Introduction
Cardiac tumors are divided into primary and
secondary tumors. Primary cardiac tumors are rare with a global
incidence of 0.0017–0.019% (1,2).
Approximately 75% of primary cardiac tumors are benign, while ~25%
are malignant (3). Among malignant
cardiac sarcomas, angiosarcoma is the most frequent type and
primary malignant fibrous histiocytoma (MFH) is the second most
common type, with an incidence of 3% (4). Cardiac sarcomas are often asymptomatic
until the tumor causes an obstruction to blood flow and/or
metastasizes to distant tissues (5).
Therefore, the symptoms of affected patients depends on the
location, size and degree of invasiveness of the tumor of the
heart, and any distant metastasis (5). The majority of cardiac tumors are
identified by transthoracic and/or transesophageal echocardiography
(6). Computed tomography (CT) and
magnetic resonance imaging (MRI) are also important to establish
the extent of invasion and metastases (7). Cardiac tumors may occur at any position
of the heart, however, primary MFH of the heart is typically found
in the left atrium (1,3).
MFH is an undifferentiated sarcoma composed of
pleomorphic spindle and epithelioid cells with a small number of
multinucleated cells. MFH is significantly more invasive than a
benign tumor, and recurrence and distant metastasis are common,
often leading to an extremely poor prognosis (8,9). Surgical
resection is the standard treatment for MFH patients, and ideal
resection involves removal of the whole tumor and a section of the
normal cardiac tissue (10). However,
multimodality treatment including radiotherapy and adjuvant
chemotherapy may achieve a better outcome (11). Mean survival for the majority of cases
of cardiac sarcoma is 9–11 months (12). A recent study revealed that the mean
survival time of MFH patients following resection (incomplete or
complete) was 22.2 ± 6.1 months (range, 0.6–36.0 months) with 1-
and 2-year survival rates of 82.5 and 41.3%, respectively (12). In the current study, the case of a
patient exhibiting a giant, rarely observed MFH of the heart with
vulvar metastases is presented. Written informed consent was
obtained from the patient's family.
Case report
In March 2009, a 37-year-old female presented to the
Yantai Yuhuangding Hospital (Yantai, Shandong, China) with a
3-month history of progressively increasing exertional dyspnea that
was relieved following rest, and a 2-day history of worsening
dyspnea. Physical examination identified a blood pressure of 140/80
mmHg (normal range, 100–120/60–80 mmHg), a body temperature of
37.0°C (normal range, 36.2–37.2°C) and a heart rate of 99 beats per
minute (bpm; normal range, 60–100 bpm) with a regular rhythm;
however, a diastolic murmur was observed upon cardiac auscultation.
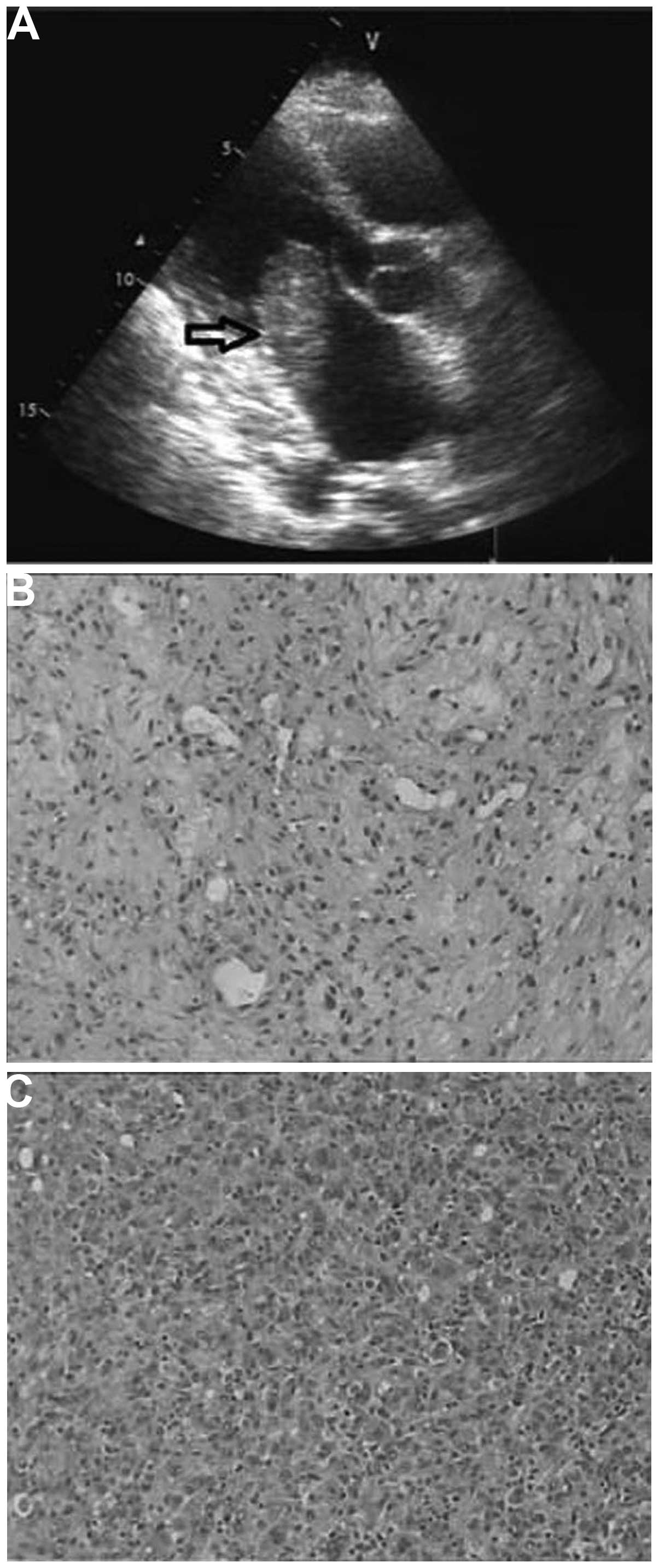
Subsequent echocardiography revealed a 5.0×2.3-cm mobile mass in
the left atrium with a short-wide stalk attached to the posterior
wall (Fig. 1A), widely attached to
the anterior mitral valve and with a small pericardial effusion.
During diastole, the mass protruded through the mitral valve
resulting in functional mitral stenosis with a peak gradient of 38
mmHg (normal range, <30 mmHg). Various auxiliary examinations,
including abdomen and brain CT and chest X-rays, showed no signs of
tumor in other organs. The initial diagnosis was a left atrial
myxoma. Upon pre-operative echocardiography, the left ventricle
ejection fraction and pulmonary artery systolic pressure were 63%
(normal range, 50–75%) and 57 mmHg (normal range, <30 mmHg),
respectively.
The gold standard therapy for atrial myxoma is
complete surgical removal of the tumor (10). In the present case, open-heart surgery
with access via a median sternotomy and cardiopulmonary bypass was
performed under cardioplegic arrest. At the incision site of the
pericardium, the pericardial effusion was yellowish and clear. The
mass appeared grey-white in color and crisp in texture, with a
short-wide pedicle attached to the posterior wall, extending into
the pericardium. Frozen section pathological examination revealed a
diagnosis of MFH; the tumor was heterogenous, composed of spindle
and epithelioid cells, interspersed with giant cells. Surgery
achieved a complete resection of the tumor of the left atrium, as
well as a partial resection of the left atrium adhering to the
tumor pedicle and peripheral adipose tissue.
Upon light microscopy (Fig. 1B), a malignant mesenchymal tumor with
areas of necrosis was identified. The tumor was composed of highly
pleomorphic spindle and epithelioid cells, with a small number of
multinucleated cells also identified. Immunohistochemistry
(Fig. 1C) was positive for cluster of
differentiation (CD)99 and vimentin, and partially positive for
CD68. The cells were immunonegative for cytokeratin, epithelial
membrane antigen, smooth muscle actin, actin, CD31 and CD34. On the
basis of the aforementioned histomorphological and
immunohistochemical findings, a diagnosis of MFH was formed.
Following cardiac surgery, the patient began six
three-weekcycles of chemotherapy (1.2 mg/m2 ifosfamide,
day 1; 60 mg/m2 epidoxorubicin, day 1) at the Department
of Oncology; however, after 1 month, the patient noticed a mass on
the left side of the vulva, with no pain. The mass had no capsule
(Fig. 2A and B) and was resected
under local anesthesia. Histopathology indicated a diagnosis of
metastatic MFH (Fig. 2C). A chest
X-ray performed after vulval surgery revealed left-sided pleural
effusion. Following surgical excision of the vulvar tumor and a
left thoracentesis, the patient immediately began four three-weekly
cycles of chemotherapy (120 mg paclitaxel, day 1; 1.6
g/m2 gemcitabine, day 1) at the Department of Oncology.
However, the combination of surgery and chemotherapy proved to be
ineffective, and the patient succumbed due to local recurrence 6
months later.
Discussion
Primary cardiac malignant neoplasmare rare tumors
that account for 25% of all primary cardiac tumors (1–3). The
prevalence of primary cardiac sarcomas was found to be 0.0017% at
autopsy in a large study of six hospitals in the USA, conducted by
the American Medical Association (1)
and the overall global incidence rate has been recorded as
0.001–0.030% (13,14). MFH of the heart in particular has an
incidence of 3% of all malignant cardiac sarcomas (4), and typically occurs in the left atrium
(3). MFH has high metastatic
potential and can spread to various tissues and viscera, including
the liver, bones, arms, skeletal muscles, lungs and brain (11,15–18).
However, to the best of our knowledge, there have been no previous
reports of primary MFH of the heart with vulvar metastases. MFH of
the vulva is a rare type of vulvar sarcoma, constituting <1% of
all vulvar sarcomas (19,20). In 2011, Iwakawa et al reported
that there had been nine case reports of MFH of the vulva to date
(21) and to the best of our
knowledge, no other cases have been reported since.
Echocardiography is a sensitive and non-invasive
modality for assessing cardiac tumors, including cardiac MFH;
however, it has a low specificity, which may lead to misdiagnosis
(6). The initial diagnosis of the
current case was left atrial myxoma. The short-wide stalk and the
small amount of pericardial effusion observed in the present case
may act as an indicator of malignant disease. CT scans and MRI are
also effective tools for assessing cardiac tumors, and facilitate
the identification of more specific structures of tumors and
adjacent invasions (7). The symptoms
of patients depend on the location, size and degree of invasiveness
of the cardiac tumor. In the current case, the mass protruded
through the mitral valve during diastole, resulting in functional
mitral stenosis that initiated the onset of dyspnea. The gold
standard therapy for cardiac MHF patients without metastases is
complete surgical resection of the tumor, with metastasis being the
only factor identified to impact survival rate in the literature
(22–24).
Ideal resection involves removal of the whole tumor
and a portion of the normal cardiac tissue. Mean survival for the
majority of cases of cardiac sarcoma is 9–11 months (12). In the current case, complete resection
of the left atrial tumor and a partial resection of the left atrial
wall and pericardium were performed. However, the tumor
metastasized to the vulva 1 month later and the patient succumbed
due to local recurrence 13 months after diagnosis. It is
hypothesized that adjuvant therapy may be indispensable to prevent
local recurrence and distant metastasis, as well as provide
improved survival, even in patients that have undergone complete
resection (25).
In conclusion, the current study reported a rare
case of a cardiac tumor. Echocardiography initially prompted a
diagnosis of a left atrial myxoma, however, the post-operative
histopathological results determined a diagnosis of MFH, and vulvar
metastases were later identified. Multicenter cooperation between
cardiac surgeons, cardiologists and oncologists may lead to the
identification of an optimal treatment strategy for cardiac MFH,
which would improve patient quality of life and increase patient
survival rates.
References
|
1
|
Straus R and Merliss R: Primary tumor of
the heart. Arch Pathol. 39:74–78. 1945.
|
|
2
|
Neragi-Miandoab S, Kim J and Vlahakes GJ:
Malignant tumours of the heart: A review of tumour type, diagnosis
and therapy. Clin Oncol (R Coll Radiol). 19:748–756. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Burke AP, Cowan D and Virmani R: Primary
sarcomas of the heart. Cancer. 69:387–395. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Stevens CW, Sears-Rogan P, Bitterman P and
Torrisi J: Treatment of malignant fibrous histiocytoma of the
heart. Cancer. 69:956–961. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Okby NT and Travis WD: Liposarcoma of the
pleural cavity: Clinical and pathologic features of 4 cases with a
review of the literature. Arch Pathol Lab Med. 124:699–703.
2000.PubMed/NCBI
|
|
6
|
Grebenc ML, de Christenson ML Rosado,
Burke AP, et al: Primary cardiac and pericardial neoplasms:
Radiologic-pathologic correlation. Radiographics. 20:1073–1103.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Levine RA, Weyman AE, Dinsmore RE, et al:
Noninvasive tissue characterization: Diagnosis of lipomatous
hypertrophy of the atrial septum by nuclear magnetic resonance
imaging. J Am Coll Cardiol. 7:688–692. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fletcher CD, Gustafson P, Rydholm A,
Willén H and Akerman M: Clinicopathologic re-evaluation of 100
malignant fibrous histiocytomas: Prognostic relevance of
subclassification. J Clin Oncol. 19:3045–3050. 2001.PubMed/NCBI
|
|
9
|
Burke A, Jeudy J Jr, Virmani R, Topol EJ
and Califf RM: Cardiac tumours. Textbook of Cardiovascular Medicine
(3rd). Lippincott Williams & Wilkins. (Philadelphia).
7102007.
|
|
10
|
Odim J, Reehal V, Laks H, et al: Surgical
pathology of cardiac tumors. Two decades at an urban institution.
Cardiovasc Pathol. 12:267–270. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Oh SJ, Yeom SY and Kim KH: Clinical
implication of surgical resection for the rare cardiac tumors
involving heart and great vessels. J Korean Med Sci. 28:717–724.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bakaeen FG, Reardon MJ, Coselli JS, Miller
CC, Howell JF, Lawrie GM, Espada R, Ramchandani MK, Noon GP,
Weilbaecher DG and DeBakey ME: Surgical outcome in 85 patients with
primary cardiac tumors. Am J Surg. 186:641–647. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
McAllister H and Fenoglio J: Tumors of the
cardiovascular system. Atlas of Tumor Pathology (2nd Series).
Fascicle 15 American Registry of Pathology/Armed Forces Institute
of Pathology. (Washington, D.C.). 1–3. 1978.
|
|
14
|
Yu K, Liu Y, Wang H, Hu S and Long C:
Epidemiological and pathological characteristics of cardiac tumors:
A clinical study of 242 cases. Interact Cardiovasc Thorac Surg.
6:636–639. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hsieh SC, Chen CY and Chan WP: Malignant
fibrous histiocytoma of the heart: A case report. Acta Cardiol.
65:85–87. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sheikh AA, Ahmad M, Lone AR and Banday MA:
Cardiac metastasis in malignant fibrous histiocytoma. Saudi Med J.
29:1041–1043. 2008.PubMed/NCBI
|
|
17
|
Milicic D, Juretic A, Bulum J, Saric N,
Bisof V, Jelic I and Jelasic D: Primary malignant fibrous
histiocytoma of the heart with skeletal muscles metastases. J Card
Surg. 22:513–516. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pimentel J, Fernandes AC, Silva R, Ferro J
and Cattoni B: Brain metastases of a malignant fibrous histiocytoma
presenting as an acute cerebral hemorrhage. Clin Neuropathol.
20:64–69. 2001.PubMed/NCBI
|
|
19
|
Grisaru D, Peyser MR, Bernstein-Lipschitz
L, Yaron Y and Lessing JB: Malignant fibrous histiocytoma with
myofibroblastic differentiation of the vulva in an adolescent
female. Eur J Obstet Gynecol Reprod Biol. 79:219–221. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Taylor RN, Bottles K, Miller TR and Braga
CA: Malignant fibrous histiocytoma of the vulva. Obstet Gynecol.
66:145–148. 1985.PubMed/NCBI
|
|
21
|
Iwakawa T, Tsuji T, Hamada T, Kamio M,
Matsuo T, Yoshinaga M, Kitajima S and Douchi T: Pleomorphic type of
malignant fibrous histiocytoma with myxoid stroma of the vulva in a
young woman. J Obstet Gynaecol Res. 37:1474–1477. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Park BJ, Bacchetta M, Bains MS, Downey RJ,
Flores R, Rusch VW and Girardi LN: Surgical management of thoracic
malignancies invading the heart or great vessels. Ann Thorac Surg.
78:1024–1030. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Eckstein R, Gössner W and Rienmüller R:
Primary malignant fibrous histiocytoma of the left atrium. Surgical
and chemotherapeutic management. Br Heart J. 52:354–357. 1984.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kim MP, Correa AM, Blackmon S,
Quiroga-Garza G, Weilbaecher D, Bruckner B, Ramlawi B, Rice DC,
Vaporciyan AA and Reardon MJ: Outcomes after right-side heart
sarcoma resection. Ann Thorac Surg. 91:770–776. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Reardon MJ, Walkes JC and Benjamin R:
Therapy insight: Malignant primary cardiac tumors. Nat Clin Pract
Cardiovasc Med. 3:548–553. 2006. View Article : Google Scholar : PubMed/NCBI
|