Introduction
Thyroid cancer is the most common type of cancer of
the endocrine system, and its incidence has increased in recent
years (1–3). Although a large proportion of patients
with thyroid cancer tend to present a good outcome, ~10% of
patients, who do not receive radioiodine, may experience recurrence
of the disease and mortality (4).
Therefore, sensitive markers for the diagnosis of thyroid cancer,
and efficient target genes for the treatment of this disease, are
required. Despite previous studies on the occurrence of thyroid
carcinoma have been published, the molecular mechanism of this type
of cancer remains to be elucidated (5–7).
Genetic mechanisms are not the only path to gene
disruption in cancer. Pathological epigenetic changes,
non-sequence-based alterations that are inherited through cell
division, are increasingly being considered as alternatives to
mutations and chromosomal alterations in disrupting gene function
(8). Certain histone demethylases
have been proved to be involved in tumor metastasis (9). Previous studies have demonstrated that
KDM5B histone demethylase controlled epithelial-mesenchymal
transition of cancer cells by regulating the expression of the
microRNA-200 family (10), and
histone demethylase KDM6B promoted epithelialmesenchymal transition
(11). Retinoblastoma binding protein
2 (RBP2) was initially identified as a retinoblastoma binding
protein that exhibited histone 3 lysine 4 (H3K4) demethylation
activity (12,13). It is a member of the Jumonji/AT-rich
interactive domain (JARID) protein family, an important epigenetic
molecule, has been implicated in cancer and other diseases
(14,15). Although it has been shown that RBP2 is
highly expressed in a number of types of cancer (16–19) and
that the deregulation of RBP2 may result in human diseases,
especially developmental disorders (14), there remains a lack of studies into
the pathological roles of RBP2 in thyroid carcinoma. Therefore, the
present study aimed to characterize the role of RBP2 in thyroid
tumors.
Materials and methods
Cell culture
Papillary thyroid carcinoma K1 cells were purchased
from The European Collection of Cell Cultures (Salisbury, UK). The
cells were cultured in Dulbecco's Modified Eagle's medium (DMEM)
(Gibco Life Technologies, Carlsbad, CA, USA), supplemented with 10%
fetal bovine serum (FBS) (GE Healthcare Life Sciences, Logan, CA,
USA), glutamine (2 mM), penicillin (100 U/ml) and streptomycin (100
mg/ml), and incubated at 37°C with 5% CO2.
Transfection of small interfering RNA
(siRNA)
The sequences of the siRNA targeting RBP2
(RBP2-siRNA) and the non-targeting scrambled siRNA (Shanghai
GenePharma Co., Ltd., Shanghai, China), used as a negative control,
were as follows: 5′-CCA GCA CCA CCU CCU UCC UUC AUAA-3′ and 5′-UUC
UCC GAA CGU GUC ACG UTT-3′, respectively. K1 cells (1.8×105) were
seeded in 6-well plates, and incubated for 18 h, prior to being
transfected with the RBP2-siRNA (siRNA group) or negative control
siRNA, using HiPerFect Transfection Reagent (Qiagen GmbH, Hilden,
Germany). Cells without transfecting were regarded as blank
group.
Immunocytochemistry (ICC) staining of
cell slides
Protein expression of RBP2 was detected by ICC
staining of K1 cells seeded on cover slips. For the experiment,
~2×105 K1 cells were added to each cover slip in a 6-well plate,
and incubated for 4 h, to allow the cells to adhere to the cover
slip in situ. Next, 3–4 ml complete culture medium (DMEM
supplemented with 10% FBS, 100 U/ml penicillin and 100 µg/ml
streptomycin) was added to each well, and the cells were cultured
for additional 36 h. The cells were then fixed with 4% (v/v)
paraformaldehyde for ≥20 min. The ICC was performed as previously
described by Kong et al (20)
using the following antibodies: Monoclonal rabbit-anti-human-RBP2,
(Cell Signaling Technology, Danvers, MA, USA) and horseradish
peroxidase-conjugated anti-rabbit secondary antibody (ZSGD-BIO,
Peking, China). The brown color observed in the cells nuclei was
regarded as positive expression of RBP2. Positive cells were
counted using an eyepiece graticule at ×400 magnification (Olympus,
Tokyo, Japan). For each slide, ≥10 views were counted. Each
experiment was performed in triplicate.
Total RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
analysis
RT-qPCR analysis was performed at 48 h
post-transfection. TRIzol reagent (Invitrogen Life Technologies)
was used to extract the total RNA content from the transfected
cells, and 1.5 µg RNA was used for the synthesis of the first
strand of cDNA with a reverse transcriptase (Promega Corporation,
Madison, WI, USA). The relative mRNA levels of RBP2 were normalized
to the levels of β-actin, and normalized using the formula
2−ΔΔCT with SYBR Green qPCR Kit (Roche Diagnostics,
Basel, Switzerland). The cycling conditions were as follows: 95°C,
5 min, 1 cycle; followed by 35 cycles of 95°C for 5 sec, 50°C for
30 sec and 72°C for 32 sec. Specific primer pairs (Shanghai
GenePharma Co., Ltd., Shanghai, China) were used for RBP2 (forward,
5′-GCT GCT GCA GCC AAA GTTG-3′; and reverse, 5′-AGC ATC TGC TAA CTG
GTC-3′) and β-actin (forward, 5′-GAG CAA GAG AGG CAT CCTCA-3′; and
reverse, 5′-AGC CTG GAT AGC AAC GTACA-3′).
Western blot analysis
Cells were harvested 48 h post-transfection, and
lysed with RIPA buffer (Beyotime Institute of Biotechnology,
Haimen, China). The protein concentration in the cell lysates was
quantified by BCA assay. The proteins were separated by 10%
SDS-PAGE, and subsequently transferred onto polyvinylidene fluoride
membranes (Bio-Rad Laboratories, Inc., Hercules, USA). The
membranes were incubated with the monoclonal rabbit-anti-human
primary antibodies (dilution 1:100; Cell Signaling Technology,
Inc., Danvers, USA) at 4°C for 24 h. Next, the membranes were
washed with Tris-buffered saline and Tween 20 (TBST) for three
times, and incubated with goat anti-rabbit HRP-conjugated secondary
antibody (Gene Tech Co., Ltd., Shanghai, China). Proteins were
detected using ECL Plus (Boster, Wuhan, China).
Cell counting Kit-8 (CCK-8) assay
CCK-8 assay (Dojindo Molecular Technologies, Inc.,
Kumamoto, Japan) was performed to evaluate the effect of RBP2-siRNA
on cell proliferation. K1 cells transfected with RBP2-siRNA or
neagtive control siRNA were seeded into 96-well plates at a density
of 3.8×103 cells/well, and cultured for 24, 48 and 72 h. At the
time points indicated above, 90 µl DMEM and 10 µl CCK-8 were added
to each well, and the cells were incubated for additional 2 h.
Subsequently, the supernatant was removed, and the absorbance at
450 nm wavelength was recorded using a microplate reader (Bio-Rad
Laboratories, Inc.). Those wells containing 10% CCK-8 (DMEM 90 µl
and 10 µl CCK-8) were regarded as blanks. The experiment was
performed with 6 repeated measures of each experimental value, and
repeated 3 times.
Invasion and migration assays
The invasion ability of K1 cells was evaluated by
Transwell chamber assay (Corning Life Sciences, New York, USA). The
chamber was covered with 50 µl Matrigel Basement Membrane Matrix (2
mg/ml; BD Biosciences, Franklin Lakes, NJ, USA). Serum-free DMEM
(200 µl) containing 2×104 cells was added to the upper chamber,
while the lower chamber was filled with 600 µl DMEM supplemented
with 15% FBS. Following incubation for 20 h, the cells on the upper
chamber were removed, and the remaining cells were fixed with
methanol for 20 min, prior to being dyed with 0.1% crystal violet
(Sigma-Aldrich, St. Louis, MO, USA). The migration assay was
conducted following the aforementioned steps, except for the
absence of the Matrigel Basement Membrane Matrix layer, and
~3.5×104 cells were added to the upper chamber. Each experiment was
performed in triplicate and repeated 3 times.
Soft agar colony formation assay
The capacity of the K1 cells to form colonies was
assessed by soft agar colony formation assay conducted in 6-well
plates. In each well, a base layer was created with 0.7% soft agar,
and a top layer was subsequently added, which contained 0.35% soft
agar and 103 suspended cells. The cells were cultured for ~14 days,
until the newly formed colonies were visible. The experiment was
performed in triplicate and repeated 3 times.
Statistical analysis
The data were evaluated by the Student's t-test for
2 groups, or analysis of variance for ≥3 groups, using SPSS
software, version 13.0 (SPSS, Inc., Chicago, IL, USA). P<0.05
was considered to indicate a statistically significant
difference.
Results
Downregulation of RBP2 by
RBP2-siRNA
RBP2-siRNA and negative control siRNA were
transfected into thyroid carcinoma K1 cells. The fluorescence
emission of green fluorescent protein in the cytoplasm of the
transfected cells was recorded and photographed (Fig. 1A). The protein expression levels of
RBP2 were detected by ICC analysis in the blank (cells without
transfecting), RBP2-siRNA-transfected and negative control
siRNA-transfected cells. The results indicated that the
transfection of RBP2-siRNA into K1 cells suppressed the expression
of RBP2 in these cells (Fig. 2A). The
expression levels of RBP2 in the nuclei of the
RBP2-siRNA-transfected cells were lower than in the blank and
negative control cells (F=26.754, P<0.01; Fig. 1B). No significant difference was
observed between the blank and control groups (P>0.05).
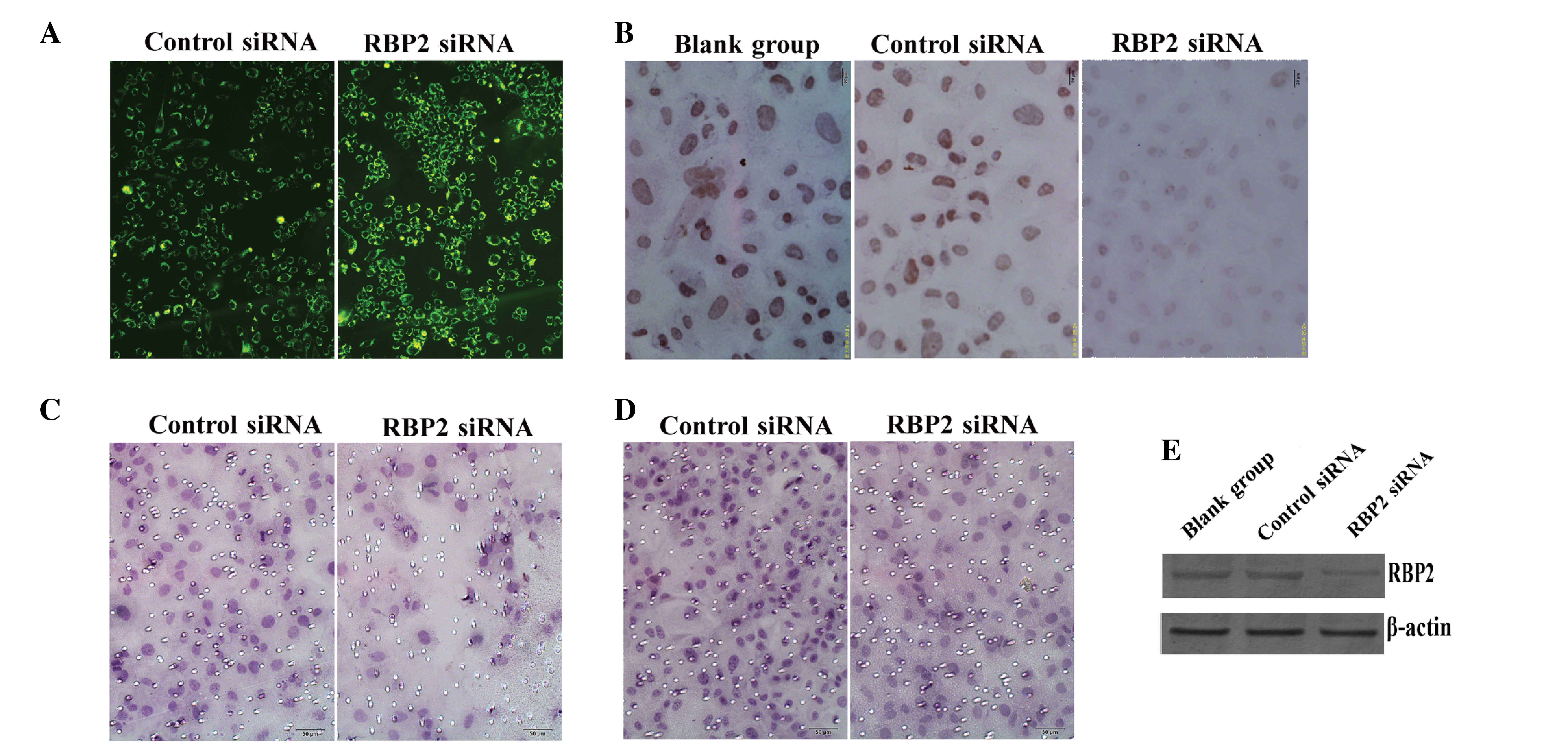 | Figure 1.(A) The fluorescence emission of
thyroid carcinoma K1 cells transfected with control siRNA and
RBP2-siRNA was recorded (magnification, ×200). (B) The protein
expression levels of RBP2 were lower in the RBP2-siRNA-transfected
cells than in the blank and negative control cells, according to
the results of immunocytochemical analysis (magnification, ×400).
(C) Transwell assay revealed the presence of invasive cells through
the membrane in the control and RBP2-siRNA groups (magnification,
×200). (D) The cell migration ability was lower in the
RBP2-siRNA-transfected cells, compared with the control cells, as
evaluated by transwell assay (magnification, ×200). (E) Western
blot analysis demonstrated that the protein expression levels of
RBP2 were markedly reduced in the RBP2-siRNA group, compared with
the blank and negative control groups. β-actin was used as
reference. siRNA, small interfering RNA; RBP2, retinoblastoma
binding protein 2; RBP2-siRNA, siRNA targeting RBP2; blank-group,
non transfected cells. |
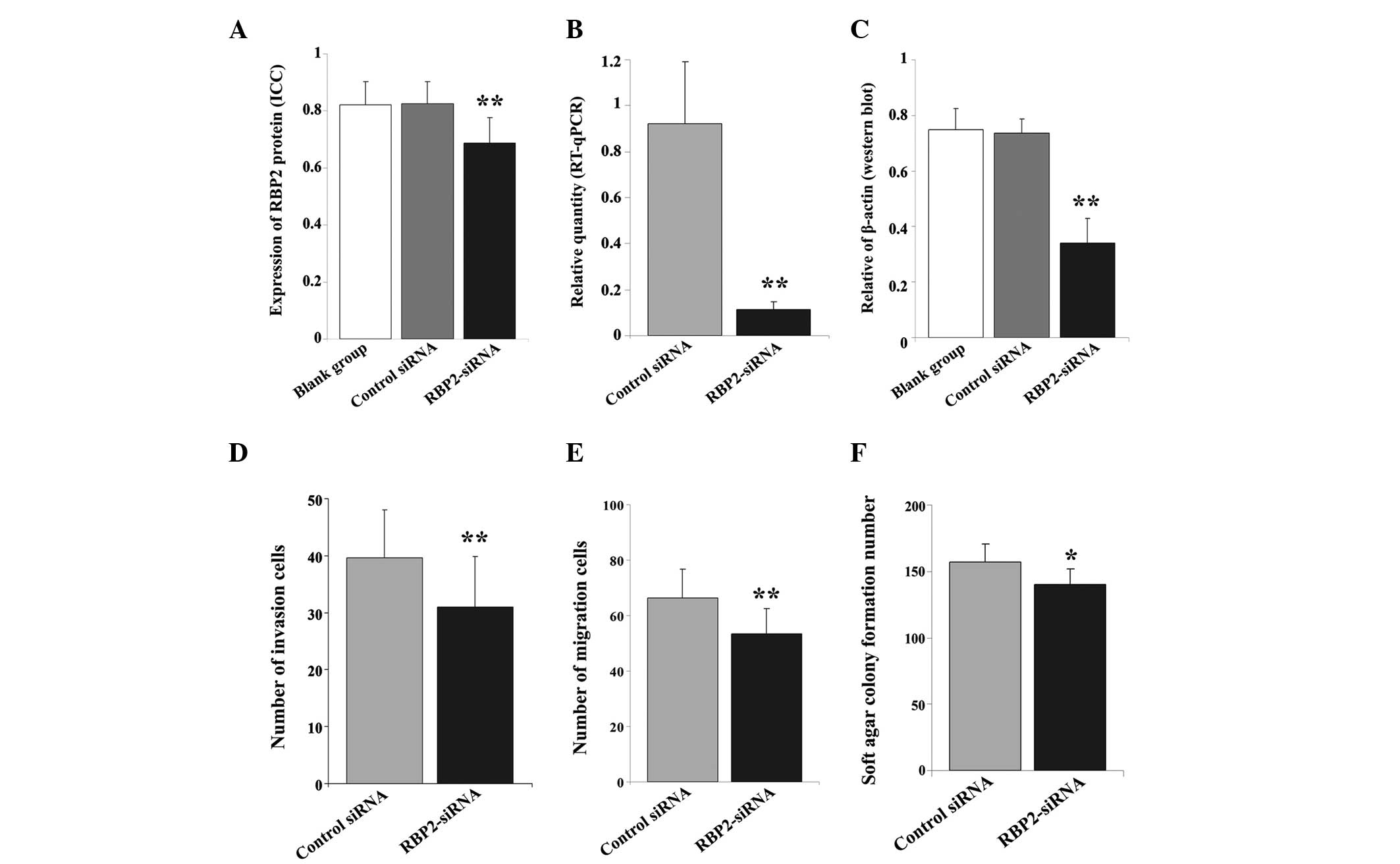 | Figure 2.(A) Immunocytochemical and (B) RT-qPCR
analyses revealed a marked difference in the (A) protein and (B)
mRNA expression levels of RBP2 in thyroid carcinoma K1 cells
transfected with RBP2-siRNA, compared with the control cells. (C)
Western blot analysis demonstrated a significant difference in the
protein levels of RBP2 across the 3 groups of cells. β-actin was
used as reference. Transwell assay identified a lower number of (D)
invasive and (E) migrated cells in the RBP2-siRNA-transfected
cells, compared with the control group. (F) The transfection with
RBP2-siRNA affected the tumorigenicity ability of K1 cells, since a
lower number of newly formed colonies were observed in the
RBP2-siRNA-transfected group, compared with the control group.
RT-qPCR, reverse transcription-quantitative polymerase chain
reaction; RBP2, retinoblastoma binding protein 2; siRNA, small
interfering RNA; RBP2-siRNA, siRNA targeting RBP2; control siRNA,
negative control siRNA targeting RBP2; blank group, non transfected
cells. **P<0.01 vs. blank group, negative control; *P<0.05
vs. negative control. |
To confirm the above data, the mRNA levels of RBP2
were quantified by RT-qPCR. The transfection of RBP2-siRNA into K1
cells significantly reduced the mRNA expression levels of RBP2 in
these cells (F=31.385, P<0.01; Fig.
3). Compared with the control group, RBP2-siRNA notably
downregulated the mRNA levels of RBP2 (t=8.869, P<0.01; Fig. 2B). The protein levels of RBP2 were
measured by western blot analysis, using β-actin as reference. The
results presented in Fig. 1E
indicated that compared with the blank cells, the expression of
RBP2 was notably inhibited in the RBP2-siRNA-transfected cells
(F=60.835, P=0.000; Fig. 2C).
Downregulation of RBP2 suppressed the
proliferation ability of K1 cells
The effect of RBP2 on cell proliferation ability was
estimated by CCK-8 assay. The data revealed that, compared with the
control cells, the optical density of the cells transfected with
RBP2-siRNA was significantly reduced at 24, 48 and 72 h
post-transfection (OD=0.325±0.067, t=7.650, P<0.01;
OD=0.605±0.118, t=2.606, P=0.016; and OD=0.906±0.070, t=2.377,
P=0.027, respectively; Table I).
 | Table I.Optical density values (mean ±
standard deviation) of Cell Counting kit-8 assay. |
Table I.
Optical density values (mean ±
standard deviation) of Cell Counting kit-8 assay.
| Cell groups | 24 h | 48 h | 72 h |
|---|
| Control siRNA | 0.507±0.048 | 0.714±0.083 | 0.978±0.078 |
| RBP2-siRNA | 0.325±0.067 | 0.605±0.118 | 0.906±0.070 |
| t/p | 7.650/0.000 | 2.606/0.016 | 2.377/0.027 |
Downregulation of RBP2 reduced the
invasion and migration ability of K1 cells in vitro
The invasion ability of K1 cells was evaluated by
transwell assay. Compared with the siRNA control group, the number
of invasive cells on the RBP2-siRNA-transfected group was
significantly reduced (39.67±8.36 vs. 30.96±8.94; t=4.774, P=0.000)
as indicated in Figs. 1C and 2D. Similarly, the number of migrated cells
was also notably reduced (66.44±10.30 vs. 53.53±8.89; t=6.366,
P=0.000), as indicated in Figs. 1D
and 2E.
Transfection of RBP2-siRNA inhibited
the colony formation ability of K1 cells
The in vitro tumorigenicity ability of K1
cells transfected with RBP2-siRNA was estimated by soft agar colony
formation assay. Compared with the control group (Fig. 4A), the number of newly formed colonies
observed in the RBP2-siRNA-transfected group was reduced (t=2.749,
P=0.014; Figs. 2F and 4B).
Discussion
Genetic and epigenetic modifications have been
demonstrated to be involved in the tumorigenesis and development of
cancer (21). Therefore, the stable
inheritance of epigenetic states is required for the cells and
tissues in the body to maintain their specific functions (21). The alteration of epigenetic
modifications has been mainly associated with the methylation of
DNA and modifications of histones (22,23). RBP2
has been recently identified as a histone demethylase, which
regulates gene expression by demethylating H3K4me3/2 (24,25). RBP2
influences cell cycle withdrawal and cell differentiation via
retinoblastoma protein (pRB) (13).
Previous studies have demonstrated that RBP2 represses the
transcription of its target genes, which are activated by pRB, by
associating with the promoters of these actively transcribed genes
(14,26). Previous studies have observed that
RBP2 was preferentially bound to the proximal promoter regions of
p27, cyclin D1, and integrin β1, whereby RBP2 repressed the
expression of p27, but acted as an activator of the gene expression
for cyclin D1 and integrin β1 (27).
In agreement with these results, previous studies have reported
that knocking down RBP2 led to overexpression of p27 (12). Since RBP2 occupies and regulates the
promoters of multiple genes containing H3K4me3, RBP2 is likely to
be involved in cancer progression (15). Previous studies have revealed that
RBP2 was overexpressed in numerous types of cancer, including
hepatocellular, non-small cell lung, breast and gastric cancer
(16–19). Previous studies have demonstrated that
RBP2 was overexpressed in hepatocellular carcinoma cells, which was
downregulated by homo sapiens microRNA-212 (hsa-miR-212). In
addition to inhibiting the expression of RBP2, the overexpression
of hsa-miR-212 in hepatocellular carcinoma cells reduced the cell
proliferation ability and induced cellular senescence (16). Furthermore, Wang et al
(17) observed that RBP2 was also
overexpressed in non-small cell lung cancer, whereby regulated the
expression of E- and N-cadherin via the activation of Akt
signaling. Previous studies also reported the overexpression of
RBP2 in human lung cancer, and revealed that RBP2 promoted
proliferation, motility, migration, invasion and metastasis in lung
cancer cells; whereas depletion of RBP2 inhibited proliferation,
motility, migration, invasion and metastasis in these cells
(17). Similarly, previous studies
observed that RBP2 was critical for the progression and metastasis
of breast cancer, since RBP2 acted as a pleiotropic positive
regulator of several genes involved in metastasis (18,27). In
gastric cancer, RBP2 was also observed to be overexpressed, and
directly regulated by miR-212 (19).
Collectively, these findings indicate that RBP2 may participate in
tumorigenesis, although its mechanism of action remains unclear
(28).
Due to the increasing incidence in thyroid cancer in
recent years (29), clinical markers
for the diagnosis of this condition are urgently required. Previous
studies have indicated that RBP2 was overexpressed in thyroid
carcinoma, and therefore, RBP2 may be considered as a clinical
marker for thyroid cancer (20). In
the present study, the effects of RBP2-siRNA on the biological
behavior of thyroid carcinoma cells, including cell proliferation,
invasion and colony formation abilities, were investigated in
vitro. The ICC results indicated that RBP2-siRNA significantly
suppressed the expression of RBP2 in RBP2-siRNA-transfected cells.
Similarly, the mRNA and protein levels of RBP2 were notably reduced
in these cells. The transfection with RBP2-siRNA also reduced the
proliferation, migration, invasion and tumorigenicity abilities of
the transfected cells. In summary, downregulation of RBP2 via
RBP2-siRNA reduced the expression levels of RBP2, and inhibited the
proliferation, invasion and tumorigenicity abilities of thyroid
carcinoma cells. These data indicate that RBP2 may be a therapeutic
target for the inhibition of tumor progression and metastasis in
thyroid cancer.
In conclusion, the results of the present study
indicate that downregulation of RBP2 notably suppressed the
biological behavior of thyroid carcinoma K1 cells. Knocking down
RBP2 markedly affected the mRNA expression levels of RBP2 in
RBP2-siRNA-transfected K1 cells, and inhibited the proliferation,
migration, invasion and colony formation abilities of these cells.
In summary, RBP2 appears to be essential for thyroid carcinoma
cells to maintain their malignant behavior. Therefore, targeting
RBP2 may be an efficient approach to control thyroid carcinoma.
Acknowledgements
The present study was supported by the Natural
Science Foundation of Shandong (Shandong, China) (grant nos.
ZR2011HM057 and ZR2009CM070).
References
|
1
|
Sherman SI: Thyroid carcinoma. Lancet.
361:501–511. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Davies L and Welch HG: Current thyroid
cancer trends in the United States. JAMA Otolaryngol Head Neck
Surg. 40:317–322. 2014. View Article : Google Scholar
|
|
3
|
Moura MA, Bergmann A, Aguiar SS and Thuler
LC: The magnitude of the association between smoking and the risk
of developing cancer in Brazil: A multicenter study. BMJ Open.
4:e0037362014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pilli T, Prasad KV, Jayarama S, Pacini F
and Prabhakar BS: Potential utility and limitations of thyroid
cancer cell lines as models for studying thyroid cancer. Thyroid.
19:1333–1342. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Medici M, Porcu E, Pistis G, Teumer A,
Brown SJ, Jensen RA, Rawal R, Roef GL, Plantinga TS, Vermeulen SH,
et al: Identification of novel genetic loci associated with thyroid
peroxidase antibodies and clinical thyroid disease. PLoS Genet.
10:e10041232014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Akeno N, Miller AL, Ma X and
Wikenheiser-Brokamp KA: p53 suppresses carcinoma progression by
inhibiting mTOR pathway activation. Oncogene. 34:589–99. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Moretti S, Menicali E, Voce P, Morelli S,
Cantarelli S, Sponziello M, Colella R, Fallarino F, Orabona C,
Alunno A, et al: Indoleamine 2,3-dioxygenase 1 (IDO1) is
up-regulated in thyroid carcinoma and drives the development of an
immunosuppressant tumor microenvironment. J Clin Endocrinol Metab.
99:E832–E840. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Egger G, Liang G, Aparicio A and Jones PA:
Epigenetics in human disease and prospects for epigenetic therapy.
Nature. 429:457–463. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tiwari N, Tiwari VK, Waldmeier L, Balwierz
PJ, Arnold P, Pachkov M, Meyer-Schaller N, Schübeler D, van
Nimwegen E and Christofori G: Sox4 is a master regulator of
epithelial-mesenchymal transition by controlling Ezh2 expression
and epigenetic reprogramming. Cancer Cell. 23:768–783. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Enkhbaatar Z, Terashima M, Oktyabri D,
Tange S, Ishimura A, Yano S and Suzuki T: KDM5B histone demethylase
controls epithelial-mesenchymal transition of cancer cells by
regulating the expression of the microRNA-200 family. Cell Cycle.
12:2100–2112. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ramadoss S, Chen X and Wang CY: Histone
demethylase KDM6B promotes epithelial-mesenchymal transition. J
Biol Chem. 287:44508–44517. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Klose RJ, Yan Q, Tothova Z, Yamane K,
Erdjument-Bromage H, Tempst P, Gilliland DG, Zhang Y and Kaelin WG
Jr: The retinoblastoma binding protein RBP2 is an H3K4 demethylase.
Cell. 128:889–900. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Christensen J, Agger K, Cloos PA, Pasini
D, Rose S, Sennels L, Rappsilber J, Hansen KH, Salcini AE and Helin
K: RBP2 belongs to a family of demethylases, specific for tri-and
dimethylated lysine 4 on histone 3. Cell. 128:1063–1076. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Benevolenskaya EV, Murray HL, Branton P,
Young RA and Kaelin WG Jr: Binding of pRB to the PHD protein RBP2
promotes cellular differentiation. Mol Cell. 18:623–635. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sharma SV, Lee DY, Li B, Quinlan MP,
Takahashi F, Maheswaran S, McDermott U, Azizian N, Zou L, Fischbach
MA, et al: A chromatin-mediated reversible drug-tolerant state in
cancer cell subpopulations. Cell. 141:69–80. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liang X, Zeng J, Wang L, Fang M, Wang Q,
Zhao M, Xu X, Liu Z, Li W, Liu S, et al: Histone demethylase
retinoblastoma binding protein 2 is overexpressed in hepatocellular
carcinoma and negatively regulated by hsa-miR-212. PLoS One.
8:e697842013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang S, Wang Y, Wu H and Hu L: RBP2
induces epithelial-mesenchymal transition in non-small cell lung
cancer. PLoS One. 8:e847352013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cao J, Liu Z, Cheung WK, Zhao M, Chen SY,
Chan SW, Booth CJ, Nguyen DX and Yan Q: Histone demethylase RBP2 is
critical for breast cancer progression and metastasis. Cell
Reports. 6:868–877. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jiping Z, Ming F, Lixiang W, Xiuming L,
Yuqun S, Han Y, Zhifang L, Yundong S, Shili L, Chunyan C and Jihui
J: MicroRNA-212 inhibits proliferation of gastric cancer by
directly repressing retinoblastoma binding protein 2. J Cell
Biochem. 114:2666–2672. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kong L, Zhang G, Wang X, Zhou J, Hou S and
Cui W: Immunohistochemical expression of RBP2 and LSD1 in papillary
thyroid carcinoma. Rom J Morphol Embryol. 54:499–503.
2013.PubMed/NCBI
|
|
21
|
Feinberg AP, Ohlsson R and Henikoff S: The
epigenetic progenitor origin of human cancer. Nat Rev Genet.
7:21–33. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Varier RA and Timmers HT: Histone lysine
methylation and demethylation pathways in cancer. Biochim Biophys
Acta. 1815:75–89. 2011.PubMed/NCBI
|
|
23
|
Fang Y, Xiao F, An Z and Hao L: Systematic
review on the relationship between genetic polymorphisms of
methylenetetrahydrofolate reductase and esophageal squamous cell
carcinoma. Asian Pac J Cancer Prev. 12:1861–1866. 2011.PubMed/NCBI
|
|
24
|
Mikkelsen TS, Ku M, Jaffe DB, Issac B,
Lieberman E, Giannoukos G, Alvarez P, Brockman W, Kim TK, Koche RP,
et al: Genome-wide maps of chromatin state in pluripotent and
lineage-committed cells. Nature. 448:553–560. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Iwase S, Lan F, Bayliss P, de la
Torre-Ubieta L, Huarte M, Qi HH, Whetstine JR, Bonni A, Roberts TM
and Shi Y: The X-linked mental retardation gene SMCX/JARID1C
defines a family of histone H3 lysine 4 demethylases. Cell.
128:1077–1088. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Defeo-Jones D, Huang PS, Jones RE, Haskell
KM, Vuocolo GA, Hanobik MG, Huber HE and Oliff A: Cloning of cDNAs
for cellular proteins that bind to the retinoblastoma gene product.
Nature. 352:251–254. 1991. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Teng YC, Lee CF, Li YS, Chen YR, Hsiao PW,
Chan MY, Lin FM, Huang HD, Chen YT, Jeng YM, et al: Histone
demethylase RBP2 promotes lung tumorigenesis and cancer metastasis.
Cancer Res. 73:4711–4721. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Blair LP, Cao J, Zou MR, Sayegh J and Yan
Q: Epigenetic regulation by lysine demethylase 5 (kdm5) enzymes in
cancer. Cancers (Basel). 3:1383–1404. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lin N, Du XL, Reitzel LR, Xu L and Strugis
EM: impact of enhanced detection on the increase in thyroid cancer
incidence in the United States: Review of incidence trends by
socioeconomic status within the surveillance, epidemiology, and end
results registry, 1980–2008. Thyroid. 23:103–110. 2013. View Article : Google Scholar : PubMed/NCBI
|