Introduction
Camptothecin (CPT) is a pentacyclic alkaloid first
isolated from a Chinese tree, Camptotheca acumincta, in the
early 1960s (1). CPT is an antitumor
agent that is clinically effective, with a broad range of antitumor
activities against solid tumors, including breast, ovarian, lung
and colorectal tumors (2). The
efficacy of CPT is associated with its ability to inhibit the
function of DNA topoisomerase I, which is vital for the
transcription of supercoiled DNA (3).
However, innovations in treatment strategies have been developed,
with targets such as oncogenes and elements of cell signaling
pathways (4). Tumors are dependent on
a switch to an angiogenic phenotype and the resultant formation of
new vasculature, and this finding has become the basis of another
potential target (5,6). Thus, the inhibition of the blood supply
to the tumor is now considered to represent a unique approach for
the cessation of tumor growth (7).
Certain studies have illustrated that CPT also produces an
inhibitory effect on the development of the vasculature (8). A previous study has also revealed that
CPT can induce the apoptosis of cancer cells (9).
A number of studies have indicated that nitric oxide
(NO) synthesized by the NO synthase (NOS) enzyme family is
significant in tumor growth, invasion and metastasis (10,11).
Mammalian systems contain three well-characterized isoforms of the
enzyme: Neuronal NOS (nNOS/NOS-1), endothelial NOS (eNOS/NOS-3) and
inducible NOS (iNOS/NOS-2). The activity of iNOS has been found to
be inducible in response to stimuli such as proinflammatory
cytokines or endotoxin (12). In a
number of solid tumors, such as colon and breast carcinoma, the
overexpression of inducible NOS has been recently documented
(13,14). It has also been suggested that the
iNOS activity in tumor tissue is associated with the tumor grade
and cell differentiation (15–17). A
previous study has indicated that CPT affects the expression of
iNOS protein and its activity in the virus-transformed mouse
macrophage-like RAW264.7 cell line when stimulated with
lipopolysaccharide (LPS) plus interferon-γ (18).
The present study investigated whether CPT alters
iNOS protein expression in the human colon adenocarcinoma SW480
cell line in order to obtain further insight into the biological
effects of CPT on iNOS.
Materials and methods
Chemicals
CPT was purchased from Sigma Aldrich (St. Louis, MO,
USA) and dissolved in dimethyl sulfoxide (DMSO; Sigma Aldrich) at 2
mg/ml, prior to being aliquoted and stored at −20°C. Further
dilutions were made in phosphate-buffered saline (PBS; Cellgro,
Herndon, VA, USA) to the appropriate concentration just prior to
use. The final concentration of DMSO in culture did not exceed 0.1%
(v/v), which is non-toxic to cells. LPS was purchased from Fluka
Chemical Corporation (Buchs SG, Switzerland) and interleukin
(IL)-1β was purchased from Cytolab Ltd. (Rehovot, Isael).
Dulbecco's modified Eagle's medium (DMEM) was purchased from Gibco
Life Technologies (Carlsbad, CA, USA). All other chemicals were
dissolved in distilled water.
Cell culture
SW480 cells were gained from the Basic Science
department at Zhejiang Medical College (Hangzhou, China) and were
cultured in DMEM containing 10% fetal calf serum (Sijiqing Company,
Zhejiang, China) in a humidified incubator at 37°C in an atmosphere
containing 5% CO2. The cells were used between passages
9 and 20.
Measurement of nitrite
Nitrite production, an indicator of NO synthesis,
was measured in the supernatant as described previously (19). The SW480 cells were plated into
96-well tissue culture plates at 2×104 cells/well and
grown under standard culture conditions. After 12 h, in order to
induce iNOS, old culture medium was replaced by fresh culture
medium containing LPS (10 mg/l) plus IL-1β (20 ng/ml). To assay the
effect of CPT on nitrite production, CPT (0, 0.032, 0.125, 0.5,
1.0, 2.0 µg/ml) was added in the presence of LPS/IL-1β for 12 h.
Nitrite was measured based on the Griess reaction. Cell culture
medium (100 µl) was mixed with 100 µl Griess reagent (1%
sulfanilamide and 0.1% naphthylenediamine in 5% phosphoric acid)
and incubated at room temperature for 10 min. The optical density
at 490 nm (OD490) was measured with a microplate reader
(SmartSpec™3000; Bio-Rad Laboratories Inc., Hercules, CA, USA). A
standard curve was prepared for calculating the concentration using
the OD490.
Cell viability
Cell viability was determined by MTT assay. A total
of 100 µl culture medium was present per well following the Griess
reaction. MTT was purchased from Sigma Aldrich and was dissolved
with PBS. MTT solution (5 mg/ml) was added in every well. The cells
were incubated for 4 h. The supernatant was discarded and then 150
µl DMSO was added. Agitation was performed for 10 min and the
OD490 was measured. The viability percentage was
calculated.
Reverse transcription-polymerase chain
reaction (RT-PCR)
The SW480 cells were cultured in 100-ml culture
bottles. After 12 h, fresh medium with LPS/IL-1β was added to
replace the old medium. Next, two concentrations of CPT (0.032 and
0.125 µg/ml) were added after 12 h, and 24 h later, total cellular
RNA was extracted from control and treated cells using TRIzol
reagent (Bio Basic Inc., Markham, ON, Canada). Total RNA
preparation (2 µg) was mixed with oligo(dT)18
(Invitrogen Life Technologies, Carlsbad, California, USA) for
reverse transcription using MMLV to derive the first-strand cDNA. A
pair of gene-specific PCR primers (Invitrogen Life Technologies)
were designed for iNOS and GAPDH as follows: iNOS,
5′-GATCAATAACCTGAACG-3′ and 5′-GCCCTTTTTTGCTCCATAGC-3′; and GAPDH,
5′-ACCACAGTCCATGCCATCAC-3′ and 5′-TCCACCACCATGTTGCTGTAA-3′. PCR was
performed with an S1000™ Thermal Cycler (Bio-Rad Laboratories Inc.)
at 94°C for 3 min for denaturing, followed by multiple cycles at
94°C for 45 sec, 50°C for 1 min and 72°C for 1 min. For iNOS, 36
cycles were performed and for GAPDH, 35 cycles were performed. The
PCR products were separated by electrophoresis and their quantity
was determined by Quantity One software (Bio-Rad Laboratories
Inc.).
Western blot analysis
The SW480 cells were cultured in 100-ml culture
bottles. After 12 h, fresh medium with LPS/IL-1β was added to
replace the old medium. Next, two concentrations of CPT (0.032 and
0.125 µg/ml) were added after 12 h, and 24 h later, the cells were
solubilized with lysis buffer. A BCA Protein Assay Kit (Amersham,
Uppsala, Sweden) was applied to detect the concentration of
protein. Lysates containing 5 µg protein were separated by SDS-PAGE
on 7.5% polyacrylamide gels with perpendicurity electrophoretic
apparatus (EPS 2A200; Amersham) and transferred onto nitrocellulose
membranes with a transmembrane machine (semi-dry transfer unit;
Amersham). Subsequent to blocking, the membrane was incubated with
rabbit polyclonal anti-iNOS body (1:2,000; Santa Cruz Inc., Dallas,
TX, USA) for 2 h at room temperature. Blots were washed with
Tris-buffered saline plus Tween-20 and incubated with horseradish
peroxidase-conjugated goat anti-rabbit immunoglobulin G (1:5,000;
Santa Cruz Inc.) for 2 h at room temperature. Next, immunoreactive
bands were detected with an enhanced chemiluminescence mixture
(Sigma Aldrich). Thereafter, the same membrane was stripped and
reprobed with rabbit anti-β-actin (1:2,000; Santa Cruz Inc.). The
membrane was placed into a magazine with a film. Following
exposure, the film was immersed in development agent for 1–2 min,
then in fixer. The film was scanned and the OD value was calculated
by Quantity One software.
Statistical evaluation
The results are expressed as the mean ± standard
deviation. Statistical comparisons were made between groups using a
one-way analysis of variance. P<0.05 was considered to indicate
a statistically significant difference.
Results
Effect of CPT on nitrite production in
SW480
As illustrated in Tables
I and II, when the cells were
stimulated with LPS/IL-1β, the ability of CPT to cause changes in
nitrite production was dependent on the concentration of CPT and
the duration of incubation with CPT. As shown in Table I, when the cells were incubated in the
presence of CPT for 18 h, nitrite production was significantly
reduced only at concentration of 2 µg/ml. As shown in Table I, all concentrations of CPT did not
affect the cell viability of the SW480 cells, even at 2 µg/ml. This
result indicates that the inhibition of nitrite production by 2
µg/ml CPT was not due to cell death. However, significant
inhibition of nitrite production by CPT was observed at all
concentrations after 24 h incubation and these effects occurred in
a dose-dependent manner. Additionally, there was no significant
difference in cell viability among the cells treated with vehicle
and 0.032 or 0.125 µg/ml CPT (Table
II). This result indicates that lower concentrations of CPT
(0.032 and 0.125 µg/ml) could reduce nitrite production, but not
cause cell death.
 | Table I.Nitrite production and cell viability
of SW480 cell in the presence of CPT for 18 h. |
Table I.
Nitrite production and cell viability
of SW480 cell in the presence of CPT for 18 h.
| Concentration of CPT,
µg/ml | Nitrite production,
µg/ml | Cell viability after
18 h, % |
|---|
| 0.000 | 26.68±1.15 | 99.98±2.09 |
| 0.032 | 24.71±1.71 | 93.93±6.39 |
| 0.125 | 24.16±1.37 | 94.56±4.91 |
| 0.500 | 23.88±1.40 | 93.21±8.78 |
| 1.000 | 23.68±0.87 | 91.04±4.85 |
| 2.000 |
21.63±4.59a | 92.58±4.69 |
 | Table II.Nitrite production and cell viability
of SW480 cells in the presence of CPT for 24 h. |
Table II.
Nitrite production and cell viability
of SW480 cells in the presence of CPT for 24 h.
| Concentration of CPT,
µg/ml | Nitrite production,
µg/ml | Cell viability after
24 h, % |
|---|
| 0.000 | 31.26±2.49 | 100.00±3.98 |
| 0.032 |
24.57±1.48a |
93.67±6.46 |
| 0.125 |
24.02±1.93a |
92.70±5.31 |
| 0.500 |
23.29±1.08a |
83.41±5.68a |
| 1.000 |
22.79±3.39a |
60.58±8.33a |
| 2.000 |
24.71±1.36a |
41.02±7.26a |
Effect of CPT on iNOS mRNA
expression
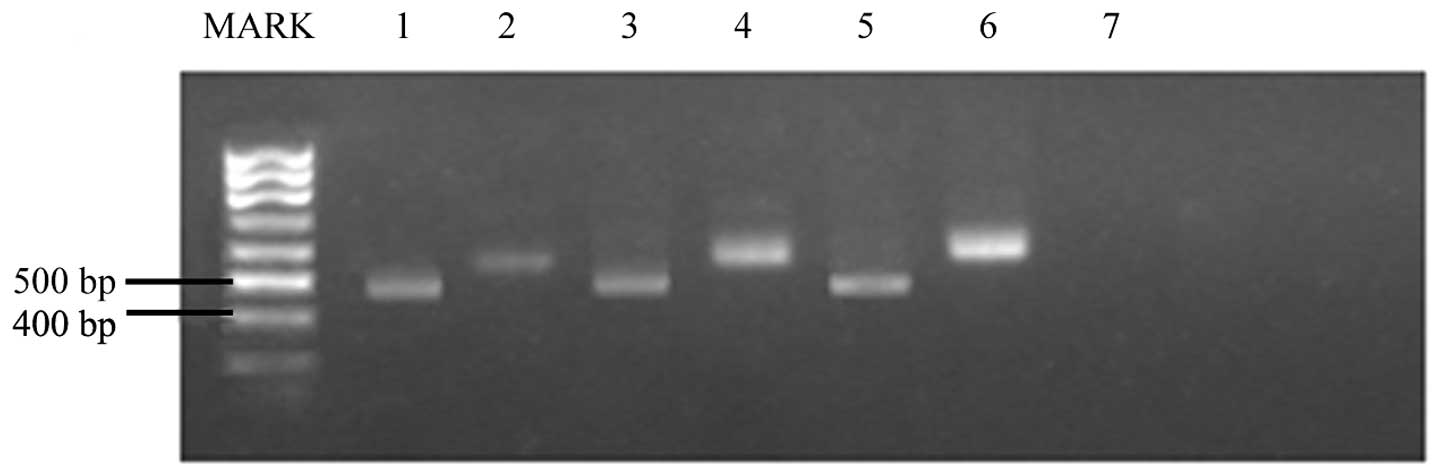
To examine whether CPT could inhibit iNOS mRNA
expression, RT-PCR was performed, as shown in Fig. 1. The house-keeping gene, GAPDH, was
also amplified from each RNA preparation to enable comparisons of
the PCR productions in different samples. As shown in Fig. 1 and Table
III, iNOS mRNA was significantly suppressed in the presence of
CPT, while GAPDH mRNA was not inhibited significantly. This result
indicates that CPT inhibits NO production at the transcription
level.
 | Table III.Optical density ratio of RT-PCR in the
presence of CPT for 24 h. |
Table III.
Optical density ratio of RT-PCR in the
presence of CPT for 24 h.
| Concentration of CPT,
µg/ml | Optical density ratio
(iNOS/GAPDH) |
|---|
| 0.000 | 1.284±0.008 |
| 0.032 |
1.179±0.003a |
| 0.125 |
1.071±0.017a |
Effect of CPT on iNOS protein
expression
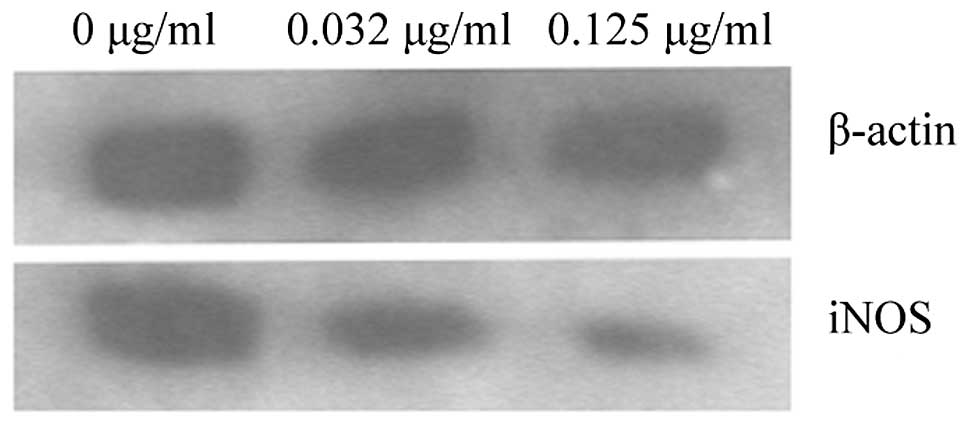
To determine whether the NO inhibitory effect of CPT
was due to the inhibition of iNOS protein expression, western blot
analysis was performed. The inhibition of 142-kDa iNOS protein
expression by CPT is shown in Fig. 2.
Marked suppression was observed at the two concentrations. As
β-actin protein was not markedly affected, cell viability was not
changed (Fig. 2; Table IV). This result indicates that the
inhibition of iNOS protein expression is one of the mechanisms of
NO inhibition.
 | Table IV.Optical density ratio of western
blotting in the presence of CPT for 24 h. |
Table IV.
Optical density ratio of western
blotting in the presence of CPT for 24 h.
| Concentration of CPT,
µg/ml | Optical density ratio
(iNOS/β-actin) |
|---|
| 0.000 | 1.051±0.024 |
| 0.032 |
0.934±0.078a |
| 0.125 |
0.366±0.016a |
Discussion
A number of the anticancer mechanisms of CPT have
previously been revealed. Studies have shown that the antitumor
activity is associated with the inhibition of topoisomerase I
(3), and that CPT may exhibit an
inhibitory effect on the development of the vasculature (8) and the apoptosis of cancer cells
(9). In the present study, it was
shown that CPT can effect the nitrite production of SW480 cells in
a process that is independent of cytotoxicity. In order to further
understand the mechanisms of action for NO inhibition, the levels
of iNOS mRNA and protein expression were determined. RT-PCR and
western blotting data showed that interference with iNOS mRNA and
protein expression may be a factor contributing to the inhibitory
effect of CPT on iNOS enzyme activity and NO production in SW480
cells. This result has not been reported in previous studies.
iNOS is overexpressed in colon tumors, and NO is
important during the progression of colon carcinoma. The associated
mechanisms involve inhibiting apoptosis, improving angiogenesis and
enhancing the expression of proto-oncogenes. This suggests that
iNOS can be the target of anticancer agents. As an effective
anticancer agent, CPT can inhibit colon tumors. From the present
experiments, it can be concluded that the inhibition of nitrite
production should be a novel mechanism underlying the effect of CPT
against colon cancer.
References
|
1
|
Wall ME, Wani MC, Cook CE, Palmer KH,
McPhail AT and Sim GA: Plant antitumor agents. I. The isolation and
structure of camptothecin, a novel alkaloidal leukemia and tumor
inhibitor from Camptotheca accuminata. J Am Chem Soc. 88:3888–3890.
1966. View Article : Google Scholar
|
|
2
|
Garcia-Carbonero R and Supko JG: Current
perspectives on the clinical experience, pharmacology and continued
development of the camptothecins. Clin Cancer Res. 8:641–661.
2002.PubMed/NCBI
|
|
3
|
Hsiang YH, Wu HY and Liu LF:
Proliferation-dependent regulation of DNA topoisomerase II in
cultured human cells. Cancer Res. 48:3230–3235. 1988.PubMed/NCBI
|
|
4
|
Abounader R, Reznik T, Colantuoni C,
Martinez-Murillo F, Rosen EM and Laterra J: Regulation of
c-Met-dependent gene expression by PTEN. Oncogene. 23:9173–9182.
2004.PubMed/NCBI
|
|
5
|
Hamano Y and Kalluri R: Tumstatin, the NC1
domain of alpha3 chain of type IV collagen, is an endogenous
inhibitor of pathological angiogenesis and suppresses tumor growth.
Biochem Biophys Res Commun. 333:292–298. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fox SB, Gatter KC, Bicknell R, Going JJ,
Stanton P, Cooke TG and Harris AL: Relationship of endothelial cell
proliferation to tumor vascularity in human breast cancer. Cancer
Res. 53:4161–4163. 1993.PubMed/NCBI
|
|
7
|
D'Amato RJ, Loughnan MS, Flynn E and
Folkman J: Thalidomide is an inhibitor of angiogenesis. Proc Natl
Acad Sci U S A. 91:4082–4085. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bezerra Y, Fuselier JA, Peyman GA, Oner H,
Drouant G and Coy DH: Study of inhibitory effects of an
antiangiogenic somatostatin-camptothecin conjugate on laser-induced
choroidal neovasculrization in rats. Retina. 25:345–354. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Panrazis P, Han Z, Balan K, Wang Y and
Wyche JH: Camptothecin and 9-nitrocamptothecin (9NC) as anti-cancer
anti-HIV and cell-differentiation agents. Development of
resistance, enhancement of 9NC-induced activities and combination
treatments in cell and animal models. Anticancer Res. 23:3623–3638.
2003.PubMed/NCBI
|
|
10
|
Jenkins DC, Charlies IG, Thomsen LL, Moss
DW, Holmes LS, Baylis SA, Rhodes P, Westmore K, Emson PC and
Moncada S: Roles of nitric oxide in tumor growth. Proc Natl Acad
Sci USA. 92:4392–4396. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Klotz T, Bloch W, Jacobs G, Niggemann S,
Engelmann U and Addicks K: Immunolocalization of inducible and
constitutive nitric oxide synthases in human bladder cancer.
Urology. 54:416–419. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang GL, Wang YH, Teng HL and Lin ZB:
Effects of aminoguanidine on nitric oxide production induced by
inflammatory cytokines and endotoxin in cultured rat hepatocytes.
World J Gastroenterol. 7:331–334. 2001.PubMed/NCBI
|
|
13
|
Jenkins DC, Charles IG, Baylis SA, Lelchuk
R, Radomski MW and Moncada S: Human colon cancer cell lines show a
diverse pattern of nitric oxide synthase gene expression and nitric
oxide generation. Br J Cancer. 70:847–849. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sherman PA, Lanbach VE, Reep BR and Wood
ER: Purification and cDNA sequence of an inducible nitric oxide
synthase from a human tumor cell line. Biochemistry.
32:11600–11605. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Franchi A, Gallo O, Paglierani M, Sardi I,
Magnelli L, Masini E and Santucci M: Inducible nitric oxide
synthase expression in laryngeal neoplasia: Correlation with
angiogenesis. Head Neck. 24:16–23. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Son HJ, Kim YH, Park DI, Kim JJ, Rhee PL,
Paik SW, Choi KW, Song SY and Rhee JC: Interaction between
cyclooxygenase-2 and inducible nitric oxide synthase in gastric
cancer. J Clin Gastroenterol. 33:383–388. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ray GN, Shahid M and Hasain SA: Effect of
nitric oxide and malondialdehyde on sister-chromatid exchanges in
breast cancer. Br J Biomed Sci. 58:169–176. 2001.PubMed/NCBI
|
|
18
|
Chiou WF, Chou CJ and Chen CF:
Camptothecin suppresses nitric oxide biosynthesis in RAW 264.7
macrophages. Life Sci. 69:625–635. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Green LC, Wagner DA, Glogowski J, Skipper
PL, Wishnok JS and Tannenbaum SR: Analysis of nitrate, nitrite and
15 Nnitrate in biological fluids. Anal Biochem. 126:131–138. 1982.
View Article : Google Scholar : PubMed/NCBI
|