Introduction
Pancreatic carcinoma (PaCa) is the fourth leading
cause of cancer-related mortality in the USA (1) and is associated with ~227,000
mortalities per year worldwide (2).
It is one of the most aggressive malignancies, with a 5-year
survival rate of <2% (3). Research
has confirmed that PaCa may be considered a disease of genetic
mutation, involving tumor-suppressor gene inactivation and oncogene
activation (4). Alterations in
microRNA (miRNA) expression appear to contribute to pancreatic
cancer development and progression; overexpression of a number of
miRNAs, including miR-21, miR-34, miR-155 and miR-200, is reported
to be important for neoplastic progression (5–8).
miRNAs are a class of small, conservative and
endogenous non-coding single-stranded RNAs of 17–24 nucleotides in
length. These molecules regulate the expression of protein-coding
genes at the translational level via complementary sequences in the
3′-untranslated region (3′UTR) of mRNA: miRNAs form part of an
RNA-induced silencing complex (9),
which binds to the 3′UTR of a target gene, triggering degradation
or preventing translation of the target mRNA (10,11).
miRNA-183 (miR-183) is located on chromosome 7q32
and is dysregulated in numerous types of tumor (12). Studies have demonstrated that miR-183
is involved in the modulation of various cellular processes and is
important in the differentiation of malignant tumors (13–15). In
addition, miR-183 is involved in the regulatory mechanisms of tumor
invasion and metastasis (16).
Studies have reported that miR-183 is overexpressed in the majority
of tumor types, including breast cancer (17) and prostate cancer (18). A number of experimental studies have
found that miRNAs are involved in multiple processes of cancer
progression, including cancer cell proliferation and metastasis
(19–21). However, we also found that miR-183 is
downregulated in certain tumors, such as retinoblastoma (22) and lung cancer (23), and may act as a tumor suppressor. A
number of studies have demonstrated that the miR-183 carcinogenic
mechanism depends on the regulation of oncogenes or
tumor-suppressor genes: Ueno et al (24) identified Dkk-3 and SMAD4 as potential
target genes of miR-183, whilst Tanaka et al (25) reported that the upregulation of
miR-183 in glioblastomas is associated with the expression of
hypoxia-inducible factor 1α. In addition, Sarver et al
(26) confirmed miR-183 acts as an
oncogene through regulation of two tumor-suppressor genes, early
growth response 1 and phosphatase and tensin homolog.
The literature indicates that miR-183 may be an
oncogene in a number of cancer types. High expression levels of
miR-183 have also been reported in pancreatic cancer (27); however, the biological characteristics
and targets of miR-183 are not well understood. Meanwhile,
suppressor of cytokine signaling 6 (SOCS-6) is a known tumor
suppressor. Based on findings from target gene detection software
(miRDB, PicTar and TargetSCAN), we hypothesized that the
differential expression of miR-183 may result in the downregulation
of SOCS-6 proteins, which are important mediators of cellular
growth, invasion and metastasis.
Materials and methods
Tissue samples and cell lines
Pancreatic adenocarcinoma tissues and respective
adjacent normal ductal epithelial tissues were obtained
postoperatively from 24 patients (18 males and 6 females; mean age,
59.8 years; range, 48–75 years), following pancreaticoduodenal
resection, who were pathologically diagnosed with stage I disease,
according to Hermeck staging (28),
at Fujian Medical University Union Hospital (Fuzhou, China) between
January 2009 and August 2013. All diagnoses were based on
pathological evidence. The tissue samples were paraffin-embedded
and stored prior to use. The human pancreatic cancer cell line
PANC-1 and pancreatic ductal cell line HPDE6-C7 were obtained from
the Institute of Liver and Gallbladder Surgery of Union Hospital,
and were maintained in Dulbecco's modified Eagle's medium (DMEM)
supplemented with 10% fetal bovine serum (FBS; Gibco Life
Technologies, Grand Island, NY, USA). Cells were grown in an
incubator at 37°C in a humidified atmosphere of 5% CO2.
This study was approved by the ethics committee of Fujian Medical
University Union Hospital.
Target prediction
Target gene detection software, TargetSCAN
(http://www.targetscan.org/mamm_31/;
Whitehead Institute for Biomedical Research, Cambridge, MA, USA),
miRDB (http://www.mirdb.org/miRDB/)
(29) and PicTar (http://www.pictar.org/; Max Delbrück Center for
Molecular Medicine, Berlin, Germany) were used to identify
complementary sequences between the miR-183-5p and SOCS-6 genes,
using the miRNA gene name ‘has-miR-183’ to predict miRNA
targets.
Cell transfections
The miR-183-5p inhibitor and negative control (NC)
gene fragments were obtained from Shanghai GenePharma, Co.. Ltd.,
(Shanghai, China). Transfections were performed using Lipofectamine
2000 (Invitrogen Life Technologies, Carlsbad, CA, USA) according to
the manufacturer's protocol. Cells were grown in 6-well culture
plates until 70–80% confluence. For each well, 5 µl human
miR-183-5p inhibitor or NC were added to 250 µl DMEM with 5 µl
Lipofectamine 2000. The mixture was added to the cells and
incubated for 24–48 h. Total RNA and protein were used for
quantitative polymerase chain reaction (qPCR) or western blot
analysis following transfection.
qPCR
Total RNA was extracted from cells using Trizol
reagent according to the manufacturer's instructions (Invitrogen
Life Technologies). The miR-183-5p and SOCS-6 levels in PANC-1
cells were quantified and validated by qPCR using Maxima® SYBR
Green/ROX qPCR Master Mix (2X) (#K0221; Thermo Fisher Scientific,
Pittsburgh, PA, USA), with U6 small nuclear RNA as an internal
normalized reference. For mRNA detection, reverse transcription was
performed according to the protocol provided with the RevertAid
First Strand cDNA Synthesis Kit (#K1622; Thermo Fisher Scientific).
Using GAPDH mRNA levels for normalization, relative levels of
miR-183-5p and SOCS-6 were measured in triplicate in a StepOne™
Real-Time PCR System (Applied Biosystems Life Technologies, Foster
City, CA, USA) according to the supplier's instructions. The
primers used were as follows: miR-183-5p forward,
5′-CGCGGTATGGCACTGGTAGA-3′, and reverse,
5′-AGTGCAGGGTCCGAGGTATTC-3′; U6 forward, 5′-CTCGCTTCGGCAGCACATA-3′,
and reverse, 5′-CGAATTTGCGTGTCATCCT-3′; SOCS-6 forward,
5′-CCCGAGGATGAGAGTCAGGTAG-3′, and reverse,
5′-TGGAGGTAGCAATGGTGAGAGTG-3′; and GAPDH forward,
5′-TGCACCACCAACTGCTTAGC-3′, and reverse,
5′-AGCTCAGGGATGACCTTGCC-3′. The qPCR conditions consisted of a
uracil-N-glycosylase carry-over protection step of 55°C for 2 min,
10 min of DNA polymerase activation at 95°C, followed by 40 cycles
of 95°C for 15 sec and 60°C for 60 sec. The expression level of
miRNA was statistically evaluated by a Student's t-test
using GraphPad Prism 5 software (GraphPad Software, Inc., La Jolla,
CA, USA).
Western blotting and
immunohistochemistry (IHC)
Total protein extracted from the untransfected,
miR-183-5p inhibitor-transfected and NC-transfected PANC-1 cells
was subjected to 10% SDS-PAGE (Bio/West, Inc., Salt Lake City, UT,
USA) at 80 V and transferred to polyvinylidene fluoride membranes
(Millipore, Billerica, MA, USA) at 120 V. The transferred membranes
were blocked for 2 h with 5% non-fat powdered milk and incubated
with the following primary antibodies, diluted 1:1,000, overnight
at 4°C: Monoclonal mouse anti-human SOCS6 (#ab56516; Abcam,
Cambridge, MA, USA) and monoclonal mouse anti-human β-actin
antibody (#sc-47778; Santa Cruz Biotechnology, Inc., Dallas, TX,
USA). The membranes were subsequently incubated for 30 min at room
temperature with the respective monoclonal goat anti-mouse
horseradish peroxidase-conjugated IgG secondary antibodies (#A0216;
Beyotime Institute of Biotechnology, Shanghai, China; dilution,
1:5,000) following the manufacturer's instructions, and then
exposed to X-ray film (Kodak, Rochester, NY, USA). β-actin levels
were used to standardize protein loading, with the assumption that
the level of β-actin would be similar in all cells. IHC (30) was performed with a rabbit polyclonal
IgG antibody against human SOCS-6 (#sc-5608; Santa Cruz
Biotechnology, Inc.; dilution, 1:200) and MaxVision™ HRP-Polymer
anti-Mouse IHC Kit (Fuzhou Maixin Biotech, Co., Ltd., Fuzhou,
China) using the standard method.
Cell proliferation assay
The Cell Counting Kit-8 (CCK-8; Beyotime Institute
of Biotechnology) was used for the detection of cell proliferation.
In 96-well plates, ~103 cells per well were incubated in
DMEM for 48 h prior to the addition of 10 µl CCK-8 solution. The
mixture was incubated at 37°C for 4 h, during which time CCK-8 was
cleaved to an orange formazan dye by metabolically active cells.
Following incubation, the absorbance of the formazan product was
measured using an enzyme-linked immunosorbent assay reader (550;
Bio-Rad Laboratories, Inc., Hercules, CA, USA) at 450 nm.
Wound-healing assay
Pancreatic cancer cells were seeded in 6-well plates
and transfected with miR-183-5p inhibitor or NC, or untreated.
After 24 h, a wound was formed by scraping the cells with a 20 µl
pipette tip and washing twice with DMEM. The cells were kept
without bovine serum albumin (BSA) in DMEM. Cells were observed at
0 and 48 h after scraping and images were captured under a
microscope (TE2000-U; Nikon Corporation, Tokyo, Japan).
Cell invasion assays
Invasion assays were performed using 24-well
transwell chambers (8 µm; Corning Life Sciences, Corning, NY, USA),
fibronectin (FN; BD Biosciences, San Jose, CA, USA) and Matrigel™
(BD Biosciences). The upper chambers were covered with 50 mg/ml
Matrigel™ inside and FN outside. PANC-1 cells transfected with
miR-183-5p inhibitor or NC were suspended in DMEM without FBS in
the upper chambers, and the cell concentration was adjusted to
5×105 cells/ml. Aliquots of the suspension (~200 µl)
were seeded into the upper chambers, and 1 ml of DMEM containing
10% FBS was added to the lower chambers. After 48 h, the migrated
cells were fixed with 95% ethanol and 5% acetic acid before
staining with hematoxylin (Beyotime Institute of Biotechnology) for
5 min.
Statistical analysis
Data collected from at least three independent
experiments are expressed as the mean ± standard deviation.
Differences between groups were analyzed using two-tailed Student's
t-test after fitting a normal distribution using an F-test.
All tests performed were two-sided. All statistical analyses were
performed using GraphPad Prism 5 software and P<0.05 was
regarded to indicate statistical significance.
Results
SOCS-6 may be a target gene of
miR-183
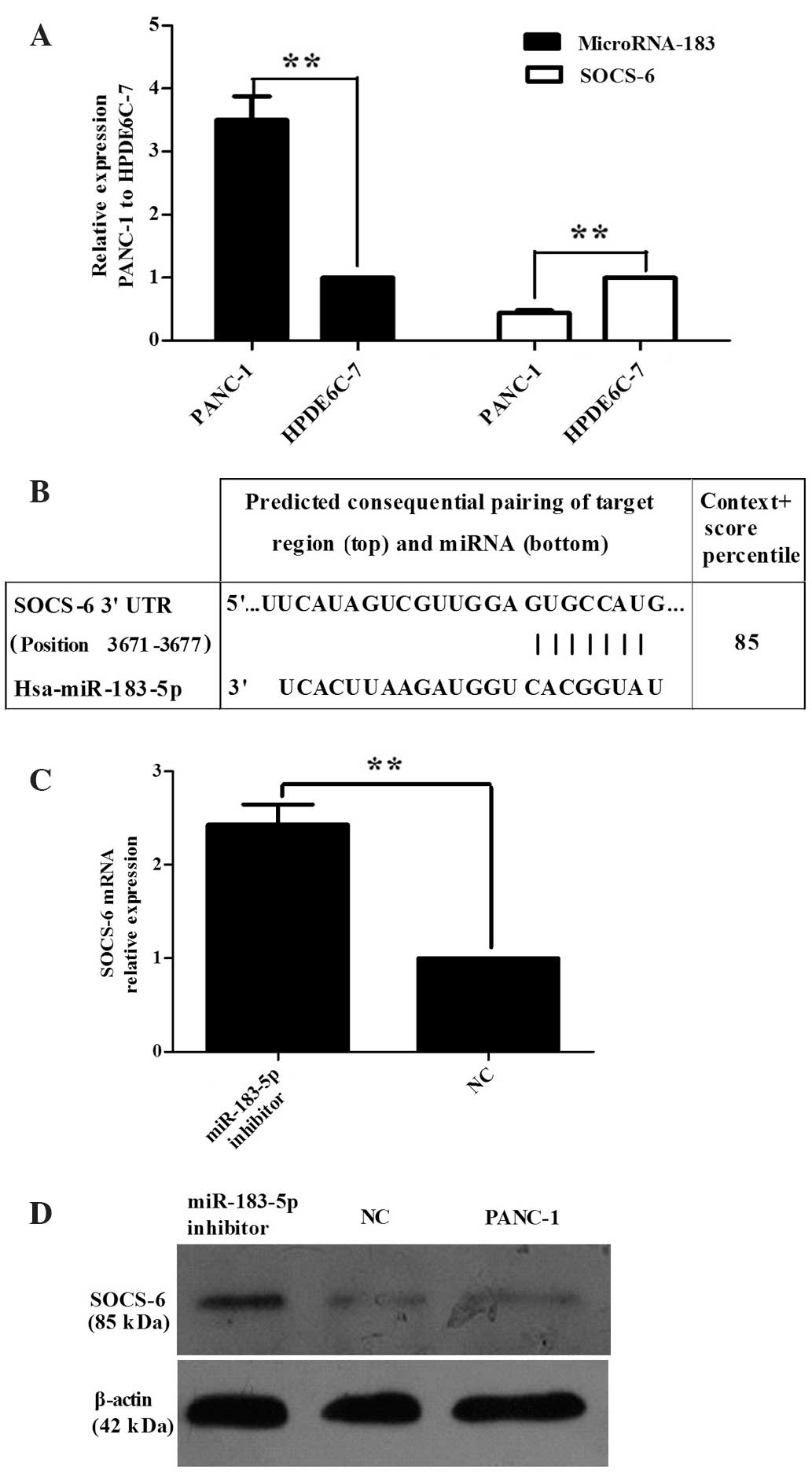
qPCR was used to detect the expression of miR-183 in
the PaCa cell line PANC-1 and in the normal pancreatic cell line
HPDE6-C7 to confirm whether miR-183-5p is dysregulated in
pancreatic cancer. The expression of miR-183-5p in PANC-1 cells was
observed to be significantly higher than that in HPDE6-C7 cells
(P=0.0003; Fig. 1A).
To establish more regarding the biological
relationship between SOCS-6 and miR-183-5p in pancreatic cancer,
the miRNA target gene prediction software TargetSCAN was used to
forecast the target genes of miR-183-5p. miR-183 was found to be
complementary to the 3′-UTR of SOCS-6 (Fig. 1B). qPCR was subsequently used to test
SOCS-6 expression in PANC-1 and HPDE6-C7 cells, revealing that
SOCS-6 expression was downregulated in PANC-1 compared with
HPDE6-C7 (Fig. 1A, P<0.0001).
However, transfection of PANC-1 cells with a miR-183-5p inhibitor
resulted in an increase in SOCS-6 expression relative to that of
untransfected PANC-1 cells (Fig. 1C,
P=0.0028). Western blot analysis (Fig.
1D) was also used to analyze the change in SOCS-6 expression in
cells transfected with the miR-183-5p inhibitor. The outcome was
consistent with that of the qPCR. All results indicated that SOCS-6
may be a target gene of miR-183-5p. Thus, further investigation of
the changes in SOCS-6 expression in pancreatic cancer tissue must
be conducted.
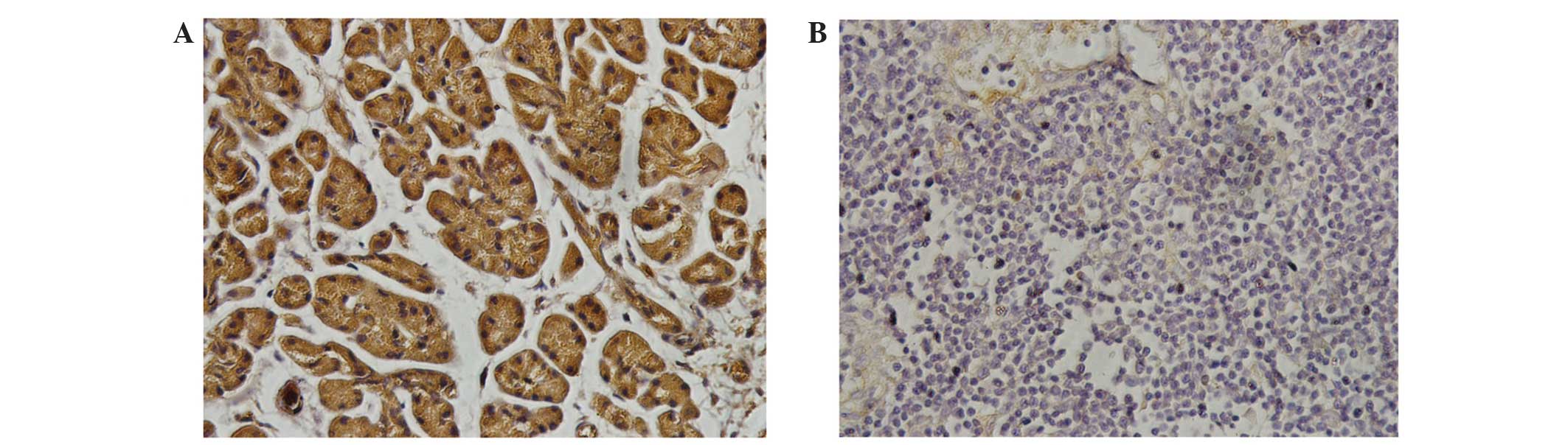
To further confirm these findings, IHC was performed
on PaCa tissue and adjacent normal pancreatic tissue from PaCa
patients, revealing that SOCS-6 expression was significantly higher
in normal pancreatic tissue compared with that in PaCa cells
(Fig. 2). The result is consistent
with those of the western blot and qPCR.
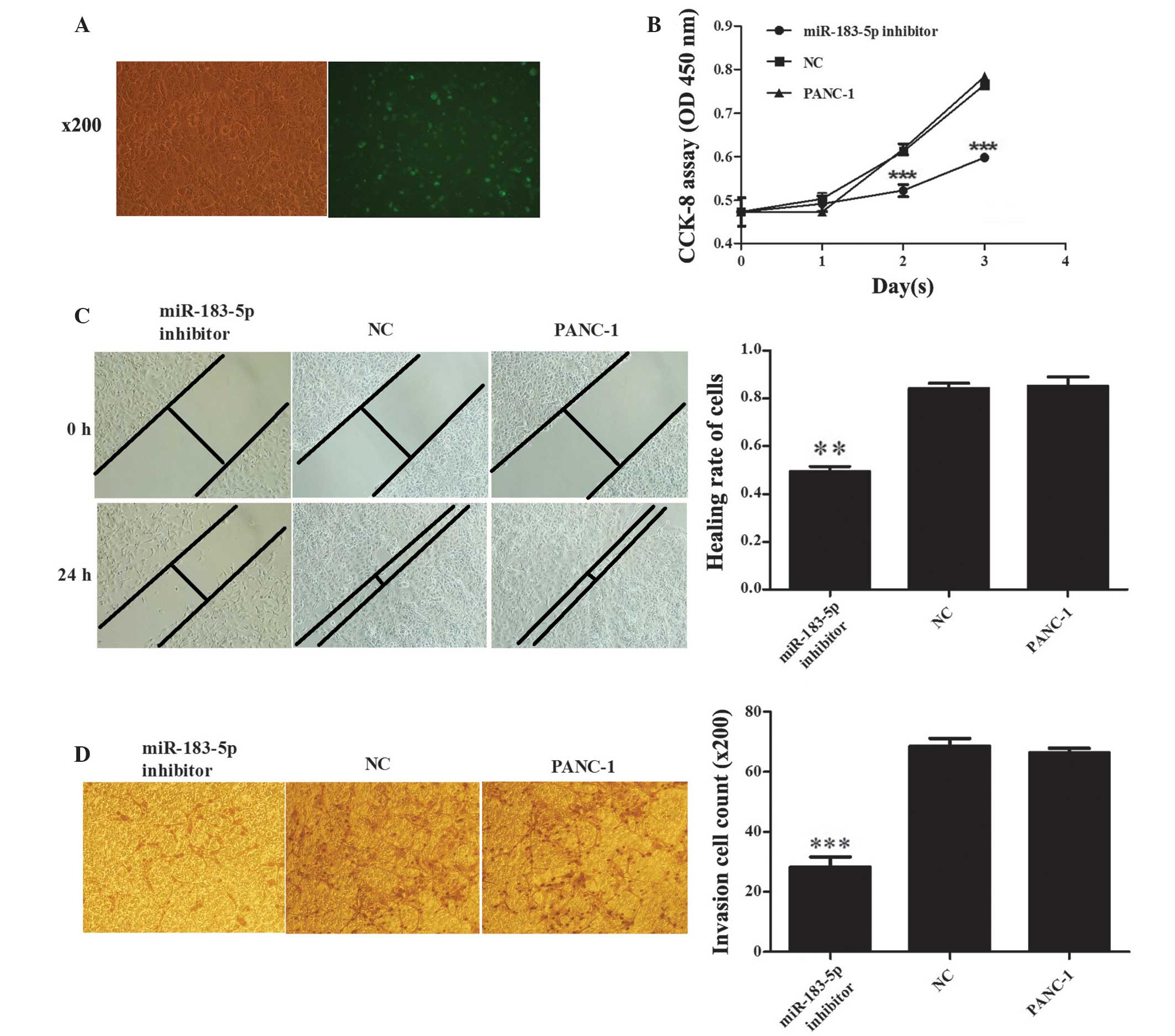
Inhibition of miR-183-5p expression
inhibits cell proliferation in PANC-1 cells
The SOCS family has been proposed to act
predominantly as negative regulator of signaling by cytokines and
certain growth factors (31). We
hypothesized that SOCS6 is a direct target of miRNA-183-5p and,
therefore, that miR-183-5p is likely to be connected with tumor
proliferation. To confirm this, CCK-8 assays were performed in
PANC-1 human PaCa cells, which highly express miR-183-5p. The
miR-183-5p inhibitor was observed to reduce cell proliferation at
1–3 days after transfection (transfection efficiency, ~60%;
Fig. 3A) compared with the
NC-transfected and untransfected PANC-1 cells (P≤0.001; Fig. 3B), which remained similar throughout
the 72 h assay period. These results indicate that anti-miR-183-5p
may repress the proliferation of PANC-1 cells.
Inhibition of miR-183-5p expression
represses PANC-1 cell migration and invasion
To study the role of miR-183-5p in the invasion and
metastasis of pancreatic cancer, a transwell assay and wound
healing/scratch test were used to detect the capacity of invasion
and migration in PANC-1 cells following transfection with a
miR-183-5p inhibitor. As shown in Fig.
2C, transfection with the miR-183-5p inhibitor reduced the
migratory ability of PANC-1 cells compared with the untreated
(P=0.0012) and NC cells (P=0.0004). Invasive capacity was also
repressed by the miR-183-5p inhibitor compared with that in NC and
untreated cells (P<0.001; Fig.
2D). These results indicate that downregulation of miR-183-5p
inhibits the migration and invasion of PANC-1 cells in
vitro.
Discussion
Various miRNAs have been reported to be dysregulated
in PaCa; these molecules may function as tumor-suppressor genes
(Let-7, miR-20a, miR-143) (32–34) or
oncogenes (miR-181, miR-21, miR-10a, miR-196a) (30,35–40). A
number of these miRNAs are associated with the invasive and
metastatic abilities of pancreatic cancer cells. For example,
downregulation of miR-218 has been demonstrated in PANC-1 cells.
Forced expression of miR-218 was found to inhibit cell migration
and invasion in a roundabout homolog 1 (ROBO1)-dependent manner and
ROBO1 has previously been identified as a functional target of
miRNA-218's downstream pathway, which is involved in the invasion
and migration of pancreatic cancer (41). miR-26a has also been reported to
impair cell migration, invasion and apoptosis in PANC-1 cells by
controlling the expression of high mobility group AT-hook 1
(HMGA1); the authors demonstrated that the oncogene HMGA1 was a
direct target of miR-26a (42). In
addition, a previous study revealed that expression of miR-126 was
promoted in pancreatic carcinoma cells, and the expression of the
tumor suppressor gene, disintegrin and metalloproteinase
domain-containing protein 9 (ADAM9), was suppressed; blocking
miR-126 decreased migration and invasion (43). These results indicated that the
miR-126/ADAM9 axis exerts a critical role in pancreatic carcinoma
(43). Another previous study showed
that endogenous miR-224 and miR-468 inhibited the expression of the
tumor suppressor gene, CD40, in PANC-1 and promoted cell metastasis
and invasion, indicating that they exhibited an important role in
the progression of pancreatic cancer (44). These findings demonstrate that miRNA
expression is dysregulated in PaCa cell lines, and miRNAs may
promote or inhibit PaCa cell migration and invasion by targeting
certain mRNAs.
Invasion and metastasis are the main determinants of
poor prognosis in pancreatic cancer (7). The functional study of miR-183 in
malignancy has been previously reported in most tumors (15,45–47).
miR-183 is downregulated in HeLa cells, and has been demonstrated
to mediate the invasive and metastatic ability of HeLa cells by
directly targeting integrin β1 (48).
miR-183 is likely to have numerous targets via which it modulates
biological functions in cancer cells (45,46,49).
However, miR-183 is rarely reported in similar research reports
regarding pancreatic carcinoma and did not show relevant biological
characteristics and molecular mechanisms. In the current study, the
expression of miR-183-5p was found to be significantly upregulated
in PaCa cell lines, whilst expression of SOCS-6 was markedly
downregulated in PaCa tissues and cell lines. To investigate the
mechanism by which miR-183 may promote the metastasis of PaCa, the
miRNA target prediction programs TargetScan, PicTar and miRDB were
employed to identify the direct targets of miR-183 (50). All three programs predicted SOCS-6 to
be a target of miR-183. To verify the effect of miR-183 on SOCS-6
expression, a miR-183 inhibitor was transfected into PANC-1 cells.
Notably, following transfection with the miR-183 inhibitor, SOCS-6
expression these cells increased significantly, as indicated by
qPCR and western blot analysis. By contrast, PANC-1 cells
transfected with NC or without transfection exhibited no
significant change in SOCS-6 expression. The current study also
found that suppressing the expression of miR-183-5p significantly
repressed the proliferative, invasive and metastatic abilities of
PANC-1 cells. Taken together, these findings suggest that miR-183
may promote proliferation, invasion and migration of PaCa cells. In
addition, SOCS-6 expression is inversely associated with the
proliferation, invasion and migration of PaCa cells. By
downregulating SOCS-6, miR-183 may act as an accelerant factor in
the progression of PaCa cells.
The SOCS family of proteins consist of eight
members: SOCS1 to SOCS7 and cytokine-inducible SH2-containing
protein (CISH) (51). Similar to
other SOCS family members, the degradation of target proteins is
the predominant regulatory effect of SOCS-6 (52). Sriram et al (53) reported that loss of SOCS-6 is
associated with significantly shorter overall survival time in lung
small cell carcinoma. The downregulation of SOCS-6 mRNA expression
has been observed in >50% of patients with gastric or colorectal
cancer (54,55). Wu et al (56) proposed that SOCS-6 is a
tumor-suppressor gene in pancreatic cancer. Through the collection
of clinical specimens and detecting SOCS-6 expression, the present
study identified a significant reduction in SOCS-6 expression in
pancreatic cancer tissues compared with that of adjacent normal
pancreatic tissues. This result was consistent with that of Wu
et al, which also demonstrated that SOCS-6 was downregulated
in pancreatic cancer.
In the current study, the expression level of
miR-183-5p was observed to be inversely correlated with SOCS-6
expression in PaCa cell lines. To the best of our knowledge, this
study is the first in vitro study to demonstrate the
regulation of metastasis and progression of PaCa cell lines by
downregulation of miR-183-5p to target the expression of SOCS-6.
The expression of miR-183-5p in PaCa cell lines is consistent with
the study by Park et al (57),
which revealed that miR-183 was upregulated in pancreatic cancer.
The findings of the current study indicate a promotional role for
miR-183 in cell proliferation, migration and invasion of PaCa cells
by downregulating SOCS-6. This may provide an important basis for
further analysis in vivo with the aim of developing a novel
potential diagnostic and therapeutic target for the screening and
treatment of metastatic PaCa. Further studies are necessary in
order to elucidate the regulatory mechanisms of miR-183-5p and
SOCS-6 in PaCa cells, in vitro and in vivo.
In conclusion, the present study has demonstrated
the upregulation of miR-183-5p in PaCa cell lines compared with
normal pancreatic cancer cells, and in vitro experiments
indicated that this miRNA was able to enhance the proliferation,
migration and invasion of PaCa cells. Downregulation of miR-183-5p
partially attenuated oncogenic effects, indicating that this
molecule may act as an oncogene. In addition, SOCS-6 was identified
as a target of miR-183-5p, and may present a potential target for
PaCa therapy. Therefore, miR-183-5p may be a key molecule for the
diagnosis and treatment of pancreatic cancer in the future.
Acknowledgements
This study was supported by grants from the Key
Project of Science and Technology Research Program in Fujian
Province (no. 2014J01323) and the National Clinical Key Specialty
Construction Project (General Surgery) of China.
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Raimondi S, Maisonneuve P and Lowenfels
AB: Epidemiology of pancreatic cancer: An overview. Nat Rev
Gastroenterol Hepatol. 6:699–708. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li D, Xie K, Wolff R and Abbruzzese JL:
Pancreatic cancer. Lancet. 363:1049–1057. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Maitra A, Kern SE and Hruban RH: Molecular
pathogenesis of pancreatic cancer. Best Pract Res Clin
Gastroenterol. 20:211–226. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Szafranska AE, Doleshal M, Edmunds HS,
Gordon S, Luttges J, Munding JB, Barth RJ Jr, Gutmann EJ,
Suriawinata AA and Marc Pipas J: Analysis of microRNAs in
pancreatic fine-needle aspirates can classify benign and malignant
tissues. Clin Chem. 54:1716–1724. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li A, Omura N, Hong SM, Vincent A, Walter
K, Griffith M, Borges M and Goggins M: Pancreatic cancers
epigenetically silence SIP1 and hypomethylate and overexpress
miR-200a/200b in association with elevated circulating miR-200a and
miR-200b levels. Cancer Res. 70:5226–5237. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bloomston M, Frankel WL, Petrocca F, et
al: MicroRNA expression patterns to differentiate pancreatic
adenocarcinoma from normal pancreas and chronic pancreatitis. JAMA.
297:1901–1908. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kent OA, Mullendore M, Wentzel EA,
López-Romero P, Tan AC, Alvarez H, West K, Ochs MF, Hidalgo M and
Arking DE: A resource for analysis of microRNA expression and
function in pancreatic ductal adenocarcinoma cells. Cancer Biol
Ther. 8:2013–2024. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sarkar S, Dubaybo H, Ali S, Goncalves P,
Kollepara SL, Sethi S, Philip PA and Li Y: Down-regulation of
miR-221 inhibits proliferation of pancreatic cancer cells through
up-regulation of PTEN, p27 (kip1), p57 (kip2) and PUMA. Am J Cancer
Res. 3:465–477. 2013.PubMed/NCBI
|
|
10
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
He L and Hannon GJ: MicroRNAs: Small RNAs
with a big role in gene regulation. Nat Rev Genet. 5:522–531. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lumayag S, Haldin CE, Corbett NJ, et al:
Inactivation of the microRNA-183/96/182 cluster results in
syndromic retinal degeneration. Proc Natl Acad Sci USA.
110:E507–E516. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Leung WK, He M, Chan AW, Law PT and Wong
N: Wnt/β-Catenin activates MiR-183/96/182 expression in
hepatocellular carcinoma that promotes cell invasion. Cancer Lett.
362:97–105. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang L, Quan H, Wang S, Li X and Che X:
MiR-183 promotes growth of non-small cell lung cancer cells through
FoxO1 inhibition. Tumour Biol. May 17–2015.(Epub ahead of print).
View Article : Google Scholar
|
|
15
|
Zhang Q, Di W, Dong Y, et al: High serum
miR-183 level is associated with poor responsiveness of renal
cancer to natural killer cells. Tumour Biol. Jun 20–2015.(Epub
ahead of print).
|
|
16
|
Kundu ST, Byers LA, Peng DH, et al: The
miR-200 family and the miR-183~96~182 cluster target Foxf2 to
inhibit invasion and metastasis in lung cancers. Oncogene. Mar
23–2015.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ouyang M, Li Y, Ye S, Ma J, Lu L, Lv W,
Chang G, Li X, Li Q, Wang S and Wang W: MicroRNA profiling implies
new markers of chemoresistance of triple-negative breast cancer.
PLoS One. 9:e962282014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mihelich BL, Khramtsova EA, Arva N,
Vaishnav A, Johnson DN, Giangreco AA, Martens-Uzunova E, Bagasra O,
Kajdacsy-Balla A and Nonn L: MiR-183-96-182 cluster is
overexpressed in prostate tissue and regulates zinc homeostasis in
prostatecells. J Biol Chem. 286:44503–44511. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ventura A and Jacks T: MicroRNAs and
cancer: Short RNAs go a long way. Cell. 136:586–591. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wojtas B, Ferraz C, Stokowy T, Hauptmann
S, Lange D, Dralle H, Musholt T, Jarzab B, Paschke R and Eszlinger
M: Differential miRNA expression defines migration and reduced
apoptosis in follicular thyroid carcinomas. Mol Cell Endocrinol.
388:1–9. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lowery AJ, Miller N, Dwyer RM and Kerin
MJ: Dysregulated miR-183 inhibits migration in breast cancer cells.
BMC Cancer. 10:5022010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang J, Wang X, Li Z, Liu H and Teng Y:
MicroRNA-183 suppresses retinoblastoma cell growth, invasion and
migration by targeting LRP6. FEBS J. 281:1355–1365. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang G, Mao W and Zheng S: MicroRNA-183
regulates Ezrin expression in lung cancer cells. FEBS Lett.
582:3663–3668. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ueno K, Hirata H, Shahryari V, Deng G,
Tanaka Y, Tabatabai ZL, Hinoda Y and Dahiya R: MicroRNA-183 is an
oncogene targeting Dkk-3 and SMAD4 in prostate cancer. Br J Cancer.
108:1659–1667. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tanaka H, Sasayama T, Tanaka K, et al:
MicroRNA-183 upregulates HIF-1α by targeting isocitrate
dehydrogenase 2 (IDH2) in glioma cells. J Neurooncol. 111:273–283.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sarver AL, Li L and Subramanian S:
MicroRNA miR-183 functions as an oncogene by targeting the
transcription factor EGR1 and promoting tumor cell migration.
Cancer Res. 70:9570–9580. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Park YG, Lee KH, Lee JK, et al: MicroRNA
expression pattern in intraductal papillary mucinous neoplasm.
Korean J Gastroenterol. 58:190–200. 2011.(In Korean). View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wong N and Wang X: miRDB: An online
resource for microRNA target prediction and functional annotations.
Nucleic Acids Res. 43:D146–D152. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jia A, Xiao J and Liu X: Serum tumor
marker CA199, CA125, CA242 and CEA. Correlational analysis and
detection of different stages of pancreatic cancer. Chongqing Med.
35:3605–3607. 2011.(In Chinese).
|
|
30
|
Hwang JH, Voortman J, Giovannetti E, et
al: Identification of microRNA-21 as a biomarker for
chemoresistance and clinical outcome following adjuvant therapy in
resectable pancreatic cancer. PloS One. 5:e106302010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Trengove MC and Ward AC: SOCS proteins in
development and disease. Am J Clin Exp Immunol. 2:1–29.
2013.PubMed/NCBI
|
|
32
|
Torrisani J, Bournet B, du Rieu MC,
Bouisson M, Souque A, Escourrou J, Buscail L and Cordelier P: Let-7
microRNA transfer in pancreatic cancer-derived cells inhibits in
vitro cell proliferation but fails to alter tumor progression.
Hu Gene Ther. 20:831–844. 2009. View Article : Google Scholar
|
|
33
|
Yan H, Wu J, Liu W, Zuo Y, Chen S, Zhang
S, Zeng M and Huang W: MicroRNA-20a overexpression inhibited
proliferation and metastasis of pancreatic carcinoma cells. Hum
Gene Ther. 21:1723–1734. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hu Y, Ou Y, Wu K, Chen Y and Sun W:
miR-143 inhibits the metastasis of pancreatic cancer and an
associated signaling pathway. Tumour Biol. 33:1863–1870. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bloomston M, Frankel WL, Petrocca F,
Volinia S, Alder H, Hagan JP, Liu CG, Bhatt D, Taccioli C and Croce
CM: MicroRNA expression patterns to differentiate pancreatic
adenocarcinoma from normal pancreas and chronic pancreatitis. JAMA.
297:1901–1908. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Panarelli NC, Chen YT, Zhou XK,
Kitabayashi N and Yantiss RK: MicroRNA expression aids the
preoperative diagnosis of pancreatic ductal adenocarcinoma.
Pancreas. 41:685–690. 2012.PubMed/NCBI
|
|
37
|
Wang JI, Chen J, Chang P, LeBlanc A, Li D,
Abbruzzesse JL, Frazier ML, Killary AM and Sen S: MicroRNAs in
plasma of pancreatic ductal adenocarcinoma patients as novel
blood-based biomarkers of disease. Cancer Prev Res (Phila).
2:807–813. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Dillhoff M, Liu J, Frankel W, Croce C and
Bloomston M: MicroRNA-21 is overexpressed in pancreatic cancer and
a potential predictor of survival. J Gastrointest Surg.
12:2171–2176. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Moriyama T, Ohuchida K, Mizumoto K, Yu J,
Sato N, Nabae T, Takahata S, Toma H, Nagai E and Tanaka M:
MicroRNA-21 modulates biological functions of pancreatic cancer
cells including their proliferation, invasion and chemoresistance.
Mo Cancer Ther. 8:1067–1074. 2009. View Article : Google Scholar
|
|
40
|
Moriyama T, Ohuchida K, Mizumoto K, Yu J,
Sato N, Nabae T, Takahata S, Toma H, Nagai E and Tanaka M:
MicroRNA-10a is overexpressed in human pancreatic cancer and
involved in its invasiveness partially via suppression of the HOXA1
gene. Ann Surg Oncol. 19:2394–2402. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
He H, Hao SJ, Yao L, Yang F, Di Y, Li J,
Jiang YJ, Jin C and Fu DL: MicroRNA-218 inhibits cell invasion and
migration of pancreatic cancer via regulating ROBO1. Cancer Biol
Ther. 15:1333–1339. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Li W, Yuan Y, Huang L, Qiao M and Zhang Y:
Metformin alters the expression profiles of microRNAs in human
pancreatic cancer cells. Diabetes Res Clin Pract. 96:187–195. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hamada S, Satoh K, Fujibuchi W, Hirota M,
Kanno A, Unno J, Masamune A, Kikuta K, Kume K and Shimosegawa T:
MiR-126 acts as a tumor suppressor in pancreatic cancer cells via
the regulation of ADAM9. Mol Cancer Res. 10:3–10. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Mees ST, Mardin WA, Sielker S, Willscher
E, Senninger N, Schleicher C, Colombo-Benkmann M and Haier J:
Involvement of CD40 targeting miR-224 and miR-486 on the
progression of pancreatic ductal adenocarcinomas. Ann Surg Oncol.
16:2339–2350. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Chang CW, Wu HC, Terry MB and Santella RM:
microRNA expression in prospectively collected blood as a potential
biomarker of breast cancer risk in the BCFR. Anticancer Res.
35:3969–3977. 2015.PubMed/NCBI
|
|
46
|
Pak MG, Lee CH, Lee WJ, Shin DH and Roh
MS: Unique microRNAs in lung adenocarcinoma groups according to
major TKI sensitive EGFR mutation status. Diagn Pathol. 10:992015.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Van Keuren-Jensen K, Malenica I,
Courtright A, et al: microRNA changes in liver tissue associated
with fibrosis progression in patients with Hepatitis-C. Liver Int.
Jul 19–2015.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Li G, Luna C, Qiu J, Epstein DL and
Gonzalez P: Targeting of integrin beta1 and kinesin 2alpha by
microRNA 183. J Biol Chem. 285:5461–5471. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Cao Z, Zhang N, Lou T, et al: microRNA-183
down-regulates the expression of BKCaβ1 protein that is related to
the severity of chronic obstructive pulmonary disease. Hippokratia.
18:328–332. 2014.PubMed/NCBI
|
|
50
|
Krek A, Grün D, Poy MN, Wolf R, Rosenberg
L, Epstein EJ, MacMenamin P, da Piedade I, Gunsalus KC, Stoffel M
and Rajewsky N: Combinatorial microRNA target predictions. Nat
Genet. 37:495–500. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
51
|
Hilton DJ, Richardson RT, Alexander WS,
Viney EM, Willson TA, Sprigg NS, Starr R, Nicholson SE, Metcalf D
and Nicola NA: Twenty proteins containing a C-terminal SOCS box
form five structural classes. Proc Natl Acad Sci USA. 95:114–119.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Krebs DL, Uren RT, Metcalf D, Rakar S,
Zhang JG, Starr R, De Souza DP, Hanzinikolas K, Eyles J, Connolly
LM, et al: SOCS-6 binds to insulin receptor substrate 4 and mice
lacking the SOCS-6 gene exhibit mild growth retardation. Mol Cell
Biol. 22:4567–4578. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Sriram KB, Larsen JE, Savarimuthu Francis
SM, Wright CM, Clarke BE, Duhig EE, Brown KM, Hayward NK, Yang IA,
Bowman RV and Fong KM: Array-comparative genomic hybridization
reveals loss of SOCS6 is associated with poor prognosis in primary
lung squamous cell carcinoma. PLoS One. 7:e303982012. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Lai RH, Hsiao YW, Wang MJ, Lin HY, Wu CW,
Chi CW, Li AF, Jou YS and Chen JY: SOCS6, down-regulated in gastric
cancer, inhibits cell proliferation and colony formation. Cancer
Lett. 288:75–85. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Storojeva I, Boulay JL, Heinimann K,
Ballabeni P, Terracciano L, Laffer U, Mild G, Herrmann R and
Rochlitz C: Prognostic and predictive relevance of microsatellite
instability in colorectal cancer. Oncol Rep. 14:241–249.
2005.PubMed/NCBI
|
|
56
|
Wu K, Hu G, He X, Zhou P, Li J, He B and
Sun W: MicroRNA-424-5p suppresses the expression of SOCS6 in
pancreatic cancer. Pathol Oncol Res. 19:739–748. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Park YG, Lee KH, Lee JK, Lee KT, Choi DW,
Choi SH, Heo JS, Jang KT, Lee EM, Kim JO, et al: MicroRNA
expression pattern in intraductal papillary mucinous neoplasm.
Korean J Gastroenterol. 58:190–200. 2011.(In Korean). View Article : Google Scholar : PubMed/NCBI
|