Introduction
Malignant mesothelioma is a rare but fatal disease
that arises from the epithelial lining of the pleura, peritoneum,
pericardium and tunica vaginalis. Malignant pleural mesothelioma
(MPM) is the most common form, accounting for 80–90% of malignant
mesotheliomas (1,2). A history of heavy and long-term exposure
to asbestos is the established cause of MPM (3). However, MPM may result from other
factors, including genetics, erionite, radiation and simian virus
40 (SV40), which may work alone or in combination (4). SV40 is a polyomavirus of which the
natural hosts are rhesus monkeys. SV40 may infect human mesothelial
cells, and may transform the cells using a mechanism whereby the
tumor antigens, large T antigen (Tag) and small t antigen (tag),
bind and inactivate the cellular tumor suppressors tumor protein
p53 and retinoblastoma 1. These interactions may contribute to the
development of malignant mesotheliomas by rendering mesothelial
cells more susceptible to other carcinogens (5–7).
The role of SV40 in the pathogenesis of MPM remains
unclear (1,2,4). Certain
studies have detected SV40 DNA sequences or SV40 Tag in
mesothelioma cells (8–10), but others have not (11–14).
Geographical variation may be one reason for the discrepancy in
SV40 detection, as SV40-contaminated polio vaccines, which had
varied availability between countries, have been suspected as a
major source of human infection (15). The association between SV40 and MPM
remains unclear. The interaction between SV40 and asbestos exposure
in the pathogenesis of MPM is unknown. The present study was
conducted in order to investigate the proportion of SV40 presence
in the histological specimens of Vietnamese patients with MPM.
Materials and methods
The present retrospective study was conducted at
Pham Ngoc Thach Hospital, a referral chest hospital, in Ho Chi Minh
City, Vietnam. The study protocol was approved by the Ethics
Committee of the hospital.
Patients
The records of patients that were diagnosed with MPM
between January 2008 and June 2012 were searched for on the patient
database of the Department of Pathology, Pham Ngoc Thach Hospital.
The medical records and histological specimens of the patients were
archived. The patients or close relatives of the patients were
asked to participate in the study, and all participants provided
informed written consent. Patients that met the following criteria
were enrolled: i) Definitively diagnosed as MPM; ii) the
formaldehyde-fixed, paraffin-embedded tissues of the pleural
specimens were eligible for additional immunohistochemical
analysis; and iii) the patients or close relatives were available
for a face-to-face or telephone interview. Patients were excluded
for the following reasons: i) The formaldehyde-fixed,
paraffin-embedded tissues of the pleural specimens were not
eligible for immunohistochemical analysis due to small size or a
lack of tumor tissue; and ii) the patients or the close relatives
were not available or contactable.
Patients were definitively diagnosed as MPM based on
histological examinations and immunohistochemical staining
(16,17). In total, 4 positive markers, including
calretinin, desmin, monoclonal mouse anti-human mesothelial cell
clone HBME-1 and Wilms tumor 1 were used to definitively diagnose
MPM. Various negative markers were used to rule out other cancers
metastasized to the pleura, including: Keratin 7, carcinoembryonic
antigen, transcription termination factor, RNA polymerase I and
epidermal growth factor receptor for adenocarcinoma; enolase 2,
gamma neuronal, synaptrophysin and mouse monoclonal EpCAM antibody
for small cell lung cancer; and clathrin, light chain A, cluster of
differentiation (CD)3, CD20, CD30, CD68 and myeloperoxidase for
lymphoma and leukemia.
Patients or close relatives were interviewed in
order to determine a history of asbestos exposure. A history of
asbestos exposure was designated to patients that had ever lived in
a fiber cement-roofed house or worked in asbestos-associated
industries, including the manufacture of fiber cement, ceramic
tiles, insulating materials or other construction materials,
shipbuilding and mineral mining.
All patients were followed up until August 31, 2013
to determine the survival time. The survival time was measured
between the date of clinical diagnosis and mortality or censoring
(the last date the patients were lost to follow-up or the last date
the patients could be contacted, whether they remained alive or
not). The date of clinical diagnosis was defined as the date on
which MPM was diagnosed at Pham Ngoc Thach Hospital.
Detection of SV40 Tag expression
The formaldehyde-fixed, paraffin-embedded tissues of
the pleural specimens of the patients were immunostained for SV40
Tag expression. The Lab Vision mouse monoclonal antibody pAb101
(dilution, 1:100; catalog no., MS-1832-P; Thermo Fisher Scientific
Inc., Waltham, MA, USA) was used to detect SV40 Tag expression. The
secondary antibody used was Lab Vision™ biotinylated goat
anti-polyvalent anti-mouse/rabbit immunoglobulin G (ready to use;
catalog no., TP-125-BN; Thermo Fisher Scientic, Inc.). The
detection system used was a Thermo Scientific™ Lab Vision™ DAB Plus
Substrate System (Thermo Fisher Scientific, Inc.). The staining
procedure was performed according to the manufacturer's protocol.
Specimens with nuclear immunoreactive tumor cells were considered
to express SV40 Tag (Fig. 1). The
positive results were scored according to the Allred score system
as follows: 1–25% of tumor cells demonstrate SV40 Tag expression,
1+; 26–50%, 2+; and >50%, 3+
(18).
Detection of asbestos bodies
A light microscope (ECLIPSE 50i; Nikon Corp., Tokyo,
Japan) was used to examine and count the presence of asbestos
bodies in specimens of lung tissue or bronchoalveolar lavage fluid.
The lung tissue specimens were stained using hematoxylin-eosin
(Thermo Fisher Scientific, Inc.). The fluid specimens were stained
using the papanicolaou method (19)
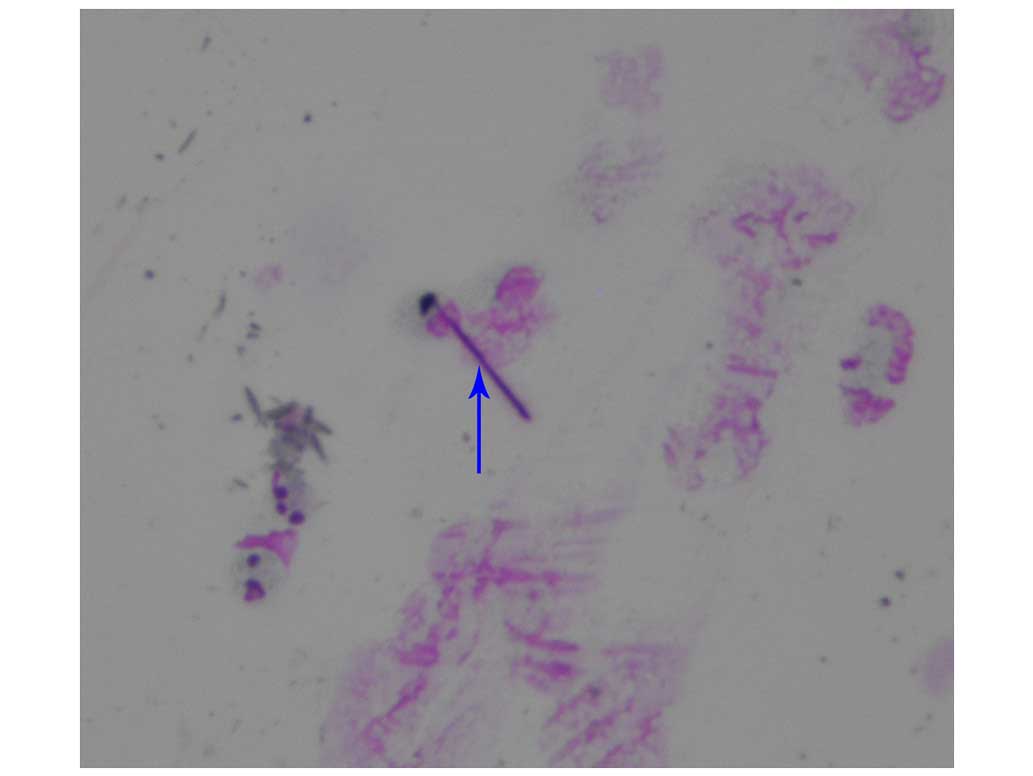
(Fig. 2), subsequent to breaking down
the mucus in the fluid using 10% NaOH (Thermo Fisher Scientific,
Inc.). A specimen with >5 asbestos bodies in every 10 examined
fields with a ×40 magnification was considered to contain asbestos
bodies (20).
Statistical analysis
The categorical variables are expressed as frequency
and percent. The comparisons of the proportions of SV40 Tag
expression between 2 groups were examined using the Fisher's exact
test. Comparisons of the survival time between two groups were
examined using the Log-Rank test of the Kaplan-Meier analysis. The
Cox regression survival analysis was used to examine the effect of
chemotherapy on the survival time, adjusting for the clinical
stages of MPM. A P-value of <0.05 was considered to indicate a
statistically significant difference. All statistical analyses were
performed using the JMP 9.0.2 statistical software (SAS Institute
Inc., Cary, NC, USA).
Results
Study population
Of the patients diagnosed with MPM at Pham Ngoc
Thach Hospital between January 2008 and June 2012, 45 patients met
the inclusion criteria. The mean (± standard deviation) and median
age were 59 (±15) and 58 years, respectively. The youngest patient
was 25 and the eldest was 88 years old. Over half of the patients
were female and 89% were in stage IV disease (Table I). Only 44% of the patients had a
history of asbestos exposure. For the definitive diagnosis of MPM,
60% of the patients required a transcutaneous needle biopsy, while
40% required a thoracoscopic biopsy. Epithelioid was the most
common histological subtype (51%) of MPM. Only 22 patients had
clinical specimens available for the examination of asbestos
bodies, 21 of which possessed bronchoalveolar lavage fluid
specimens and 1 of which possessed a lung tissue specimen. Asbestos
bodies were identified in 10 (45%) out of 22 patients.
 | Table I.Characteristics of 45 patients with
malignant pleural mesothelioma. |
Table I.
Characteristics of 45 patients with
malignant pleural mesothelioma.
| Characteristic | No. of patients | % of total |
|---|
| Gender |
|
|
| Male | 19 | 42 |
|
Female | 26 | 58 |
| History of asbestos
exposure |
|
|
| Yes | 20 | 44 |
| No | 25 | 56 |
| Method of pleural
biopsy |
|
|
|
Transcutaneous needle | 27 | 60 |
|
Thoracoscopy | 18 | 40 |
| Histological
subtypes |
|
|
|
Epithelioid | 23 | 51 |
|
Biphasic | 7 | 16 |
|
Sarcomatoid | 6 | 13 |
|
Desmoplastic | 4 | 9 |
|
Papillary | 4 | 9 |
|
Anaplastic | 1 | 2 |
| SV40 Tag
expression |
|
|
|
Positive | 9 | 20 |
|
Negative | 36 | 80 |
| Asbestos bodies |
|
|
|
Positive | 10 | 45 |
|
Negative | 12 | 55 |
| Clinical stage |
|
|
| II | 3 | 7 |
| III | 2 | 4 |
| IV | 40 | 89 |
Proportion of SV40 Tag expression
In total, 9 (20%) out of 45 patients exhibited SV40
Tag expression in the histological specimens: 2 patients with an
Allred score of 1+, 4 patients with an Allred score of
2+; and 3 patients with an Allred score of
3+. However, only 1 (5%) out of 22 patients exhibited
SV40 Tag expression and asbestos bodies.
The proportion of SV40 Tag expression was decreased
in males compared with females (5 vs. 31%), but this difference was
not significant (P=0.0578). Similarly, the proportion of SV40 Tag
expression was not significantly different between the patients
with and without asbestos bodies (P=0.5940), with the epithelioid
subtype and other subtypes (P=1.000), or the patients with stage IV
and other stages of disease (P=1.000) (Table II). There was no significant
difference in the mean age between the patients with and without
SV40 Tag expression (58.8±16.6 vs. 59.1±14.6; P=0.7227).
 | Table II.Comparison of the proportions of SV40
Tag expression between groups. |
Table II.
Comparison of the proportions of SV40
Tag expression between groups.
|
| SV40 Tag
expression |
|
|---|
|
|
|
|
|---|
| Characteristic | Expressed, frequency
(%) | Not expressed,
frequency (%) | P-valuea |
|---|
| Gender |
|
|
|
| Male | 1/19 (5) | 18/19 (95) | 0.0578 |
|
Female | 8/26
(31) | 18/26 (69) |
|
| Asbestos bodies |
|
|
|
|
Positive | 1/10
(10) | 9/10
(90) | 0.5940 |
|
Negative | 3/12
(25) | 9/12
(75) |
|
| Histological
subtype |
|
|
|
|
Epithelioid | 5/23
(22) | 18/23 (78) | 1.0000 |
|
Other | 4/22
(18) | 18/22 (82) |
|
| Stage |
|
|
|
| Stage
IV | 8/40
(20) | 32/40 (80) | 1.0000 |
| Stages
II–III |
1/5 (20) |
4/5 (80) |
|
Survival time
Among the 45 patients, 34 succumbed to the disease,
5 dropped out of the study and 6 survived during the follow-up. The
median survival time was 236 days [95% confidence interval (CI),
125–366]. The proportions of patients surviving for 1 and 2 years
were 35% (95% CI, 22–51%) and 23% (95% CI, 12–38%), respectively.
The median survival time was not significantly different between
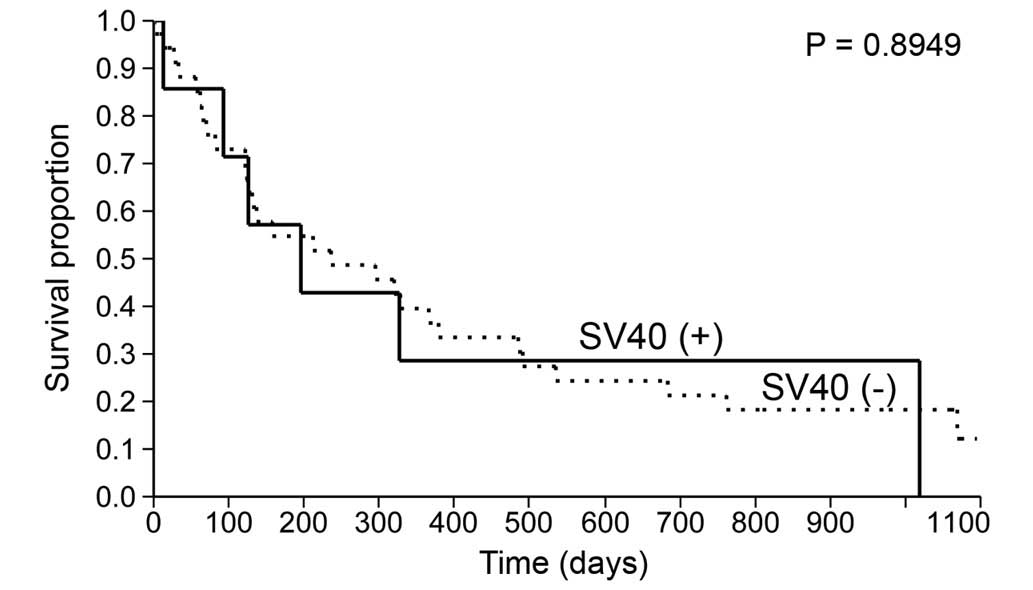
the patients with or without SV40 Tag expression (196 vs. 236 days;
P=0.8949) (Table III; Fig. 3). Similarly, the median survival time
was not significantly different between the patients with the
epithelioid subtype and other subtypes (327 vs. 131 days;
P=0.7803). By contrast, the median survival time was significantly
increased in the patients receiving chemotherapy compared with the
patients not receiving chemotherapy (435 vs. 196 days; P=0.0397)
(Fig. 4A). The mortality in the
patients receiving chemotherapy decreased by 52% compared with the
patients not receiving chemotherapy (hazard ratio, 0.48; 95% CI,
0.23–0.96) (Table III). However,
the Cox regression survival analysis indicated that only the
clinical stages of MPM had significant effect on the survival time
(P<0.0001), while the chemotherapy did not (adjusted hazard
ratio, 0.75; 95% CI, 0.36–1.52; P=0.4317). In a subset of patients
with stage IV disease, the median survival time was not
significantly different between the patients receiving chemotherapy
and the patients not receiving chemotherapy (227 vs. 137 days;
P=0.4344) (Fig. 4B).
 | Table III.Comparisons of the survival time
between groups. The total number of patients was 45. |
Table III.
Comparisons of the survival time
between groups. The total number of patients was 45.
| Characteristic | No. of cases | No. of
mortalities | No. of survivors, n
(%) | Median survival
time, days (95% CI) |
P-valuea |
|---|
| SV40 Tag
expression |
|
|
|
| 0.8949 |
|
Expressed | 9 | 6 | 3 (33) | 196
(13–1,019) |
|
| Not
expressed | 36 | 28 | 8 (22) | 236
(122–379) |
|
| Histological
subtype |
|
|
|
| 0.7803 |
|
Epithelioid | 23 | 19 | 4 (17) | 327
(125–491) |
|
|
Other | 22 | 15 | 7 (32) | 131 (62–485) |
|
| Management |
|
|
|
| 0.0397b |
|
Chemotherapy | 17 | 12 | 5 (29) |
435
(125–1,019) |
|
| No
chemotherapy | 28 | 22 | 6 (21) | 196 (62–327) |
|
Discussion
The present study shows that a 5th of the Vietnamese
patients with MPM demonstrated SV40 Tag expression in their
histological specimens. This finding indicates that a 5th of
patients with MPM may be associated with SV40, which may explain
why not all patients with MPM are associated with asbestos
exposure. Only half of the patients had evident asbestos exposure,
indicated by either the history of asbestos exposure or the
examination of asbestos bodies in clinical specimens.
To the best of our knowledge, the present study is
the first to propose the association between SV40 and MPM in
Vietnamese patients and to register Vietnam as one of the countries
in which SV40 may potentially affect the pathogenesis of MPM.
However, the proportion of patients with SV40 in the present study
is decreased compared with previously published studies (8,10,15). This finding may result from the
varying prevalence of SV40 infection between countries. The
immunohistochemical methods used in the present study may also not
be as sensitive as the molecular methods of SV40 detection, used in
previous studies (9,11).
For the patients that demonstrated SV40 Tag
expression, the means by which they contracted the virus was
unknown. In previous studies, SV40 infection was hypothesized to be
a result of receiving the SV40-contaminated polio vaccines that
were produced before 1961 (8,10,15). In
the present study, only 2 patients with SV40 Tag expression were
born prior to 1961. Therefore, the remaining patients may have
possibly contracted the virus through SV40-contaminated polio
vaccines that remained available in Vietnam subsequent to 1961, as
was observed in other Eastern European countries (21). Another explanation is that the
patients were infected by other unknown sources (22).
In the present study, only half of the patients
exhibited evidence of asbestos exposure, which is a lower figure
compared with other reports (70–80%) (3). There are several potential reasons for
this low prevalence: The method used to detect asbestos bodies may
not be sensitive enough (23); only
22 (49%) out of 45 patients had clinical specimens available for
asbestos body examination; and there may be a recalled bias
regarding the history of asbestos exposure during the interviews of
patients or close relatives.
Notably, only 5% of the patients exhibited
overlapping results for the presence of asbestos bodies and SV40
Tag expression. This finding may imply that the interaction between
SV40 and asbestos exposure is not the prerequisite for the
development of mesothelioma in humans, which has been previously
demonstrated in hamsters (24). The
finding also supports the speculation that SV40 may be an
independent carcinogen (25) or a
co-carcinogen, and interact with other environmental or genetic
factors in the pathogenesis of malignant mesothelioma (6).
The proportion of patients that survived for 1 and 2
years in the present study was similar to other populations
(26,27). The finding that only the clinical
stages of MPM significantly affected the survival time, whereas
chemotherapy did not, partly explains why the prognosis of MPM
remains poor, regardless of current therapies for MPM, particularly
as the majority of MPM patients are diagnosed at stage IV of
disease. In the present study, the median survival time was not
significantly different between the patients with or without SV40
Tag expression. This finding may be explained by the lack of
significant differences in the mean age, histological subtypes and
clinical stages of MPM between the 2 groups of patients (Table II).
There are certain strengths of the present study.
First, the present study is the first to propose the association
between SV40 and MPM in Vietnam. Second, the present study included
a balanced number of male and female patients, making the results
more generalizable compared with other studies. Third, SV40
detection was based on immunohistochemical analysis, which avoids
the potential false expression that may occur in polymerase chain
reaction tests due to the presence of SV40 sequence-contaminated
plasmids in pathological laboratories (13).
However, the present study has certain limitations.
First, as the present study is retrospective, not all patients had
clinical specimens available for asbestos body examination.
Therefore, the prevalence of asbestos exposure may be
underestimated in the present study. Second, SV40 was only detected
using immunohistochemistry, which may not be as sensitive as other
molecular methods. Third, immunohistochemistry results may yield
false expression due to the immunostaining procedure and result
interpretation. However, the immunostaining procedure was performed
following the antibody manufacturer's protocols to minimise the
risk of false expression. In addition, SV40 expression was strictly
defined as the presence of strong immunoreactive tumor nuclei,
which indicates the nuclear expression of SV40 Tag. Therefore, the
possibility of false expression may be limited. Fourth, since the
sample size is relatively small, the power to detect statistically
significant differences between groups of patients was not
sufficient. Finally, the present study is limited by the lack of
published data regarding the general levels of SV40 in the
Vietnamese population.
In conclusion, a 5th of the Vietnamese patients with
MPM were infected with SV40. SV40 may be another potential cause of
MPM in Vietnam and this potential association requires thorough
investigation with a larger sample size and more reliable methods
of SV40 detection.
References
|
1
|
Robinson BM: Malignant pleural
mesothelioma: An epidemiological perspective. Ann Cardiothorac
Surg. 1:491–496. 2012.PubMed/NCBI
|
|
2
|
Robinson BW, Musk AW and Lake RA:
Malignant mesothelioma. Lancet. 366:397–408. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cugell DW and Kamp DW: Asbestos and the
pleura: A review. Chest. 125:1103–1117. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yang H, Testa JR and Carbone M:
Mesothelioma epidemiology, carcinogenesis, and pathogenesis. Curr
Treat Options Oncol. 9:147–157. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Carbone M, Pannuti A, Zhang L, Testa JR
and Bocchetta M: A novel mechanism of late gene silencing drives
SV40 transformation of human mesothelial cells. Cancer Res.
68:9488–9496. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Carbone M, Pass HI, Miele L and Bocchetta
M: Novel developments about the association of SV40 with human
mesothelioma. Oncogene. 22:5173–5180. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jaurand MC and Fleury-Feith J:
Pathogenesis of malignant pleural mesothelioma. Respirology.
10:2–8. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Comar M, Rizzardi C, de Zotti R, Melato M,
Bovenzi M, Butel JS and Campello C: SV40 multiple tissue infection
and asbestos exposure in a hyperendemic area for malignant
mesothelioma. Cancer Res. 67:8456–8459. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jin M, Sawa H, Suzuki T, Shimizu K, Makino
Y, Tanaka S, Nojima T, Fujioka Y, Asamoto M, Suko N, et al:
Investigation of simian virus 40 large T antigen in 18 autopsied
malignant mesothelioma patients in Japan. J Med Virol. 74:668–676.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Testa JR, Carbone M, Hirvonen A, Khalili
K, Krynska B, Linnainmaa K, Pooley FD, Rizzo P, Rusch V and Xiao
GH: A multi-institutional study confirms the presence and
expression of simian virus 40 in human malignant mesotheliomas.
Cancer Res. 58:4505–4509. 1998.PubMed/NCBI
|
|
11
|
Aoe K, Hiraki A, Murakami T, Toyooka S,
Shivapurkar N, Gazdar AF, Sueoka N, Taguchi K, Kamei T, Takeyama H,
et al: Infrequent existence of simian virus 40 large T antigen DNA
in malignant mesothelioma in Japan. Cancer Sci. 97:292–295. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hirvonen A, Mattson K, Karjalainen A,
Ollikainen T, Tammilehto L, Hovi T, Vainio H, Pass HI, Di Resta I,
Carbone M and Linnainmaa K: Simian virus 40 (SV40)-like DNA
sequences not detectable in finnish mesothelioma patients not
exposed to SV40-contaminated polio vaccines. Mol Carcinog.
26:93–99. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
López-Ríos F, Illei PB, Rusch V and
Ladanyi M: Evidence against a role for SV40 infection in human
mesotheliomas and high risk of false-positive PCR results owing to
presence of SV40 sequences in common laboratory plasmids. Lancet.
364:1157–1166. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pilatte Y, Vivo C, Renier A, Kheuang L,
Greffard A and Jaurand MC: Absence of SV40 large T-antigen
expression in human mesothelioma cell lines. Am J Respir Cell Mol
Biol. 23:788–793. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
De Rienzo A, Tor M, Sterman DH, Aksoy F,
Albelda SM and Testa JR: Detection of SV40 DNA sequences in
malignant mesothelioma specimens from the United States, but not
from Turkey. J Cell Biochem. 84:455–459. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kushitani K, Takeshima Y, Amatya VJ,
Furonaka O, Sakatani A and Inai K: Immunohistochemical marker
panels for distinguishing between epithelioid mesothelioma and lung
adenocarcinoma. Pathol Int. 57:190–199. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sandeck HP, Røe OD, Kjærheim K, Willén H
and Larsson E: Re-evaluation of histological diagnoses of malignant
mesothelioma by immunohistochemistry. Diagn Patho. 5:472010.
View Article : Google Scholar
|
|
18
|
Allred DC, Harvey JM, Berardo M and Clark
GM: Prognostic and predictive factors in breast cancer by
immunohistochemical analysis. Mod Pathol. 11:155–168.
1998.PubMed/NCBI
|
|
19
|
Nguyen GK and Kline TS: Cytology
laboratory and quality assurance practice. Essentials of
Exfoliative Cytology (New York, NY). Igaku-Shoin Medical
Publishers, Inc. 6–13. 1992.
|
|
20
|
De Vuyst P, Dumortier P, Moulin E,
Yourassowsky N, Roomans P, de Francquen P and Yernault JC: Asbestos
bodies in bronchoalveolar lavage reflect lung asbestos body
concentration. Eur Respir J. 1:362–367. 1988.PubMed/NCBI
|
|
21
|
Cutrone R, Lednicky J, Dunn G, Rizzo P,
Bocchetta M, Chumakov K, Minor P and Carbone M: Some oral
poliovirus vaccines were contaminated with infectious SV40 after
1961. Cancer Res. 65:10273–10279. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Martini F, Corallini A, Balatti V,
Sabbioni S, Pancaldi C and Tognon M: Simian virus 40 in humans.
Infect Agent Cancer. 2:132007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xaubet A, Rodriguez-Roisín R, Bombí JA,
Marín A, Roca J and Agustí-Vidal A: Correlation of bronchoalveolar
lavage and clinical and functional findings in asbestosis. Am Rev
Respir Dis. 133:848–854. 1986.PubMed/NCBI
|
|
24
|
Kroczynska B, Cutrone R, Bocchetta M, Yang
H, Elmishad AG, Vacek P, Ramos-Nino M, Mossman BT, Pass HI and
Carbone M: Crocidolite asbestos and SV40 are cocarcinogens in human
mesothelial cells and in causing mesothelioma in hamsters. Proc
Natl Acad Sci USA. 103:14128–14133. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cicala C, Pompetti F and Carbone M: SV40
induces mesotheliomas in hamsters. Am J Pathol. 142:1524–1533.
1993.PubMed/NCBI
|
|
26
|
Milano MT and Zhang H: Malignant pleural
mesothelioma: A population-based study of survival. J Thorac Oncol.
5:1841–1848. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
van der Bij S, Koffijberg H, Burgers JA,
Baas P, van de Vijver MJ, de Mol BA and Moons KG: Prognosis and
prognostic factors of patients with mesothelioma: A
population-based study. Br J Cancer. 107:161–164. 2012. View Article : Google Scholar : PubMed/NCBI
|