Introduction
Worldwide, colorectal cancer (CRC) is the third most
common cancer (1). Currently, the
standard regimen for newly diagnosed patients with locally advanced
rectal cancer (grade, cT3/T4 and cN+) is surgery in
combination with neoadjuvant radiochemotherapy (2,3). However,
the majority of patients have mid to advanced stage CRC at the time
of diagnosis. Neoadjuvant radiochemotherapy improves the survival
and anus-preservation rates by shrinking tumors, decreasing the
clinical stage and reducing the pathological grade (4). While patients with local CRC have a more
favorable outcome, with a 5-year survival rate of 90%, patients
with metastatic CRC have a poor 5-year survival rate of 12%,
despite the good therapeutic regimens that are available, including
surgical resection, adjuvant radiotherapy and chemotherapy
(5).
Hepatocyte growth factor receptor (c-MET) is a
tyrosine kinase located on cell membranes, and is composed of two
disulfide-linked chains, an extracellular 50-kD α-chain and a
transmembrane 140-kD β-chain with tyrosine kinase activity
(6). The ligand of c-MET is
hepatocyte growth factor (HGF), also termed scatter factor. HGF is
secreted by fibroblasts in the tumor stroma, which acts on c-MET in
tumor cells, leading to the activation of downstream signaling
pathways, therefore implicating c-MET in the development,
progression and metastasis of cancer (7). c-MET promotes the mitosis, migration and
morphogenesis of multiple cells (8),
and aberrant expression of c-MET is associated with the development
and progression of multiple human malignancies (9). There are two important phenotypes that
appear to be more pronounced with the activation of c-MET,
metastasis and drug resistance (10).
High c-MET expression has been detected in CRC and has been
observed to be associated with tumor invasion and lymph node and
liver metastasis (11,12). It has been revealed that c-MET may
upregulate the expression of hypoxia-inducible factor (HIF) in
tumor cells, therefore increasing the resistance of tumor cells to
radiochemotherapy (13). In addition,
c-MET was revealed to induce epithelial-mesenchymal transition
(EMT) in tumor cells (14). Cells
that develop EMT exhibit stem cell characteristics and are
resistant to radiochemotherapy (6).
However, little is known concerning the effect of HGF/c-MET
inhibition on the sensitivity of CRC cells to radiotherapy
(15,16).
The present study hypothesized that concurrent c-MET
inhibition may sensitize CRC cells to irradiation. The current
study investigated the effect of c-MET inhibition using short
hairpin (sh)RNA or the c-MET inhibitor PHA665752 on the viability
of human colon carcinoma cells and xenografts exposed to
irradiation.
Materials and methods
Cells lines
The human colorectal adenocarcinoma HT-29 and colon
carcinoma SW620 cell lines were purchased from the Cell Bank of
Type Culture Collection of Chinese Academy of Sciences (Shanghai,
China). The cells were incubated at 37°C in a 100% atmosphere and
in complete Leibovitz's L-15 medium (M&C Gene Technology,
Beijing, China) with Gibco tetracycline-free 10% fetal bovine serum
(Thermo Fisher Scientific, Inc., Waltham, MA, USA), 100 units/ml
penicillin and 100 µg/ml streptomycin (M&C Gene
Technology).
Lentiviral infections
shRNA targeting the c-MET gene (sequence,
5′-AGAATGTCATTCTACATGAGC-3′; Promega Corporation, Madison, WI, USA)
and a scrambled shRNA (sequence, 5′-ATCAGAACCAGAGGCTTGGTC-3′) were
separately cloned into the BamHI site of a TA Cloning®
vector. The scrambled shRNA and TA Cloning vector were kindly
donated by Dr. Demin Zhou (School of Pharmaceutical Sciences,
Peking University, Beijing, China). The lentiviral vector plasmids
pSD400-c-MET-shRNA and pSD400-scr-shRNA were generated by cloning
the BamHI fragment of the TA Cloning vector containing
appropriate shRNAs into the BamHI site of the lentiviral
vector pSD400 (Dr Demin Zhou; School of Pharmaceutical Sciences,
Peking University, Beijing, China). The recombinant lentiviruses
were produced by co-transfecting human embryonic kidney 293T cells
with pSD400-c-MET-shRNA or pSD400-scr-shRNA and the packaging
vectors pMDL, pRSV and VSV-G (human embryonic kidney 293T cells and
packaging vectors were obtained from the School of Pharmaceutical
Sciences, Peking University, Beijing, China). This was achieved
using Invitrogen Lipofectamine® 2000 kit (Thermo Fisher Scientific,
Inc.). The HT-29 cells were then infected with the lentiviral
vectors, consisting of pSD400-c-MET-shRNA expressing shRNA against
c-MET or pSD400-scr-shRNA expressing scrambled shRNA at a final
concentration at 10 µg/ml, and transfected cells were selected with
0.85 µg/ml puromycin (M&C Gene Technology).
Immunoblotting assays
Cellular lysates were prepared using the RIPA lysis
buffer (Dakewe Biotech Co., Ltd, Beijing, China) containing a
cocktail of protease inhibitors (Roche Diagnostics, Basel,
Switzerland). The cellular proteins were resolved by SDS-PAGE and
electrotransferred to a polyvinyl difluoride transfer membrane. The
immunoblotting procedure was performed as previously described
(17), using the rabbit anti-human
c-MET monoclonal antibody (dilution, 1:2,000; catalog no., ab51067;
Abcam, Cambridge, MA, USA) and rabbit anti-human glyceraldehyde
3-phosphate dehydrogenase (GAPDH) monoclonal antibody (dilution,
1:1,000; catalog no., ab181602; Abcam). The protein bands were
visualized using Immobilon Western Chemiluminescent HRP Substrate
(catalog no., WBKLS0500; EMD Millipore, Billerica, MA, USA).
Densitometry was performed by calculating the optical density ×
optical region using Molecular Analyst™ (Bio-Rad Laboratories,
Inc., Hercules, CA, USA) and Image J software (version 1.410;
National Institutes of Health, Bethesda, MD, USA). GAPDH acted as
an internal control and c-MET protein expression was described as
the c-MET/GAPDH ratio.
alamarBlue® assays
Transfected HT-29 cells were irradiated at a dose of
0, 2, 4, 6 or 8 Gy and cell viability was assessed using Invitrogen
alamarBlue assays, according to the manufacturer's protocol (Thermo
Fisher Scientific, Inc.). Subsequent to a 72 h incubation, the
cells were stained with alamarBlue dye and the fluorescence
intensities were measured at 540 nm using SpectraMax Gemini XS
(Molecular Devices, LLC., Sunnyvale, CA, USA), and the values were
calculated with the controls set at 100%. The experiments were
performed ≥3 times independently in 6 pairs.
Mouse xenograft studies
In total, 32 4-week-old female nude mice of the
Balb/c strain were purchased from the Institute of Laboratory
Animal Sciences, Chinese Academy of Medical Sciences [Beijing,
China; permission no. SCXK (Jing) 2005–0013]. The mice were bred in
a clean environment at the Experimental Animal Center of the Fourth
Hospital Affiliated to Hebei Medical University (Shijiazhuang,
Hebei, China) and fed a cobalt-60-irradiated mouse diet. The
experimental protocol for the present animal study was approved by
the Institutional Animal Care and Use Committee. The animal
experiments were conducted in accordance with the USA National
Institutes of Health guidelines for the care and use of laboratory
animals (18).
In total, 5×106 SW620 cells in suspended
in 200 µl dimethyl sulfoxide (DMSO; Tokyo Chemical Industry, Tokyo,
Japan) were subcutaneously inoculated into the left
costal-abdominal region of the mice. The xenograft growth was
monitored and when it reached a size of ~100 mm3 at 7
days post-inoculation, the mice were randomly assigned to receive
various agents, as follows: 2.5% DMSO intraperitoneally once every
2 days for 3 weeks; PHA665752 (Pfizer, Inc., New York, NY, USA) at
25 mg/kg intraperitoneally once every 2 days for 3 weeks;
irradiation at a total dose of 10 Gy; or PHA665752 at 25 mg/kg
intraperitoneally followed 24 h later by irradiation at a total
dose of 10 Gy. The long and short diameters of the tumor were
measured using a beam caliper every 3 days and the tumor volume was
calculated using the following formula: Tumor size = (π × long
diameter × short diameter2) / 6. The nude mice were
anesthetized by intraperitoneal injection with 10% chloral hydrate
(Shanghai Seebio Biotechnology, Inc., Shanghai, China), sacrificed
and dissected, and the tumor xenografts were collected for
subsequent experiments.
Terminal deoxynucleotidyl
transferase-mediated dUTP nick-end labeling (TUNEL) assays
Apoptosis of tumor cells was detected by TUNEL
assay, according to the manufacturer's protocol (Roche
Diagnostics), and the tissues were incubated with
peroxidase-labeled mouse anti-human anti-digoxin polyclonal
antibody (dilution, 1:100; catalog no., 11684817910; In Situ
Cell Death Detection kit; Sigma-Aldrich, St. Louis, MO, USA). The
tissue sections were observed under a fluorescence microscope
(Zeiss EM 109; Carl Zeiss AG, Oberkochen, Germany) and images were
captured. In total, 5 slides were selected per treatment and 10
fields of view were randomly selected. The number of apoptotic
cells was counted and averaged by two experienced pathologists, who
assessed all slides and were blind to the treatment administered,
under an optical microscope (BX61; Olympus Corp., Tokyo, Japan) at
a magnification of x100. The percentage of apoptotic cells
[apoptotic index (AI)] was estimated using the following formula:
AI (%) = (number of apoptotic cells / total cell number) × 100.
Immunohistochemistry
Immunohistochemistry was performed using the
streptavidin peroxidase method. The tumor tissues were incubated
with rabbit anti-human double stranded break antibody H2AX
monoclonal antibody and rabbit anti-human hypoxia inducible factor
(HIF)-1α monoclonal antibody (Abcam) at 4°C overnight. The tissues
were conjugated with a secondary monoclonal rabbit anti-biotin
antibody (catalog no. SP-9001; dilution, 1:200; SPlink HRP Rabbit
Detection (DAB) kit; Hebei Bio-High Technology Development Co.,
Shijiazhuang, China), and visualized with 3,3′-diaminobenzidine.
H2AX is indicated by brown-yellow staining of the nuclei, while
HIF-1α is indicated by brown-yellow staining of the cytoplasm and
membrane of cells. Image-Pro® plus image analysis software version
6.0 (Media Cybernetics, Inc., Rockville, MD, USA) was used for
quantitative analysis. The integrated optical density of the
positively stained cell per unit region at each field of view was
calculated, and the mean density was estimated from 3 randomly
selected fields of view.
Statistical analysis
All statistical analyses were performed using SPSS
software version 13.0 (SPSS, Inc., Chicago, IL, USA). All numerical
variables were expressed as the mean ± standard deviation, and were
analyzed using one-way analysis of variance. Pairwise comparisons
were calculated using Fisher's least significant difference or
Student-Newman-Keuls test, and differences of proportions were
tested for statistical significance with the χ2 test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Downregulation of c-MET expression
sensitizes human colon carcinoma cells to irradiation in vitro
The immunoblotting assays revealed marked
suppression of the expression of c-MET upon DOX treatment (400 and
1,000 nM; Fig. 1A). The present study
evaluated the effect of c-MET downregulation on irradiation-induced
cytotoxicity against human colorectal adenocarcinoma HT-29 cells.
alamarBlue assays demonstrated that the irradiation caused a
significant dose-dependent decrease in the viability rate of HT-29
cells (2 Gy, 69.0±7.90%; 8 Gy, 37.2±8.02%; Fig. 1B). c-MET downregulation by shRNA
markedly accentuated irradiation-induced reduction in the viability
of HT-29 cells compared with HT-29 cells irradiated at the same
doses (P<0.05), which indicates that c-MET downregulation
sensitizes HT-29 cells to irradiation in vitro.
c-MET inhibition accentuates the
suppression of tumor xenograft growth in nude mice
In mice bearing human colon carcinoma SW620 cells,
irradiation markedly reduced the MTV (998.0±180.6 mm3) compared to
DMSO (2,231.1±855.6 mm3; P<0.01; Fig.
2A). In addition, PHA665752 caused a significant reduction in
the MTV (844.8±190.0 mm3; P<0.01 vs. DMSO). The combination of
irradiation and PHA665752 caused a markedly greater reduction in
the MTV (382.8±42.4 mm3; P<0.01 vs. irradiation or PHA665752
alone). These results demonstrate that c-MET inhibition may
accentuate the suppression by irradiation of tumor xenograft growth
in vivo.
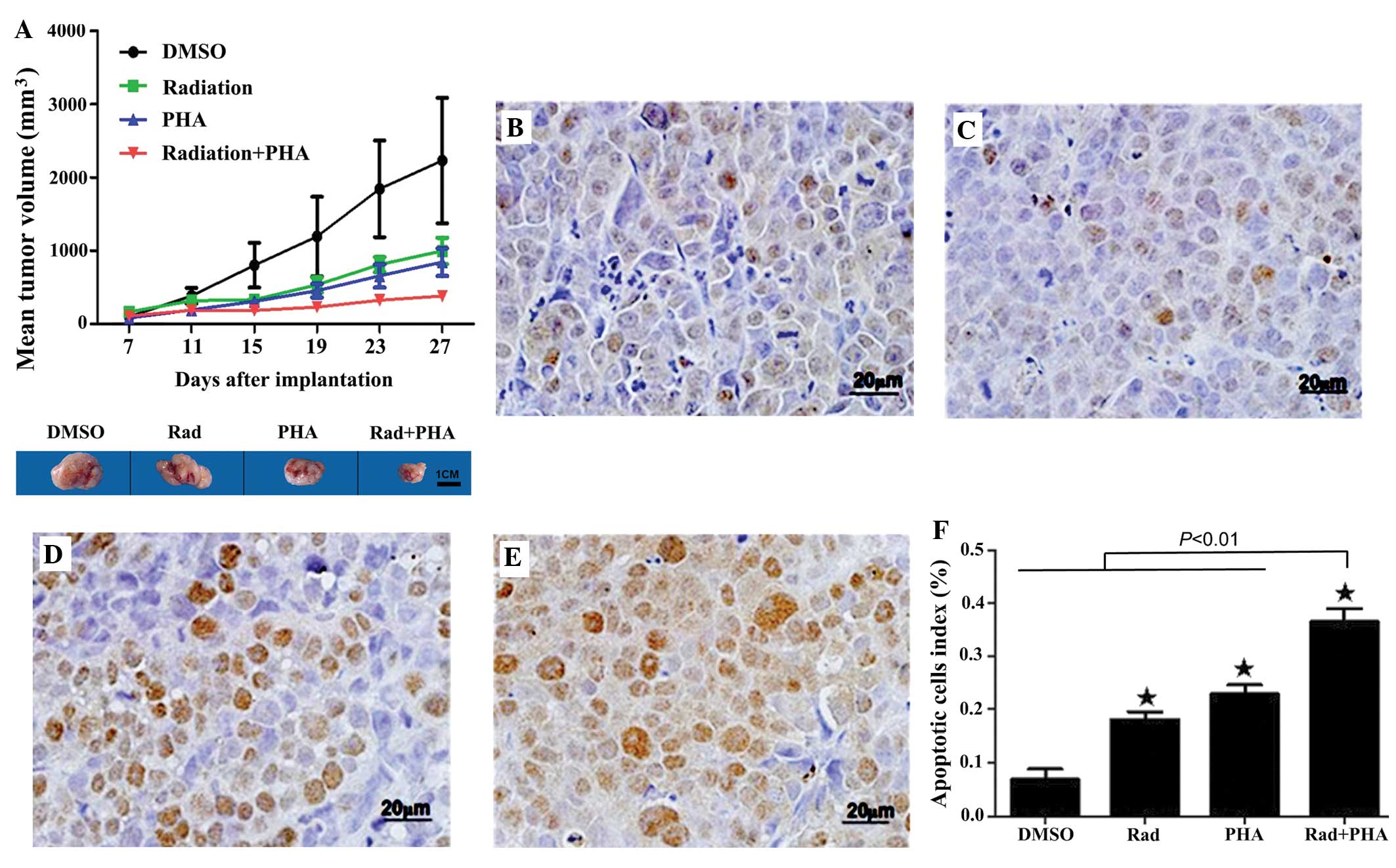 | Figure 2.(A) Mice bearing human colon carcinoma
SW620 xenografts were treated with 2.5% DMSO, PHA665752,
irradiation or the combination of irradiation and PHA665752. Tumor
growth was monitored by measuring the mean tumor volume. P<0.01,
PHA665752, irradiation or a combination of irradiation and
PHA665752 vs. DMSO; P<0.01, the combination of irradiation and
PHA665752 vs. irradiation or PHA665752. (B-E) Mice were treated as
in (A) and the apoptotic rate of the xenograft tissue was examined
by terminal deoxynucleotidyl transferase-mediated dUTP nick-end
labeling assay: (B) The DMSO group; (C) the irradiation group; (D)
the PHA665752 group; and (E) the combination of irradiation and
PHA665752 group. (F) The percentage of apoptotic cells in the tumor
xenografts. *P<0.01, the combination of irradiation and
PHA665752 vs. DMSO, irradiation or PHA665752. DMSO; dimethyl
sulfoxide; PHA665752, hepatocyte growth factor receptor inhibitor;
PHA, PHA665752; Rad, irradiation. |
c-MET inhibition enhances the
apoptosis of human colon carcinoma cells in the tumor xenograft by
promoting irradiation-induced formation of DNA double strand
breaks
TUNEL assays demonstrated that irradiation and
PHA665752 alone caused significant apoptosis of SW620 cells in the
tumor xenograft (irradiation, 18.14%; PHA665752, 22.90%; P<0.01
vs. DMSO; Fig. 2B–E). The AI in the
tumor xenograft of mice treated with a combination of irradiation
and PHA665752 was significantly higher (36.43%) compared with mice
treated with either agent alone (P<0.01; Fig. 2F).
The present study examined the expression of γ-H2AX
in SW620 cells. Irradiation of the tumor xenografts bearing SW620
cells caused a marked increase in the expression of γ-H2AX
(P<0.01 vs. DMSO; Fig. 3A, B and
E). PHA665752 also caused a clear increase in the expression of
γ-H2AX (P<0.01 vs. DMSO; Fig. 3C and
E). The combination of irradiation and PHA665752 leads to the
greatest increase in the expression of γ-H2AX (P<0.01 vs.
irradiation or PHA665752; Fig. 3D and
E). The present findings reveal that concurrent targeted
inhibition of c-MET aggravates irradiation-induced formation of DNA
double strand breaks in mouse tumor xenografts.
 | Figure 3.Mice bearing human colon carcinoma
SW620 xenografts were treated with 2.5% DMSO, PHA665752,
irradiation or the combination of irradiation and PHA665752. γ-H2AX
expression in the tumor xenograft was examined using
immunohistochemistry in the (A) DMSO, (B) irradiation, (C)
PHA665752, and (D) combination of irradiation and PHA665752 groups.
(E) Quantification of γ-H2AX expression. *P<0.01, combination of
irradiation and PHA665752 vs. DMSO, irradiation or PHA665752.
γ-H2AX, a double stranded break marker; DMSO; dimethyl sulfoxide;
PHA665752, hepatocyte growth factor receptor inhibitor; Rad,
irradiation; PHA, PHA665752. |
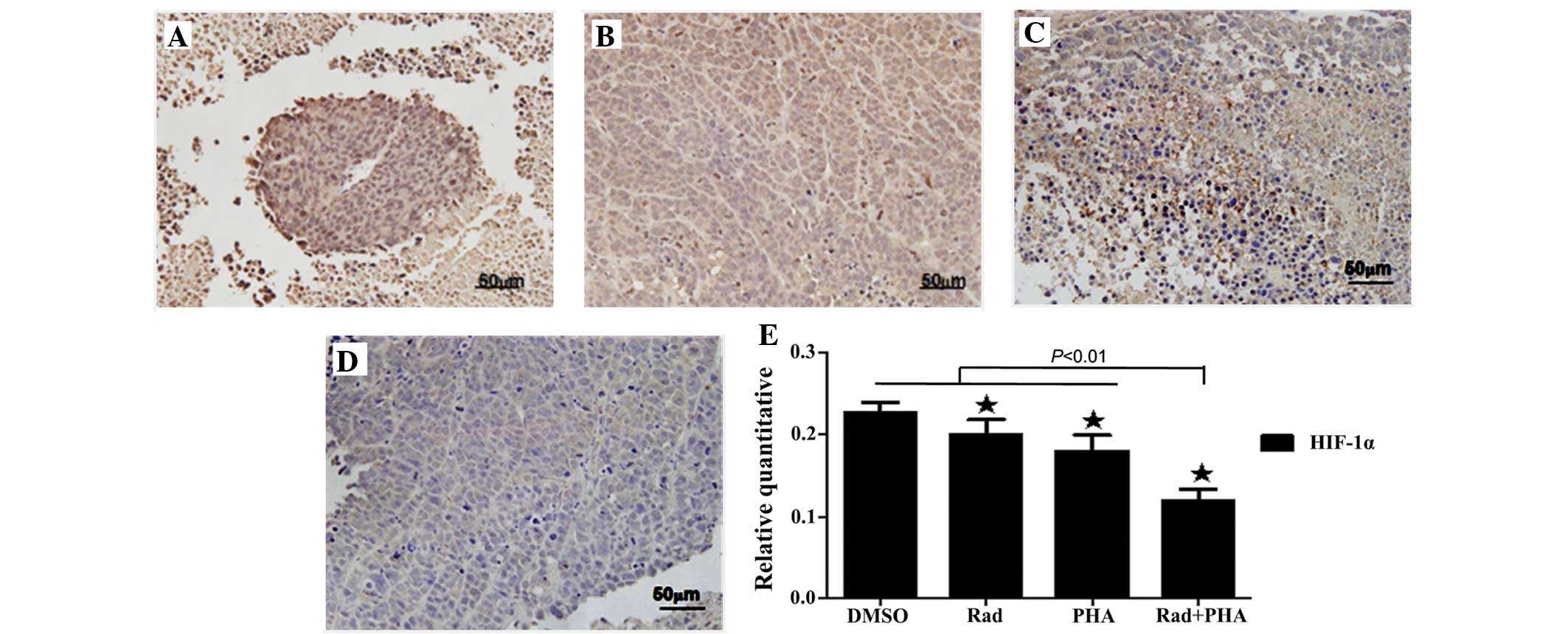
c-MET inhibition suppresses the
expression of HIF-1α in mouse tumor xenografts
Immunohistochemistry revealed that irradiation and
PHA665752 alone significantly suppressed the expression of HIF-1α
in the tumor xenografts (P<0.01 vs. DMSO; Fig. 4A–C and E). The combination of
irradiation and PHA665752 caused a significantly greater inhibition
of HIF-1α expression than either agent alone (P<0.01 vs.
irradiation or PHA665752; Fig. 4D and
E). The results demonstrate that treatment with a c-MET
inhibitor alleviates hypoxia in tumor tissues.
Discussion
CRC is a common malignant tumor of the
gastrointestinal tract (19). This
type of cancer often reaches mid to advanced stages prior to
diagnosis (20). Recently, there has
been a shift in the paradigm for the treatment of CRC between
conventional chemotherapy and combination therapy, consisting of
chemotherapy and radiotherapy, and targeted therapy, including
antibodies against vascular endothelial growth factor (VEGF), such
as bevacizumab, and antibodies against epidermal growth factor
receptor, such as cetuximab and panitumumab (11,21).
However, the outcome of patients with advanced-stage CRC remains
poor, therefore mandating the search for alternative effective
therapies (11,21). The present study hypothesized that a
blockade of MET signaling may aid in overcoming resistance to
radiotherapy. The present study demonstrated that c-MET
downregulation by shRNA or treatment with the small-molecule c-MET
inhibitor PHA665752 accentuated the cytotoxicities of irradiation
against colon cancer cells in vitro and in vivo. This
suggests that c-MET inhibition may require investigation as a
future approach to the treatment of CRC.
Aberrant expression of c-MET is associated with the
development and progression of multiple human malignancies
(8,9)
and has been identified as a novel target for the treatment of
multiple malignant tumors (11,22).
Radiation has been observed to upregulate c-MET expression in a
HGF-dependent or independent manner, and promotes the transcription
of HGF and c-MET, while a high c-MET expression induces tumor
metastasis and prevents the apoptosis of cells, resulting in
radiochemotherapeutic resistance (15). In rectal cancer patients undergoing
concurrent chemoradiotherapy, high expression of
metastasis-associated in colon cancer-1 and c-MET was revealed to
be associated with a reduced relapse-free survival rate and an
adverse prognosis (23). Therefore,
targeting c-MET may exhibit a synergistic effect with chemotherapy
or radiotherapy (24,25). The present findings are consistent
with previous studies (26–29). It has been previously reported that
the combination therapy of the anti-HGF monoclonal antibody AMG102
with the cytotoxic agent temozolomide or docetaxel enhances the
anti-tumor action of temozolomide and docetaxel against
glioblastoma multiforme (30).
Currently, the mechanisms underlying
radiotherapeutic resistance in tumors mainly involve a reduction in
DNA repair capacity, hypoxia and tumor cell EMT. The present study
identified that PHA665752 significantly accentuated the
irradiation-induced elevation of γ-H2AX expression in human colon
carcinoma SW620 cells that led to enhanced apoptosis of the tumor
cells, which suggests that targeted inhibition of c-MET may impair
DNA repair in tumor cells caused by irradiation. It has been
demonstrated that the activation of c-MET is involved in resistance
to DNA damage, including radiation-induced DNA damage (31), and HGF/c-MET has been revealed to
protect DNA from damage via the phosphoinositide 3-kinase/protein
kinase B and proto-oncogenes RAS and RAF/mitogen-activated protein
kinase pathways (32).
In addition, the present findings demonstrated that
PHA665752 inhibited HIF-1α expression in mice bearing SW620
xenografts, which suggests that c-MET inhibition may alleviate
hypoxia in the tumor. Angiogenesis drives tumor growth (33). In addition to VEGF and the VEGF
receptor (VEGFR), the HGF/c-MET signaling pathway is involved in
tumor angiogenesis by upregulating the expression of pro-angiogenic
factors, including VEGF and VEGFR, to promote the proliferation and
migration of vascular endothelial cells, and by downregulating the
expression of the angiogenesis inhibitor thrombospondin-1 (34). It has been demonstrated that
anti-angiogenic treatments may normalize tumor vessels, which
accelerates blood circulation and improves hypoxia in the tumor,
and facilitates the aggregation of chemotherapeutic agents to the
tumor microenvironment; therefore increasing the sensitivity of the
tumor to radiochemotherapy (13,35). To
the best of our knowledge, there have been no previous studies
concerning the inhibition of HIF-1α expression in tumors by
PHA665752.
In summary, c-MET inhibition sensitizes CRC cells to
irradiation and may offer a promising approach for the treatment of
locally advanced CRC.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81172332).
References
|
1
|
Robbins AS, Siegel RL and Jemal A: Racial
disparities in stage-specific colorectal cancer mortality rates
from 1985 to 2008. J Clin Oncol. 30:401–405. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bosset JF, Collette L, Calais G, Mineur L,
Maingon P, Radosevic-Jelic L, Daban A, Bardet E, Beny A and Ollier
JC: EORTC Radiotherapy Group Trial 22921: Chemotherapy with
preoperative radiotherapy in rectal cancer. N Engl J Med.
355:1114–1123. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gérard JP, Conroy T, Bonnetain F, Bouché
O, Chapet O, Closon-Dejardin MT, Untereiner M, Leduc B, Francois E,
Maurel J, et al: Preoperative radiotherapy with or without
concurrent fluorouracil and leucovorin in T3–4 rectal cancers:
Results of FFCD 9203. J Clin Oncol. 24:4620–4625. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dhadda AS, Dickinson P, Zaitoun AM, Gandhi
N and Bessell EM: Prognostic importance of Mandard tumour
regression grade following pre-operative chemo/radiotherapy for
locally advanced rectal cancer. Eur J Cancer. 47:1138–1145. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hu T, Yao Y, Yu S, Guo H, Han L, Wang W,
Tian T, Hao Y, Liu Z, Nan K and Wang S: Clinicopathologic
significance of CXCR4 and Nrf2 in colorectal cancer. J Biomed Res.
27:283–290. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Scarpino S, d'Alena Cancellario F, Di
Napoli A, Pasquini A, Marzullo A and Ruco LP: Increased expression
of Met protein is associated with up-regulation of hypoxia
inducible factor-1 (HIF-1) in tumour cells in papillary carcinoma
of the thyroid. J Pathol. 202:352–358. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
De Wever O, Nguyen QD, Van Hoorde L,
Bracke M, Bruyneel E, Gespach C and Mareel M: Tenascin-C and SF/HGF
produced by myofibroblasts in vitro provide convergent
pro-invasive signals to human colon cancer cells through RhoA and
Rac. FASEB J. 18:1016–1018. 2004.PubMed/NCBI
|
|
8
|
Park MK, Kim DK and Lee HJ: Adenoviral
mediated hepatocyte growth factor gene attenuates hyperglycemia and
beta cell destruction in overt diabetic mice. Exp Mol Med.
35:494–500. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Comoglio PM and Trusolino L: Invasive
growth: From development to metastasis. J Clin Invest. 109:857–862.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Stellrecht CM and Gandhi V: MET receptor
tyrosine kinase as a therapeutic anticancer target. Cancer Lett.
280:1–14. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kammula US, Kuntz EJ, Francone TD, Zeng Z,
Shia J, Landmann RG, Paty PB and Weiser MR: Molecular co-expression
of the c-Met oncogene and hepatocyte growth factor in primary colon
cancer predicts tumor stage and clinical outcome. Cancer Lett.
248:219–228. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kataoka H, Hamasuna R, Itoh H, Kitamura N
and Koono M: Activation of hepatocyte growth factor/scatter factor
in colorectal carcinoma. Cancer Res. 60:6148–6159. 2000.PubMed/NCBI
|
|
13
|
Du Z, Qin R, Wei C, Wang M, Shi C, Tian R
and Peng C: Pancreatic cancer cells resistant to chemoradiotherapy
rich in “stem-cell-like” tumor cells. Dig Dis Sci. 56:741–750.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Suárez-Causado A, Caballero-Díaz D,
Bertrán E, Roncero C, Addante A, García-Álvaro M, Fernández M,
Herrera B, Porras A, Fabregat I and Sánchez A: HGF/c-Met signaling
promotes liver progenitor cell migration and invasion by an
epithelial-mesenchymal transition-independent, phosphatidyl
inositol-3 kinase-dependent pathway in an in vitro model.
Biochim Biophys Acta. 1853:2453–2463. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
De Bacco F, Luraghi P, Medico E, Reato G,
Girolami F, Perera T, Gabriele P, Comoglio PM and Boccaccio C:
Induction of MET by ionizing radiation and its role in
radioresistance and invasive growth of cancer. J Natl Cancer Inst.
103:645–661. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Buchanan IM, Scott T, Tandle AT, Burgan
WE, Burgess TL, Tofilon PJ and Camphausen K: Radiosensitization of
glioma cells by modulation of Met signalling with the hepatocyte
growth factor neutralizing antibody, AMG102. J Cell Mol Med.
15:1999–2006. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sun W, Song L, Ai T, Zhang Y, Gao Y and
Cui J: Prognostic value of MET, cyclin D1 and MET gene copy number
in non-small cell lung cancer. J Biomed Res. 27:220–230. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
National Research Council of the National
Academies: Guide for the care and use of laboratory animals (8th).
The National Academies Press. Washington, D.C., USA: 199–200.
2011.
|
|
19
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Singh H, Daci K, Petersen LA, Collins C,
Petersen NJ, Shethia A and El-Serag HB: Missed opportunities to
initiate endoscopic evaluation for colorectal cancer diagnosis. Am
J Gastroenterol. 104:2543–2554. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bekaii-Saab T and Wu C: Seeing the forest
through the trees: A systematic review of the safety and efficacy
of combination chemotherapies used in the treatment of metastatic
colorectal cancer. Crit Rev Oncol Hematol. 91:9–34. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Teicher BA: Antiangiogenic agents and
targets: A perspective. Biochem Pharmacol. 81:6–12. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kawamura M, Saigusa S, Toiyama Y, Tanaka
K, Okugawa Y, Hiro J, Uchida K, Mohri Y, Inoue Y and Kusunoki M:
Correlation of MACC1 and MET expression in rectal cancer after
neoadjuvant chemoradiotherapy. Anticancer Res. 32:1527–1531.
2012.PubMed/NCBI
|
|
24
|
Hong TS, Wo JY and Kwak EL: Targeted
therapies with chemoradiation in esophageal cancer: Development and
future directions. Semin Radiat Oncol. 23:31–37. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Akervall J, Nandalur S, Zhang J, Qian CN,
Goldstein N, Gyllerup P, Gardinger Y, Alm J, Lorenc K, Nilsson K,
et al: A novel panel of biomarkers predicts radioresistance in
patients with squamous cell carcinoma of the head and neck. Eur J
Cancer. 50:570–581. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Medová M, Aebersold DM, Blank-Liss W,
Streit B, Medo M, Aebi S and Zimmer Y: MET inhibition results in
DNA breaks and synergistically sensitizes tumor cells to
DNA-damaging agents potentially by breaching a damage-induced
checkpoint arrest. Genes Cancer. 1:1053–1062. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Medová M, Aebersold DM and Zimmer Y: MET
inhibition in tumor cells by PHA665752 impairs homologous
recombination repair of DNA double strand breaks. Int J Cancer.
130:728–734. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhuang HQ, Bo QF, Yuan ZY, Wang J, Zhao LJ
and Wang P: The different radiosensitivity when combining erlotinib
with radiation at different administration schedules might be
related to activity variations in c-MET-PI3K-AKT signal
transduction. Onco Targets Ther. 6:603–608. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Welsh JW, Mahadevan D, Ellsworth R, Cooke
L, Bearss D and Stea B: The c-Met receptor tyrosine kinase
inhibitor MP470 radiosensitizes glioblastoma cells. Radiat Oncol.
4:692009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jun HT, Sun J, Rex K, Radinsky R, Kendall
R, Coxon A and Burgess TL: AMG 102, a fully human anti-hepatocyte
growth factor/scatter factor neutralizing antibody, enhances the
efficacy of temozolomide or docetaxel in U-87 MG cells and
xenografts. Clin Cancer Res. 13:6735–6742. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fan S, Wang JA, Yuan RQ, Rockwell S,
Andres J, Zlatapolskiy A, Goldberg ID and Rosen EM: Scatter factor
protects epithelial and carcinoma cells against apoptosis induced
by DNA-damaging agents. Oncogene. 17:131–141. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Meyn RE, Munshi A, Haymach JV, Milas L and
Ang KK: Receptor signaling as a regulatory mechanism of DNA repair.
Radiother Oncol. 92:316–322. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Abdollahi A and Folkman J: Evading tumor
evasion: Current concepts and perspectives of anti-angiogenic
cancer therapy. Drug Resist Updat. 13:16–28. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Puri N, Khramtsov A, Ahmed S, Nallasura V,
Hetzel JT, Jagadeeswaran R, Karczmar G and Salgia R: A selective
small molecule inhibitor of c-Met, PHA665752, inhibits
tumorigenicity and angiogenesis in mouse lung cancer xenografts.
Cancer Res. 67:3529–3534. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Citrin D, Ménard C and Camphausen K:
Combining radiotherapy and angiogenesis inhibitors: Clinical trial
design. Int J Radiat Oncol Biol Phys. 64:15–25. 2006. View Article : Google Scholar : PubMed/NCBI
|