after transfection. The
effects of miR-34a on cell proliferation were detected 0, 24 and 48
h after seeding using the Cell Counting Kit-8 (CCK-8; Dojindo
Molecular Technologies, Kumamoto, Japan) according to the
manufacturer's instructions. Each assay was replicated 5 times.
Flow cytometry cell cycle
analysis
PC-3 cells were transfected with miR-34a for 48 h,
as descibed above. The cells were then collected, and washed with
cold phosphate-buffered saline (PBS) containing 1% FBS, fixed in
cold 70% ethanol in PBS for at least 24 h and stained with
propidium iodide (Sigma-Aldrich, St. Louis, MO, USA). Following
staining, the cell cycle statuses were determined by FACSCalibur
(Becton Dickinson, Mountain View, CA, USA) using CellQuest Pro
software (BD Biosciences, Franklin Lakes, NJ, USA). The cell cycle
fractions were analyzed by ModFit software 3.0 (Verity Software
House, Inc.).
Western blot analysis
Total protein was extracted from the cells using a
cell lysis buffer and protease inhibitor mixture (Beyotime
Institute of Biotechnology, Jiangsu, China). After centrifugation
at 10,000 × g and 4°C for 15 min, the protein concentration
was determined by the Bradford protein assay kit (Bio-Rad
Laboratories, Hercules, CA, USA), according to the manufacturer's
instructions. The sample loading buffer was added to the protein
sample and heated at 100°C for 10 min. The proteins (20 µg) were
then loaded onto a 12% SDS-PAGE gel and transferred onto
polyvinylidene difluoride (PVDF) membranes (Amersham Biosciences,
Piscataway, NJ, USA). The PVDF membranes were blocked using 5%
non-fat dry milk in a Tris-buffered sodium chloride-Tween-20 (TBST)
solution at room temperature for 1 h, and incubated with monoclonal
rabbit anti-SIRT1 (1:1,000; Abcam, Cambridge, MA, USA) overnight at
4°C. After washing, the membranes were incubated with horseradish
peroxidase-labeled secondary anti-rabbit antibody (1:2,000; Abcam)
at room temperature for 2 h. Following three 10-min washes in TBST,
the immunoreactive bands were detected using western blot
chemiluminescence luminol reagents (Santa Cruz Biotechnology, Inc.,
Santa Cruz CA, USA). The band intensities were quantified using
scanning densitometry (Bio-Rad Quantity One software; Bio-Rad).
Statistical analysis
Data are presented as the mean ± standard deviation.
The differences were analyzed using the Student's t-test.
All the analyses were performed using SPSS software, version 17.0
(SPSS Inc., Chicago, IL, USA). P<0.05 was considered to indicate
a statistically significant difference.
Results
miR-34a expression in prostate
cancer
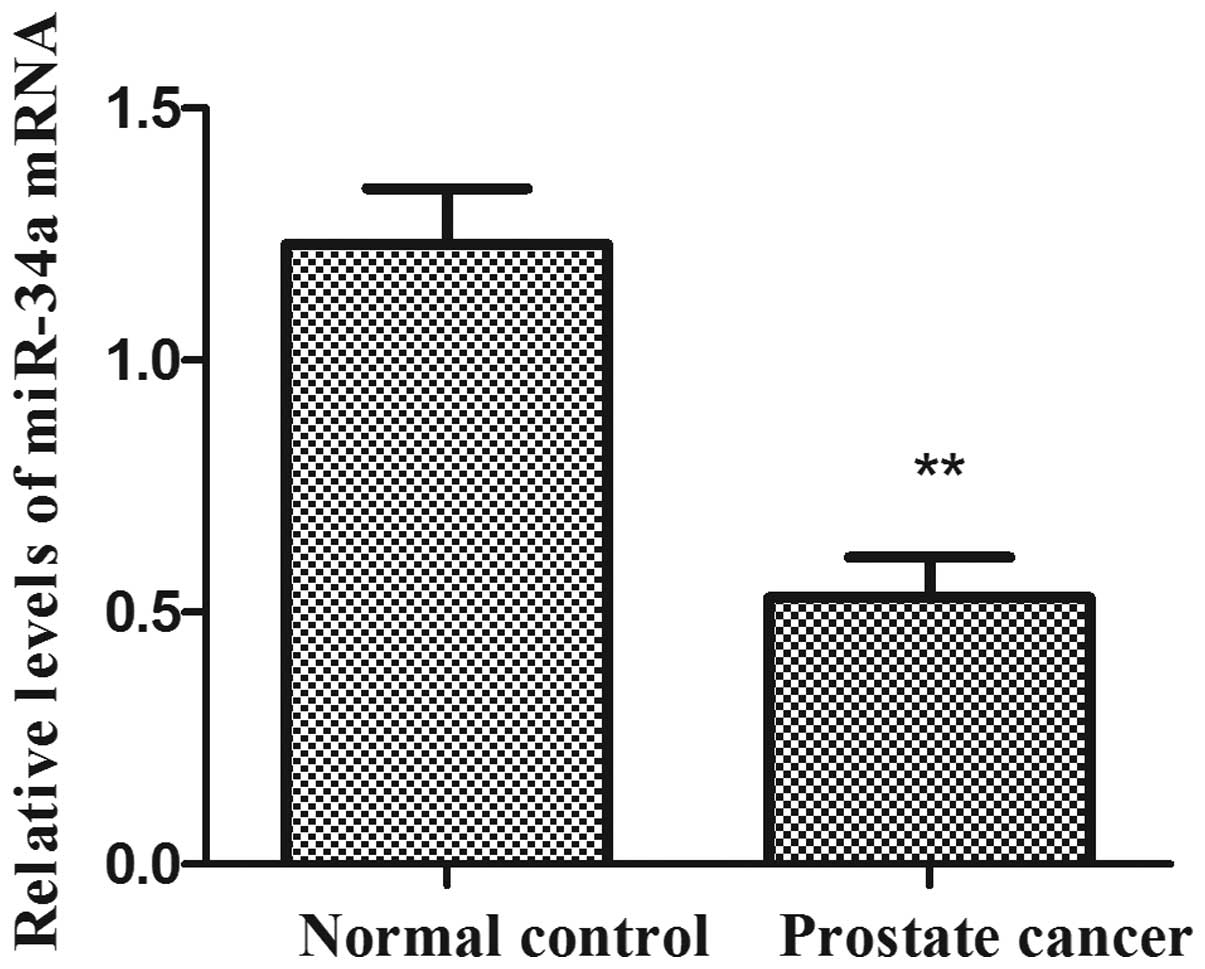
miR-34a expression levels in human prostate cancer
tissues and adjacent normal prostate tissues were examined by
RT-qPCR (Fig. 1). The expression
levels of miR-34a were significantly reduced in human prostate
cancer tissues compared with the adjacent normal prostate tissues
(P<0.01). The results indicated that the downregulation of
miR-34a may be involved in human prostate carcinogenesis.
The effect of miR-34a on cell
proliferation and cycle progression in human prostate cancer PC-3
cell
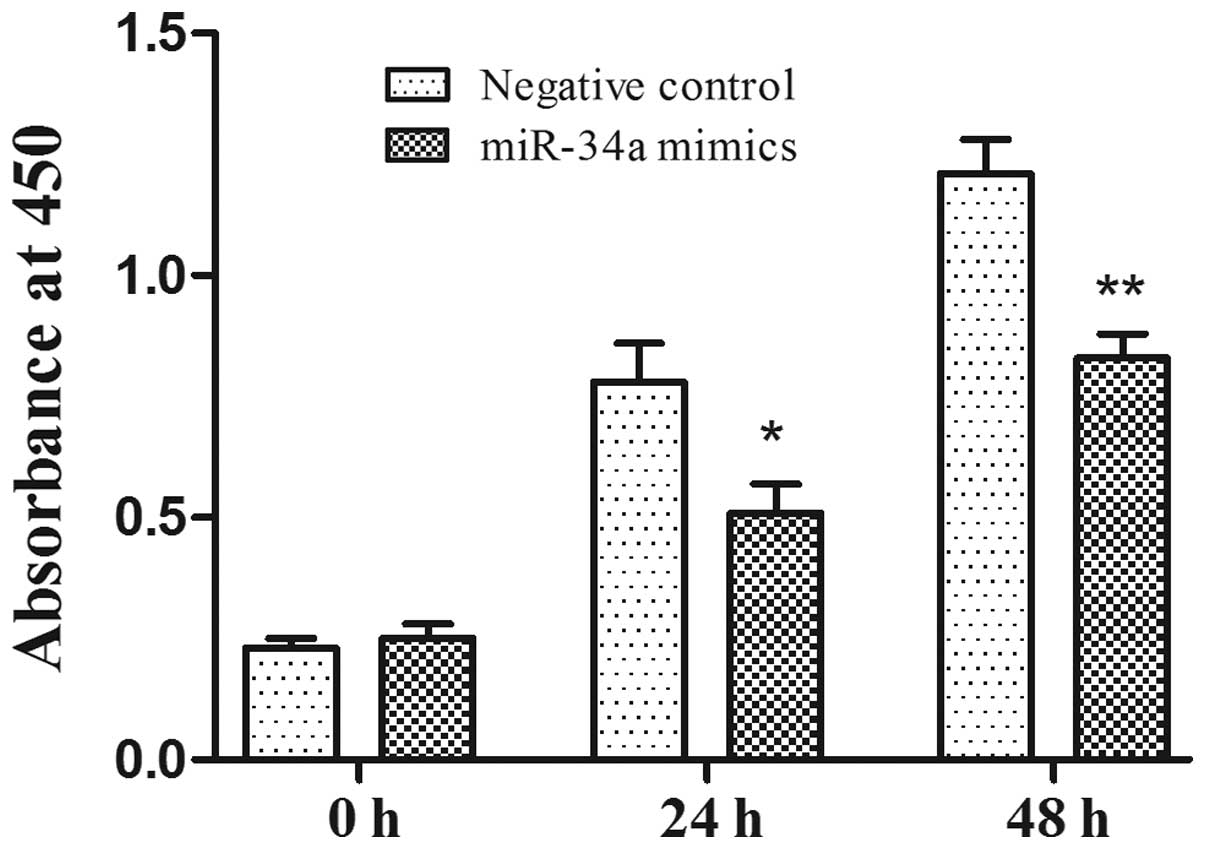
To investigate the antiproliferative function of
miR-34a in prostate cancer cells, human prostate cancer PC-3 cells
were transfected with miR-34a mimics and the negative control.
CCK-8 was used to examine the proliferation rate of PC-3 cell 24 h
after transfection and over the following 3 days. Compared with the
negative control, the overexpression of miR-34a significantly
inhibited the cell proliferation rate in the PC-3 cell lines
(Fig. 2; P<0.05). The effect of
miR-34a on cell cycle progression was examined by flow cytometry.
The percentage of PC-3 cells in S phase in the miR-34a mimics group
was increased compared with the negative control (P<0.05), and
there was a significant reduction in the proportion of cells
arrested in the G0-G1 phase and G2-M phase in the miR-34a mimics
group compared with negative control (Fig. 3) (P<0.05).
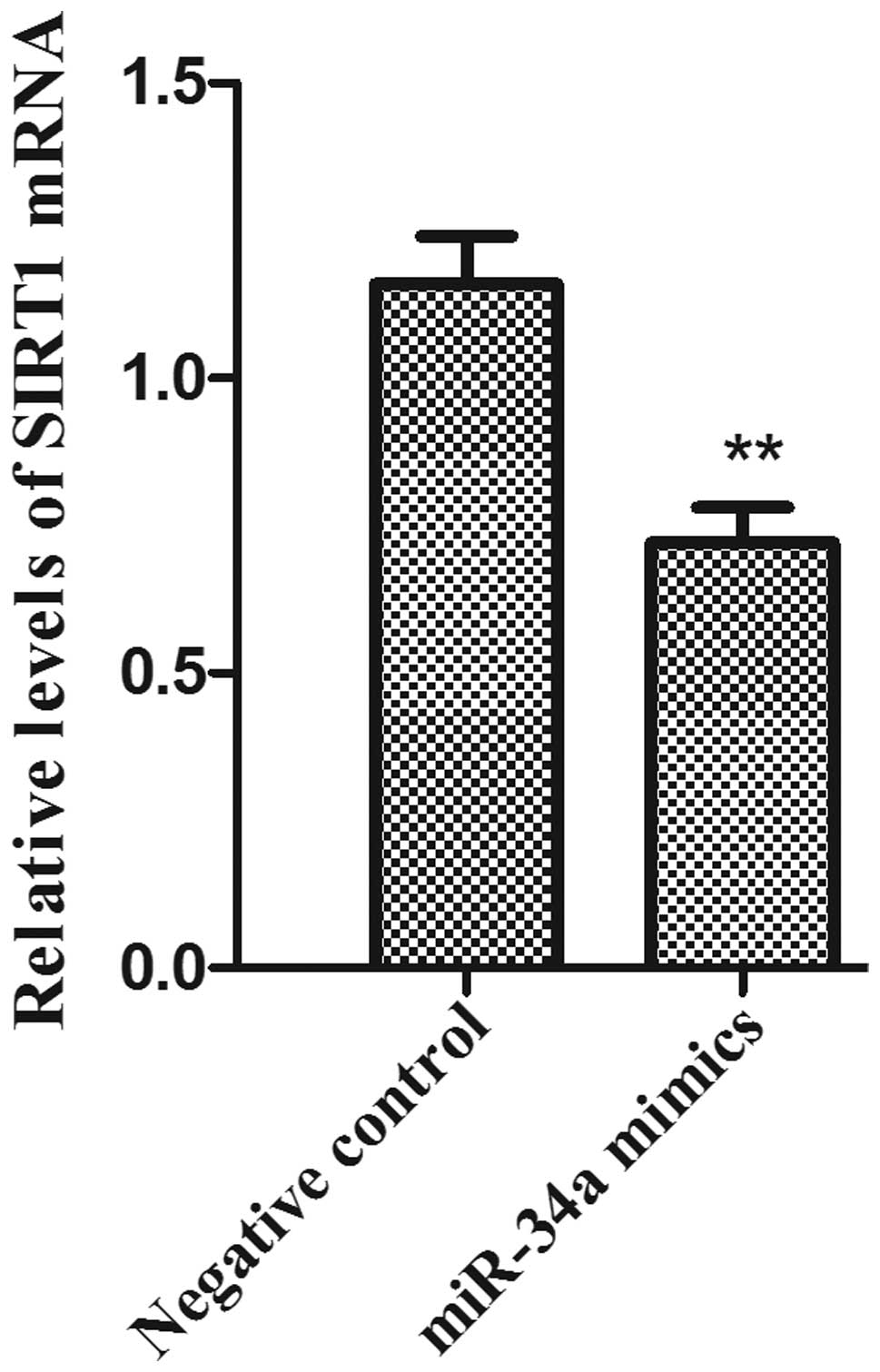
Expression levels of the miR-34a
target gene, SIRT1, in PC-3 cells
The total RNA samples was isolated from PC-3 cells.
The mRNA expression levels of SIRT1 following miR-34A transfection
was examined by RT- qPCR. The results demonstrated that the mRNA
expression levels of SIRT1 in the PC-3 cell line were significantly
reduced in the miR-34a mimics group compared with the negative
control group (Fig. 4;
P<0.01).
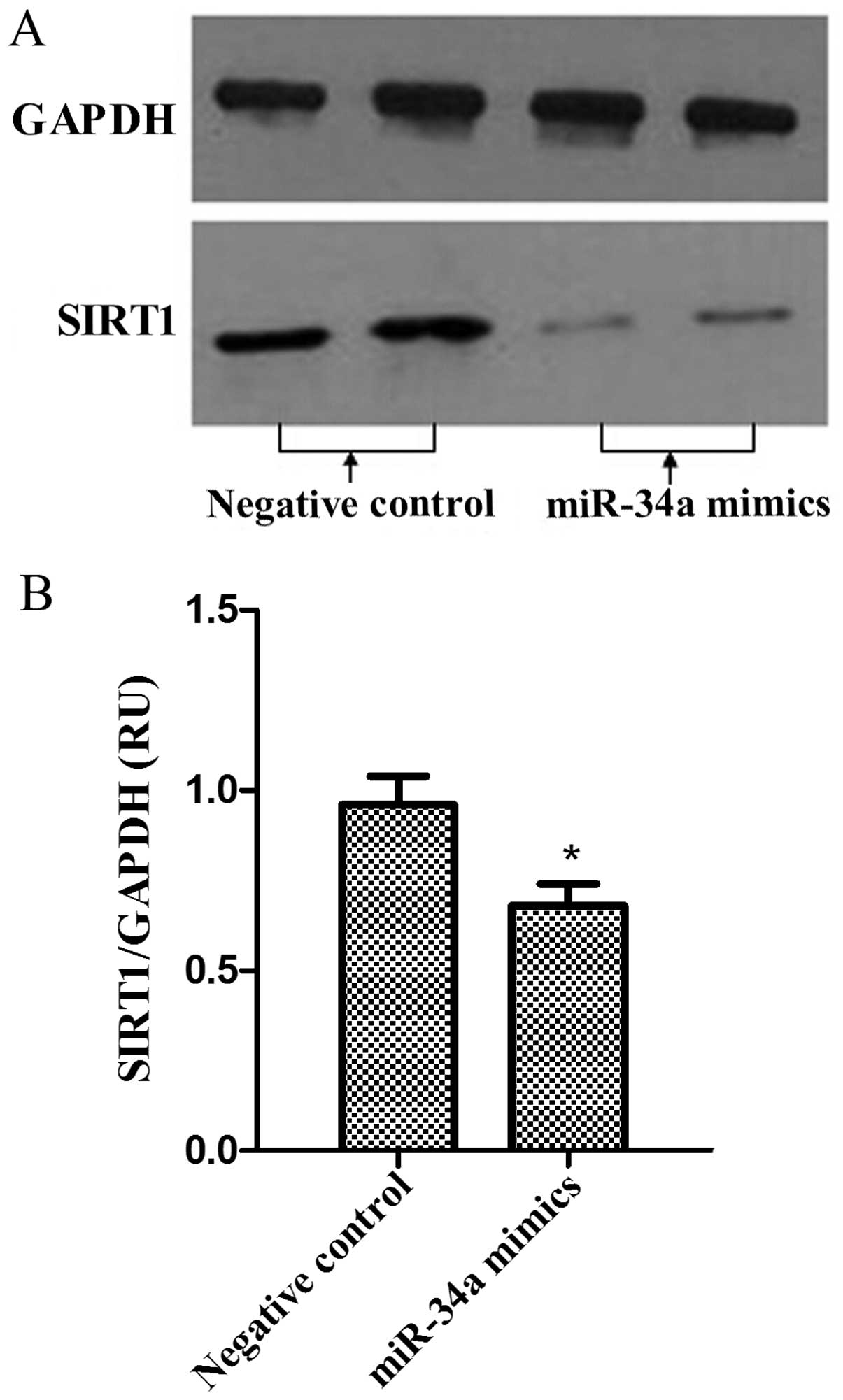
Western blot analysis was performed to analyze the
expression levels of SIRT1 proteins in PC-3 cells. The bands of
SIRT1 proteins were observed in miR-34a mimics and negative control
groups. As was observed in SIRT1 mRNA levels, the protein
expression levels of SIRT1 were reduced following miR-34a
overexpression in PC-3 cells (Fig. 5A and
B; P<0.05).
Discussion
A previous study reported that a low expression of
miR-34a in certain types of human tumor was observed (20). In the present study, the results
indicated that the expression of miR-34a was reduced in human
prostate cancer tissues compared with the adjacent normal tissues.
These results indicated that dysregulation of miR-34a may be
involved in the pathogenesis of human prostate cancer. miR-34a is
located on chromosome 1p-36; deletion or loss of heterozygosity of
this region is associated with a number of types of human tumor,
including breast cancer, lung cancer and cervical cancer (7). In addition, miR-34a is the target gene
of the p53 tumor suppressor gene, and p53 tumor suppressor gene
mutation is involved in the development of prostate cancer
(21). CpG methylation is currently
recognized as one of the mechanisms in carcinogenesis. miR-34a
represents a tumor suppressor gene which is inactivated by CpG
methylation and subsequent transcriptional silencing in various
types of cancer including bladder cancer (22). Therefore, the downregulation of
miR-34a expression may be of tumorgenesis in human prostate
cancer.
SIRT1 has been demonstrated to protect cells against
stress-induced senescence, which has been reported to be one of the
potential targets of miR-34a (23).
miR-34a is a recognized cancer cell inhibitor, was revealed as a
posttranscriptional regulator of SIRT1. Moreover, SIRT1 is also
involved in tumorigenesis, which is overexpressed in a number of
types of human tumor, including colon, renal and lung cancer
(24). In the present study,
overexpression of miR-34a was observed to reduce the expression
levels of SIRT1 compared with the negative control. miR-34a was
overexpressed in endothelial progenitor cells (EPCs), and SIRT1
protein level was found to be diminished, which indicated that
miR-34a negatively regulated SIRT1 expression in EPCs (25). Akao et al (26) reported that overexpression of miR-34a
activated acetylation of p53 by mitigating SIRT1 activation in
human colon cancer cells. However, in another study, overexpression
of miR-34a did not completely suppress SIRT1 translation (23). These inconsistent results may be
associated with the observation that miR-34a does not saturate its
SIRT1 binding site or every SIRT1 binding site does not interact
with miR-34a, so that a number of SIRT1 mRNA are still
translated.
In the present study, the proliferation rate of
human prostate cancer cells in miR-34a mimics group was
significantly reduced compared with the negative control, which
indicated that miR-34a may possess a significant antitumor effect
on prostate cancer cells. The results were similar to observations
in colon, breast and lung cancer, in which miR-34a expression was
downregulated, and overexpression of miR-34a inhibited cell
proliferation (7,26,27). The
present study selected SIRT1, an energy sensor, to validate the
antitumor mechanism of miR-34a in prostate cancer cells. The
downregulation of SIRT1 by miR-34a is considered to be part of a
positive feedback loop acting on p53. Chapman et al
(28) proposed that SIRT1 is involved
in metabolism and tolerance to oxidative stress, promoting the
growth of human urothelial cancer cell. Moreover, inhibition of
SIRT1 expression reduced cell proliferation even in p53 mutated
cells (29). The present study
demonstrated that overexpression of miR-34a significantly reduced
SIRT1 mRNA and protein expression levels. The results indicated
that the proliferation inhibitory effect of miR-34a on human
prostate cancer cell may be partly implemented by downregulating
SIRT1 expression.
A previous study reported that miR-34a could induce
the cell cycle arrest in tumors, especially in cell proliferation
(30). In the present study,
downregulation by miR-34a of SIRT1 was detected in human prostate
cancer PC-3 cells. Certain proteins involved in cell cycle control
have been identified as direct targets of miR-34a, including SIRT1,
NMYC, MET and E2F3 (31). miR34-a
induce cell cycle arrest via p-53-miR-34a-SIRT1 axis was also
observed in cancer cells and umbilical vein endothelial cells
(32). In addition, the expression of
SIRT1 mRNA was also significantly reduced in response to
overexpression miR-34a in the present study: The results indicated
that SIRT1 expression level may be regulated by miR-34a at the
transcriptional and/or post-transcriptional levels. However,
Yamakuchi et al (23) reported
that overexpression of miR-34a in HCT116 human colon carcinoma
cells downregulated SIRT1 protein expression, but did not affect
its mRNA level (23). These
inconsistent results indicate that the binding characteristics of
miR-34a to the target gene sequence and its effects on gene
regulation may vary depending on the cell lines.
In conclusion, the expression of miR-34a was reduced
in prostate cancer tissues compared with the adjacent normal
prostate tissues. The target gene SIRT1 of miR-34a was
downregulated at the protein and mRNA levels when the expression of
miR-34a was elevated in the human prostate cancer cells. The
results of the present study indicated that miR-34a may inhibit
carcinogenesis by downregulating the expression of SIRT1, therefore
inhibiting cell proliferation and inducing cell cycle arrest in the
human prostate cancer cells. Therefore, modulation of miR-34a
activity may represent a novel approach for treating prostate
cancer.
Acknowledgements
The present study was supported by grants from the
key scientific and technological project of Henan province (no.
201004022).
References
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods, and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gade TP, Hassen W, Santos E, Gunset G,
Saudemont A, Gong MC, Brentjens R, Zhong XS, Stephan M, Stefanski
J, et al: Targeted elimination of prostate cancer by genetically
directed human T lymphocytes. Cancer Res. 65:9080–9088. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Salter KH, Acharya CR, Walters KS, Redman
R, Anguiano A, Garman KS, Anders CK, Mukherjee S, Dressman HK,
Barry WT, et al: An integrated approach to the prediction of
chemotherapeutic response in patients with breast cancer. PLoS One.
3:e19082008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Denli AM, Tops BB, Plasterk RH, Ketting RF
and Hannon GJ: Processing of primary microRNAs by the
Microprocessor complex. Nature. 432:231–235. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Huntzinger E and Izaurralde E: Gene
silencing by microRNAs: Contributions of translational repression
and mRNA decay. Nat Rev Genet. 12:99–110. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kloosterman WP and Plasterk RH: The
diverse functions of microRNAs in animal development and disease.
Dev Cell. 11:441–450. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li XJ, Ren ZJ and Tang JH: MicroRNA-34a: A
potential therapeutic target in human cancer. Cell Death Dis.
5:e13272014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Guessous F, Zhang Y, Kofman A, Catania A,
Li Y, Schiff D, Purow B and Abounader R: MicroRNA-34a is tumor
suppressive in brain tumors and glioma stem cells. Cell Cycle.
9:1031–1036. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ji Q, Hao X, Meng Y, Zhang M, Desano J,
Fan D and Xu L: Restoration of tumor suppressor miR-34 inhibits
human p53-mutant gastric cancer tumorspheres. BMC Cancer.
8:2662008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Maroof H, Salajegheh A, Smith RA and Lam
AK: Role of microRNA-34 family in cancer with particular reference
to cancer angiogenesis. Exp Mol Pathol. 97:298–304. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Satoh A, Stein L and Imai S: The role of
mammalian sirtuins in the regulation of metabolism, aging and
longevity. Handb Exp Pharmacol. 206:125–162. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kabra N, Li Z, Chen L, Li B, Zhang X, Wang
C, Yeatman T, Coppola D and Chen J: SirT1 is an inhibitor of
proliferation and tumor formation in colon cancer. J Biol Chem.
284:18210–18217. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jung W, Hong KD, Jung WY, Lee E, Shin BK,
Kim HK, Kim A and Kim BH: SIRT1 Expression Is Associated with Good
Prognosis in Colorectal Cancer. Korean J Pathol. 47:332–339. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim JR, Moon YJ, Kwon KS, Bae JS, Wagle S,
Yu TK, Kim KM, Park HS, Lee JH, Moon WS, et al: Expression of SIRT1
and DBC1 is associated with poor prognosis of soft tissue sarcomas.
PLoS One. 8:e747382013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Leko V, Park G, Lao U, et al:
Enterocyte-specific inactivation of SIRT1 reduces tumor load in the
APC (+/min) mouse model. PLoS One. 8:e662832013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Knight JR, Allison SJ and Milner J: Active
regulator of SIRT1 is required for cancer cell survival but not for
SIRT1 activity. Open Biol. 3:130–135. 2013. View Article : Google Scholar
|
|
17
|
Revollo JR and Li X: The ways and means
that fine tune SIRT1 activity. Trends Biochem Sci. 38:160–167.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang XL, Chen ML and Zhou SL: Fentanyl
increases colorectal carcinoma cell apoptosis by inhibition of
NF-κB in a Sirt1-dependent manner. Asian Pac J Cancer Prev.
15:10015–10020. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
The American Joint Committee on Cancer:
The 7th edition of the AJCC cancer staging manual and the future of
TNM. Ann Surg Oncol. 17:1471–1474. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Welch C, Chen Y and Stallings RL:
MicroRNA-34a functions as a potential tumor suppressor by inducing
apoptosis in neuroblastoma cells. Oncogene. 26:5017–5022. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Moretti RM, Mareli M Montagnani, Taylor
DM, Martini PG, Marzagalli M and Limonta P: Gonadotropin-releasing
hormone agonists sensitize and resensitize, prostate cancer cells
to docetaxel in a p53-dependent manner. PLoS One. 9:e937132014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lodygin D, Tarasov V, Epanchintsev A,
Berking C, Knyazeva T, Körner H, Knyazev P, Diebold J and Hermeking
H: Inactivation of miR-34a by aberrant CpG methylation in multiple
types of cancer. Cell Cycle. 7:2591–2600. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yamakuchi M, Ferlito M and Lowenstein CJ:
MiR-34a repression of SIRT1 regulates apoptosis. Proc Natl Acad Sci
U S A. 105:13421–13426. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hwang BJ, Madabushi A, Jin J, Lin SY and
Lu AL: Histone/protein deacetylase SIRT1 is an anticancer
therapeutic target. Am J Cancer Res. 4:211–221. 2014.PubMed/NCBI
|
|
25
|
Zhao T, Li J and Chen AF: MicroRNA-34a
induces endothelial progenitor cell senescence and impedes its
angiogenesis via suppressing silent information regulator 1. Am J
Physiol Endocrinol Metab. 299:E110–E116. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Akao Y, Noguchi S, Iio A, Kojima K, Takagi
T and Naoe T: Dysregulation of microRNA-34a expression causes
drug-resistance to 5-FU in human colon cancer DLD-1 cells. Cancer
Lett. 300:197–204. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li L, Yuan L, Luo J, Gao J, Guo J and Xie
X: MiR-34a inhibits proliferation and migration of breast cancer
through down-regulation of Bcl-2 and SIRT1. Clin Exp Med.
13:109–117. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chapman EJ, Kelly G and Knowles MA: Genes
involved in differentiation, stem cell renewal and tumorigenesis
are modulated in telomerase-immortalized human urothelial cells.
Mol Cancer Res. 6:1154–1168. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Audrito V, Rossi D, Gottardi D, et al:
Nicotinamide blocks proliferation and induces apoptosis of chronic
lymphocytic leukemia cells through activation of the
p53/miR-34a/SIRT1 tumor suppressor network. Cancer Res.
71:4473–4483. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bommer GT, Feng Y, Kaczorowski AJ, Gerin
I, Kuick R, Love RE, Zhai Y, Giordano TJ, et al: p53-mediated
activation of miRNA34 candidate tumor-suppressor genes. Curr Biol.
17:1298–1307. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chen F and Hu SJ: Effect of microRNA-34a
in cell cycle, differentiation and apoptosis: A review. J Biochem
Mol Toxicol. 26:79–86. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ito T, Yagi S and Yamakuchi M:
MicroRNA-34a regulation of endothelial senescence. Biochem Biophys
Res Commun. 398:735–740. 2010. View Article : Google Scholar : PubMed/NCBI
|