Introduction
Radiation has previously been the main therapy in
tumor treatment, and may prolong the survival time of patients by
improving the local control rate of tumors (1). However, for patients with breast,
esophageal, pulmonary and mediastinal lymph node cancer masses
treated by radiation, radiation-induced pulmonary injury frequently
occurs (2,3). Patients may develop sub-acute
pneumonitis or late fibrosis subsequent to radiation exposure, which
are the most common complications of radiotherapy that result in
eventual mortality (3).
Following the treatment of patients by radiation,
acute inflammation may be resolved with the recruitment of
fibroblasts, resulting in interstitial collagen deposition and
alveolar septal thickening during alveolar epithelial regeneration
(4). Endothelial dysfunction is
mainly caused by vascular damage and endothelial barrier injury,
which may activate various pathophysiological cascades (5). Blood plasma permeates the damaged
vascular barrier, and affects structural elements of vessels by
activating the specific receptors (6). This change in tissue homeostasis may, in
turn, lead to a chronic inflammatory response that does not subside
(7). Radiation has previously been
found to change the cell phenotype and tumor microenvironment, and
as a result, an increased number of invasive residual tumor cells
demonstrated higher rates of metastasis (8). However, the pathophysiology of radiation
induced pneumonitis is complicated and remains unclear, although
studies have indicated that inflammatory mediators affect genetic
stability and cause persistent epigenetic alteration (9,10). This
indicates that inflammatory components of the tumor
microenvironment affect fundamental mechanisms responsible for the
generation of metastatic variants (11). Therefore, it was hypothesized that
radiation-induced pulmonary injury may accelerate metastasis in the
cancer patients that received chest radiation.
In the present study, the impact of
radiation-induced pulmonary injury on the lung metastasis of breast
cancer was examined in mice. Furthermore, the present study aimed
to reveal the mechanism by which radiation-induced pulmonary injury
accelerated the lung metastasis of breast cancer, and it has been
hypothesized that there may be a novel method to prevent
radiation-induced pulmonary injury and metastasis subsequent to
radiotherapy.
Materials and methods
In vivo experiments
A total of 24 BALB/c inbred strains of female mice
(age, 4–6 weeks; weight, 16–18 g) were purchased from the
Experimental Animal Center of Wuhan University (Wuhan, Hubei,
China). All animal experiments were approved by the Scientific
Ethics Committee of Renmin Hospital of Wuhan University (approval
no. KF 01–143/03; Wuhan, Hubei, China). The animals were bred in a
barrier-free animal house in the First Clinical College of Wuhan
University Laboratory Animal Center (Wuhan, Hubei, China). All mice
were housed in accordance with the Guide for the Care and Use of
Laboratory Animals (12).
The mice were randomly divided into the radiation
and control groups, with 12 mice in each group. To generate the
mammary cancer model, the mice in each group were injected with
3×105 mammary carcinoma 4T1 tumor cells (American Type
Culture Collection, Manassas, VA, USA) in the right last mammary
gland. For thoracic irradiation, the mice were anesthetized by
intraabdominal administration of chloral hydrate (dose, 400 mg/kg
in mice). The right chest in the radiation group was irradiated by
6 MV X-rays from a linear accelerator (LINAC; Elekta Oncology
Systems, Ltd., Crawley, UK), at a dose of 9 Gy when tumors grew to
measurable sizes, ranging between 3 and 5 mm in diameter, 7 days
subsequent to tumor cell transplantation. The size of the treatment
field was 1.3×0.8 cm.
The body weights of the mice were tested every day
and the survival rate was also evaluated. Following irradiation,
spontaneous lung metastasis was scored every week. The mice were
sacrificed (three/week, every week) and the lungs were removed for
weighing. Metastasis was assessed by observing the appearance of
the lung, and the white round nodules on the surface of the
yellowish lung were counted as metastatic lesions. The regions of
the lungs were separated and fixed in buffered formaldehyde
(Tianjin Kermel Chemical Reagent Co., Ltd, Tianjin, China) for
immunohistochemical analysis.
Immunohistochemistry for detecting the
chemokine (C-X-C motif) ligand 12 (CXCL12)/chemokine (C-X-C motif)
receptor 4 (CXCR4) axis
At the indicated times subsequent to irradiation, 3
or 4 mice from each group were sacrificed by cervical dislocation,
1, 2, 3 or 4 weeks following irradiation. Sections of the lungs
were formalin-fixed and paraffin-embedded. Coronal sections of the
lung were sliced into 5-mm sections, and the slides with the
largest cross section were stained with hematoxylin and eosin. The
slides were evaluated by a pathologist without knowledge of the
treatment administered. Immunohistochemical analysis of CXCL12 and
CXCR4 expression was performed according to the routine protocol
(13). Briefly, 4–5-µm sections were
de-paraffinized in xylene and rehydrated through serial solutions of
ethanol, consisting of 95, 90, 80 and 70% ethanol. Subsequent to
antigen retrieval and blockage of endogenous peroxidase activity,
the sections were incubated with polyclonal mouse or rabbit
anti-CXCL12 (#ab18919; 1:1,000; Abcam, Cambridge, UK) and
monoclonal anti-CXCR4 (#ab124824; 1:1,000; Abcam) antibodies at 4°C
for 8–12 h, followed by detection using 3,3′-diaminobenzidine
coloration.
Statistical analysis
Statistical analysis was performed using SigmaStat
software (Jandel Scientific, San Rafael, CA, USA). All results are
presented as the mean ± standard deviation of at least six
independent experiments. The data of the groups were compared using
non-paired t-test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Development of lethality following
thoracic irradiation
Firstly, the effect of various doses of radiation on
the mice was investigated. Following radiation treatment at a dose
of 9 Gy, the mice did not succumb to pneumonitis, but succumbed, in
general, 4–5 weeks later due to metastasis. In addition, sub-acute
pneumonitis occurred at 2 weeks after irradiation and progressively
increased over the next 2 weeks. Lung metastasis began to appear at
3 weeks. Finally, the possibility that radiation-induced pulmonary
injury may decrease the survival time in mice was investigated. A
passive metastatic model was used in the radiation-treated BALB/c
mice, and the mice that were intrahepatically injected with 4T1
cells 7 days subsequent to radiation treatment demonstrated a
shorter median survival time of 21 days, compared with 27 days in
the mice without radiation treatment. The survival time of the
radiation-treated mice was significantly shorter compared with the
survival time of untreated mice (Fig.
1). In addition, the effect on survival was reflected in a
statistically significant reduction in body weight in the
radiation-treated mice compared with untreated mice (P<0.05;
Fig. 2).
Metastasis formation
It was hypothesized that the mice with
radiation-induced pulmonary injury may possess an increased risk of
lung metastasis and associated cancer progression, which may
contribute to the shorter survival time in radiation-treated mice.
To test this hypothesis, mice were sacrificed weekly subsequent to
the administration of radiation. Lung metastatic progression was
evaluated by measuring the lung wet weight, counting the lung
nodules and histological examination. All mice that succumbed
demonstrated an extensive tumor burden in the lungs, but evident
metastasis was not observed in other organs, including the liver
and spleen, indicating that the mice succumbed to lung metastasis
(Fig. 3). Histological examination
revealed the infiltration of inflammatory cells in the early period
subsequent to radiation and the presence of lung metastatic nodes
at 3 weeks (Fig. 4). The present
results provide evidence that the mice with radiation-induced
pulmonary injury may demonstrate an increased risk of lung
metastasis.
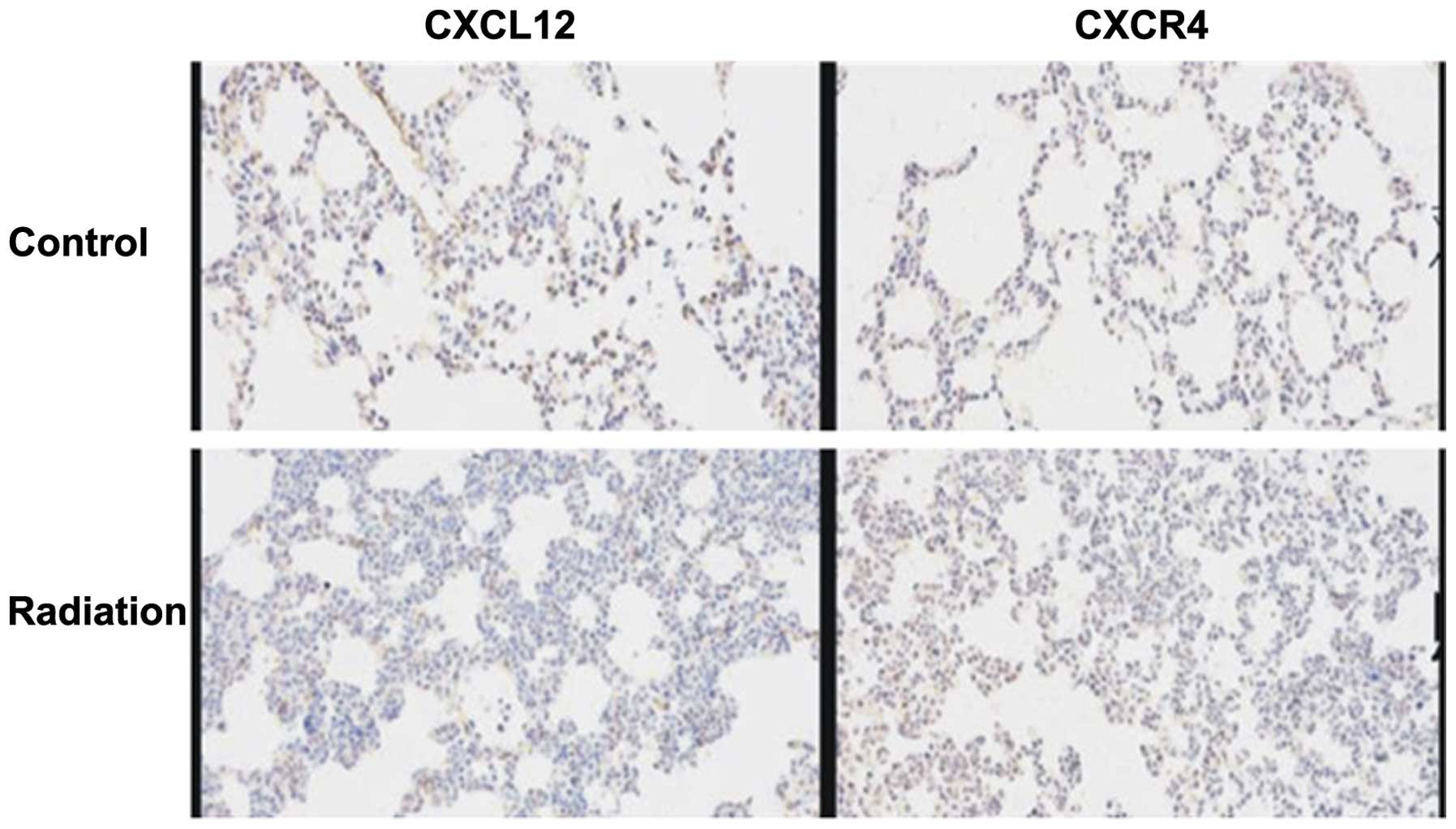
Expression of the CXCL12/CXCR4 axis in
lung tissues of mice
The contribution of the levels of the CXCL12/CXCR4
axis to the metastatic activity of mammary carcinoma 4T1 cells was
investigated. It has been reported that the activation of the
CXCL12/CXCR4 axis was important in the development of
radiation-induced pulmonary fibrosis (14). The CXCL12/CXCR4 axis participated in
the vascularization induced by radiation and was closely associated
with the recurrence and metastasis of breast cancer subsequent to
radiation. In the present study, the expression level of
CXCL12/CXCR4 in treated mice increased markedly following radiation
and was significantly increased compared with normal lung tissues
(Fig. 5). The present results
indicate that the CXCL12/CXCR4 axis was associated with pulmonary
metastasis accelerated by radiation-induced pulmonary injury.
Discussion
To the best of our knowledge, the present study
demonstrated for the first time that radiation-induced pulmonary
injury may accelerate pulmonary metastasis in a passive metastatic
breast cancer BALB/c mouse model. This finding is supported by
studies that have revealed that irradiated fibroblasts accelerate
the invasive growth of non-irradiated adenocarcinoma cells
(15,16). The aforementioned irradiated
lung-induced phenomena were elicited through a bystander mechanism
involving the interaction of cancer and normal cells, as the tumor
itself was not irradiated. This indicated that the effects of
radiation on cancer and normal cells should be considered in cancer
radiotherapy. In the present study, the precise underlying
mechanism of the aforementioned radiation-induced pulmonary
metastasis phenomena was unclear. It was hypothesized that the
activation of surrounding stromal cells and recruitment of various
inflammatory cells subsequent to radiation was involved in the
multistep process of invasion and metastasis. In addition to
promoting carcinogenesis, myelomonocytic cells and the mediators of
these cells affect the key components of the multistep process of
metastasis, including the interaction with the extracellular matrix
and the construction of a pre-metastatic niche (17).
Radiation-induced lung injury may be divided into
two phases, consisting of an acute inflammatory phase and a late
fibrotic phase (18,19). The histopathological changes to the
irradiated lung have been well described (18,20,21). The
inflammatory phase is generally characterized by alveolar cell
depletion and inflammatory cell accumulation (4,22). This
process initiated the activation of specific leukocyte subsets to
produce important biological mediators, including cytokines, growth
factors and chemokines, which participate in the majority of the
aspects of the inflammatory response (4). Bone marrow derived cells are
chemotactically recruited to sites of radiation tissue injury and
fibroblasts are directly involve in the fibrotic pathway. The
fibrotic phase includes fibroblast proliferation, collagen
accumulation and alveolar septal thickening (22). It was hypothesized that the acute
inflammation during the early phase lead to the later fibrosis
phase, but a direct and causal association between early acute
inflammation and the later fibrotic phase has not been established
(23,24). The recruitment and activation of
monocytes, macrophages and lymphocytes is a key component of
radiation-induced lung injury, and chemokines are important
mediators in the pathogenesis of lung injury in several
environments (25,26). Previous studies have reported that the
expression of chemokines and chemokine receptors is elevated in
tumor cells, fibrosis-sensitive mice and patients that have
undergone radiotherapy (27–29). In addition, blockade of the chemokine
ligand and the associated receptors may prevent lung inflammation
and fibrosis in C57BL/6J mice that received thoracic radiation
(30). Therefore, it was hypothesized
that the expression of specific chemokines and chemokine receptors
during the acute inflammatory phase induced by radiation may result
in the recruitment and activation of lymphocytes and macrophages,
which then contribute to the late fibrotic phase.
It has previously been reported that activation of
the chemotactic receptor CXCR4 by the ligand CXCL12 was important
in the development of radiation-induced pulmonary fibrosis
(14). Subsequent to
radiation-induced injury, bone marrow-derived fibroblast progenitor
cells, also termed fibrocytes, which express CXCR4, are recruited
to regions of fibrosis in the lung (25,31). A
neutralizing antibody against CXCL12 may prevent the recruitment of
circulating fibrocytes to radiation-damaged lung and suppress the
development of fibrosis. The CXCL12-CXCR4 axis was also reported to
participate in the vascularization induced by radiation (32), in addition to being closely associated
with recurrence and metastasis subsequent to radiation (33,34).
Previous studies have demonstrated that stromal fibroblast
fractions extracted from a number of invasive human breast
carcinomas were more able to promote the growth of mammary
carcinoma cells and to enhance tumor angiogenesis compared with the
comparable cells derived from outside of these tumor masses
(35,36). Furthermore, high concentrations of
CXCL12 gradients in the lung, liver and lymph nodes more easily
attracted circulating CXCR4-expressing tumor cells to such sites
(37). These findings are not unique
to breast cancer and were also found in other types of tumors
(38–42). According to previous findings, the
CXCL12/CXCR4 axis participates in the pathophysiological process of
metastasis in at least 23 types of tumors (43). High levels of the CXCR4 expression by
various types of human carcinoma cells are clinically associated
with a poor prognosis (44).
Therefore, it was hypothesized that the CXCL12/CXCR4 axis played an
important role in the acceleration of the metastasis of breast
cancer due to radiation. As a result, the expression levels of
CXCL12/CXCR4 were tested in treated mice, and were found to be
increased subsequent to radiation, compared with normal lung
tissues. To a certain extent, this result confirmed the present
hypothesis.
In summary, radiation-induced pulmonary injury leads
to a chronic inflammatory response, which produces an eligible
pre-metastatic microenvironment for cancer cells. It is possible
that the CXCL12/CXCR4 axis affects key elements in the multistep
process of invasion and metastasis. However, additional studies are
required to validate the exact mechanism.
Acknowledgements
The authors thank the doctors and nurses of the
Department of Oncology, Cancer Center, Renmin Hospital of Wuhan
University for their prodigious support.
References
|
1
|
Jeremic B, Fidarova E, Sharma V, Faheem M,
Ameira AA, Ben Nasr Ammar C, Frobe A, Lau F, Brincat S and Jones G:
The International Atomic Energy Agency (IAEA) randomized trial of
palliative treatment of incurable locally advanced non-small cell
lung cancer (NSCLC) using radiotherapy (RT) and chemotherapy (CHT)
in limited resource setting. Radiother Oncol. 116:21–26. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Abid SH, Malhotra V and Perry MC:
Radiation-induced and chemotherapy-induced pulmonary injury. Curr
Opin Oncol. 13:242–248. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mehta V: Radiation pneumonitis and
pulmonary fibrosis in non-small-cell lung cancer: pulmonary
function, prediction, and prevention. Int J Radiat Oncol Biol Phys.
63:5–24. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tsoutsou PG and Koukourakis MI: Radiation
pneumonitis and fibrosis: Mechanisms underlying its pathogenesis
and implications for future research. Int J Radiat Oncol Biol Phys.
66:1281–1293. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ghafoori P, Marks LB, Vujaskovic Z and
Kelsey CR: Radiation-induced lung injury. Assessment, management
and prevention. Oncology (Williston Park). 22:37–47; discussion
52–53. 2008.PubMed/NCBI
|
|
6
|
Alphonsus CS and Rodseth RN: The
endothelial glycocalyx: A review of the vascular barrier.
Anaesthesia. 69:777–784. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li X, Xue J and Lu Y: Current situation
and prospect of treatment for radiation-induced lung injury. Sheng
Wu Yi Xue Gong Cheng Xue Za Zhi. 27:937–940. 2010.(In Chinese).
PubMed/NCBI
|
|
8
|
Shin JW, Son JY, Raghavendran HR, Chung
WK, Kim HG, Park HJ, Jang SS and Son CG: High-dose ionizing
radiation-induced hematotoxicity and metastasis in mice model. Clin
Exp Metastasis. 28:803–810. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim JY, Kim YS, Kim YK, Park HJ, Kim SJ,
Kang JH, Wang YP, Jang HS, Lee SN and Yoon SC: The TGF-β1 dynamics
during radiation therapy and its correlation to symptomatic
radiation pneumonitis in lung cancer patients. Radiat Oncol.
4:592009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jarnicki A, Putoczki T and Ernst M: Stat3:
Linking inflammation to epithelial cancer - more than a “gut”
feeling? Cell Div. 5:142010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Solinas G, Marchesi F, Garlanda C,
Mantovani A and Allavena P: Inflammation-mediated promotion of
invasion and metastasis. Cancer Metastasis Rev. 29:243–248. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Commission on Life Sciences National
Research Council: Guide for the care and use of laboratory animals.
Washington, DC: The National Academies Press. 1996.
|
|
13
|
Li T, Li H, Wang Y, Harvard C, Tan JL, Au
A, Xu Z, Jablons DM and You L: The expression of CXCR4, CXCL12 and
CXCR7 in malignant pleural mesothelioma. J Pathol. 223:519–530.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shu HK, Yoon Y, Hong S, Xu K, Gao H, Hao
C, Torres-Gonzalez E, Nayra C, Rojas M and Shim H: Inhibition of
the CXCL12/CXCR4-Axis as preventive therapy for radiation-induced
pulmonary fibrosis. Plos One. 8:e797682013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Barcellos-Hoff MH and Ravani SA:
Irradiated mammary gland stroma promotes the expression of
tumorigenic potential by unirradiated epithelial cells. Cancer Res.
60:1254–1260. 2000.PubMed/NCBI
|
|
16
|
Ohuchida K, Mizumoto K, Murakami M, Qian
LW, Sato N, Nagai E, Matsumoto K, Nakamura T and Tanaka M:
Radiation to stromal fibroblasts increases invasiveness of
pancreatic cancer cells through tumor-stromal interactions. Cancer
Res. 64:3215–3222. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Vala Sofia I, Martins LR, Imaizumi N,
Nunes RJ, Rino J, Kuonen F, Carvalho LM, Rüegg C, Grillo IM, Barata
JT, et al: Low doses of ionizing radiation promote tumor growth and
metastasis by enhancing angiogenesis. PLoS One. 5:e112222010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Movsas B, Raffin TA, Epstein AH and Link
CJ Jr: Pulmonary radiation injury. Chest. 111:1061–1076. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xie L, Zhou J, Zhang S, Chen Q, Lai R,
Ding W, Song C, Meng X and Wu J: Integrating microRNA and mRNA
expression profiles in response to radiation-induced injury in rat
lung. Radiat Oncol. 9:1112014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Libshitz HI: Radiation changes in the
lung. Semin Roentgenol. 28:303–320. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chou CH, Teng CM, Tzen KY, Chang YC, Chen
JH and Cheng JC: MMP-9 from sublethally irradiated tumor promotes
Lewis lung carcinoma cell invasiveness and pulmonary metastasis.
Oncogene. 31:458–468. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Morgan GW and Breit SN: Radiation and the
lung: A reevaluation of the mechanisms mediating pulmonary injury.
Int J Radiat Oncol Biol Phys. 31:361–369. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ward PA and Hunninghake GW: Lung
inflammation and fibrosis. Am J Respir Crit Care Med.
157:S123–S129. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Johnston CJ, Williams JP, Elder A, Hernady
E and Finkelstein JN: Inflammatory cell recruitment following
thoracic irradiation. Exp Lung Res. 30:369–382. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Phillips RJ, Burdick MD, Hong K, Lutz MA,
Murray LA, Xue YY, Belperio JA, Keane MP and Strieter RM:
Circulating fibrocytes traffic to the lungs in response to CXCL12
and mediate fibrosis. J Clin Invest. 114:438–446. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Heinzelmann F, Jendrossek V, Lauber K,
Nowak K, Eldh T, Boras R, Handrick R, Henkel M, Martin C, Uhlig S,
et al: Irradiation-induced pneumonitis mediated by the
CD95/CD95-ligand system. J Natl Cancer Inst. 98:1248–1251. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Satoko M and Sandra D: Up-regulation of
the Pro-inflammatory chemokine CXCL16 is a common response of tumor
cells to ionizing radiation. Radiat Res. 173:418–425. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Johnston CJ, Williams JP, Okunieff P and
Finkelstein JN: Radiation-induced pulmonary fibrosis: Examination
of chemokine and chemokinereceptor families. Radiat Res.
157:256–265. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Okera M, Bae K, Bernstein E, Cheng L,
Lawton C, Wolkov H, Pollack A, Dicker A, Sandler H and Sweeney CJ:
Evaluation of nuclear factor κB and chemokine receptor CXCR4
co-expression in patients with prostate cancer in the radiation
therapy oncology group (RTOG) 8610. BJU Int. 108:E51–E58. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yang X, Walton W, Cook DN, Hua X, Tilley
S, Haskell CA, Horuk R, Blackstock AW and Kirby SL: The chemokine,
CCL3 and its receptor, CCR1, mediate thoracic radiation-induced
pulmonary fibrosis. Am J Respir Cell Mol Biol. 45:127–135. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Andersson-Sjöland A, de Alba CG, Nihlberg
K, Becerril C, Ramírez R, Pardo A, Westergren-Thorsson G and Selman
M: Fibrocytes are a potential source of lung fibroblasts in
idiopathic pulmonary fibrosis. Int J Biochem Cell Biol.
40:2129–2140. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zong ZW, Cheng TM, Su YP, Ran XZ, Shen Y,
Li N, Ai GP, Dong SW and Xu H: Recruitment of transplanted dermal
multipotent stem cells to sites of injury in rats with combined
radiation and wound injury by interaction of SDF-1 and CXCR4.
Radiat Res. 170:444–450. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Balkwill F: Cancer and the chemokine
network. Nat Rev Cancer. 4:540–550. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kulbe H, Levinson NR, Balkwill F and
Wilson JL: The chemokine network in cancer-much more than directing
cell movement. Int J Dev Biol. 48:489–496. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Orimo A and Weinberg RA: Stromal
fibroblasts in cancer: A novel tumor-promoting cell type. Cell
Cycle. 5:1597–1601. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Orimo A, Gupta PB, Sgroi DC,
Arenzana-Seisdedos F, Delaunay T, Naeem R, Carey VJ, Richardson AL
and Weinberg RA: Stromal fibroblasts present in invasive human
breast carcinomas promote tumor growth and angiogenesis through
elevated SDF-1/CXCL12 secretion. Cell. 121:335–348. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Muller A, Homey B, Soto H, Ge N, Catron D,
Buchanan ME, McClanahan T, Murphy E, Yuan W, Wagner SN, et al:
Involvement of chemokine receptors in breast cancer metastasis.
Nature. 410:50–56. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
38
|
Phillips RJ, Burdick MD, Lutz M, Belperio
JA, Keane MP and Strieter RM: The stromal derived
factor-1/CXCL12-CXC chemokine receptor 4 biological axis in
non-small cell lung cancer metastases. Am J Respir Crit Care Med.
167:1676–1686. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Scotton CJ, Wilson JL, Scott K, Stamp G,
Wilbanks GD, Fricker S, Bridger G and Balkwill FR: Multiple actions
of the chemokine CXCL12 on epithelial tumor cells in human ovarian
cancer. Cancer Res. 62:5930–5938. 2002.PubMed/NCBI
|
|
40
|
Rubin JB, Kung AL, Klein RS, Chan JA, Sun
Y, Schmidt K, Kieram MW, Luster AD and Segal RA: A small-molecule
antagonist of CXCR4 inhibits intracranial growth of primary brain
tumors. PNAS. 100:13513–13518. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Bajetto A, Barbieri F, Dorcaratto A,
Barbero S, Daga A, Porcile C, Ravetti JL, Zona G, Spaziante R,
Corte G, Schettini G and Florio T: Expression of CXC chemokine
receptor 1–5 and their ligands in human glioma tissues: role of
CXCR4 and SDF1 in glioma cell proliferation and migration.
Neurochem Int. 49:423–432. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Scala S, Ottaiano A, Ascierto PA, Cavalli
M, Simeone E, Giuliano P, Napolitano M, Franco R, Botti G and
Castello G: Expression of CXCR4 predicts poor prognosis in patients
with malignant melanoma. Clin Cancer Res. 11:1835–1841. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Balkwill F: Chemokine biology in cancer.
Semin Immunol. 15:49–55. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Staller P, Sulitkova J, Lisztwan J, Moch
H, Oakeley EJ and Krek W: Chemokine receptor CXCR4 downregulated by
von hippel-lindau tumour suppressor pVHL. Nature. 425:307–311.
2003. View Article : Google Scholar : PubMed/NCBI
|