Introduction
Gastric cancer is the fourth most common cancer in
the world, with an estimated 988,000 cases in 2008 worldwide and an
estimated 736,000 mortalities occurring as a result of the disease
(1). More than 70% of cases occur in
developing countries (1). It is
characterized by high incidence, frequent metastasis, high
mortality and relative unresponsiveness to standard oncological
therapies, including radiotherapy and chemotherapy (2). The overall five-year survival rate is
<40%, due to relapse and metastasis (2).
Yes-associated protein (YAP) is a 65 kD proline-rich
phosphoprotein, which is located at locus 11q22 (3). A previous study reported evidence that
YAP may have oncogenic functions, and an increasing amount of
literature has associated elevated YAP expression with malignant
tumors (4). In MCF-10A cells, YAP
gene overexpression induces epithelial-to-mesenchymal transition,
which is a characteristic of malignant cell transformation
(5). YAP additionally combines with
myc to promote growth of tumors in mice (6). Notably, liver-specific YAP gene
overexpression leads to hepatic carcinoma in transgenic mice
(7). YAP is a member of the Hippo
signaling cascade, which consists of protein kinases and regulatory
proteins, including merlin, the mammalian Hippo homolog,
salvador/WW45 and Lats1/2. When activated the Hippo pathway
antagonizes YAP via phosphorylation and binding to 14-3-3 protein,
resulting in cytoplasmic sequestration (8).
RNA interference (RNAi) is a novel type of genetic
tool that is able to mediate posttranscriptional sequence-specific
gene silencing (9). The authors of
the present study established the YAP knockdown SGC7901 gastric
cancer cell line using lentivirus-mediated small hairpin (sh)RNA
and observed that silencing of YAP led to effective inhibition of
tumor cell growth and invasive ability in vitro (10,11). To
identify the effect of YAP silencing in vivo and explore its
mechanism, the present study performed animal experiments using a
mouse carcinoma model.
In the present study, it was demonstrated that
inhibition of YAP expression results in the reversal of a number of
properties associated with the malignant phenotype, including
proliferation and metastasis in vivo. The results of the
present study suggest that YAP may be a potential therapeutic
target for the treatment of gastric cancer.
Materials and methods
Materials
SGC7901 cells were purchased from the Shanghai
Institute of Biochemistry and Cell Biology (Shanghai, China).
TRIzol® Reagent was purchased from Thermo Fisher Scientific, Inc.
(Waltham, MA, USA). The lentivirus system against YAP gene
consisting of pGC-LV, pHelper 1.0 and pHelper 2.0 was obtained from
GeneChem Co., Ltd. (Shanghai, China) and was constructed as
previously described (11). The YAP
rabbit anti-human polyclonal antibody (sc-15407), the cluster of
differentiation (CD)31 rabbit anti-human polyclonal antibody
(sc-8306), the vascular endothelial growth factor receptor (VEGF)
rabbit anti-human polyclonal antibody (sc-152), the cyclinD1 rabbit
anti-human polyclonal antibody (sc-753), the Ki-67 goat anti-mouse
polyclonal antibody (sc-7846), the fibroblast growth factor (FGF)-2
rabbit anti-human polyclonal antibody (sc-79), the cyclinA rabbit
anti-human polyclonal antibody (sc-751), the cyclinE rabbit
anti-rat polyclonal antibody (sc-481) and the
glyceraldehyde-3-phosphate dehydrogenase (GAPDH) rabbit anti-rat
polyclonal antibody (sc-25778) were purchased from Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA). The TEA domain family member
1 (TEAD) rabbit anti-human polyclonal antibody (13283–1-AP) was
purchased from ProteinTech Group, Inc., (Chicago, IL, USA). ECL
Western Blotting Substrate (32106) was purchased from Thermo Fisher
Scientific, Inc.. TUNEL assay kit (KGA700) was purchased from
Nanjing KeyGen Biotech Co., Ltd. (Nanjing, China). All primers were
synthesized by the Shanghai Sangon Biological Engineering
Technology and Service Co., Ltd. (Shanghai, China). RPMI-1640
medium was obtained from Gibco (Thermo Fisher Scientific, Inc.) and
fetal bovine serum was purchased from Zhejiang Tianhang
Biotechnology Co., Ltd. (Hangzhou, China).
Animals
Female severe combined immunodeficiency mice (SCID;
6 weeks old) were purchased from the Shanghai Laboratory Animal
Center of the Chinese Academy of Sciences (Shanghai, China). All 5
mice were kept in laminar flow cabinets under specific
pathogen-free conditions, with food and water ad libitum
(humidity 30–50%, temperature 20–22°C and a 12-h light-dark cycle).
The mice were split into groups for the use of the study at random.
The use of animals in this study was approved by Sichuan Medical
Experimental Animal Care Committee (Chengdu, China).
Preparation of SGC7901 cells with YAP
gene expression stably inhibited
In our previous study (11), we successfully synthesized a
lentiviral vector small hairpin (sh)RNA (5′-CTCAGGATGGAGAAATTTA-3′)
targeting the YAP gene, which efficiently inhibited YAP expression
at the messenger (m)RNA and protein level in vitro. A
control vector carrying a sequence (5′-TTCTCCGAACGTGTCACGT-3′)
unrelated to the human gene was used as a negative control.
Subsequently, these vectors were transfected into SGC7901 cells
separately. During transfection, cells were seeded in a 24 well
plate with confluence reaching 80% prior to transfection. A total
of 4 µl Lipofectamine 2000® (11668–027; Thermo Fisher Scientific,
Inc.) and 5 µg plasmid DNA were mixed in Opti-MEM medium
(31985–088; Thermo Fisher Scientific, Inc.) and added to the cells
of each well according to manufacturer's protocol. Cells were
selected for stable expression by culturing in puromycin medium
(Thermo Fisher Scientific, Inc.) for 6 weeks. A total of 3
experimental groups were designed as follows: The YAP shRNA
vector-transfected cells (YAP-shRNA group), negative control
vector-transfected cells (NC group) and untransfected cells (CON
group).
Gastric orthotopic implantation tumor
model
SCID mice were injected subcutaneously into the
dorsal scapula region with 1×106 SGC7901 cells from one
of the three groups (YAP-shRNA, NC or CON). Once xenografts were
established, the tumors were removed and washed twice with
phosphate-buffered saline (PBS). Cancer tissue was divided into
small pieces (~1 mm3) and gastric surgical orthotopic
implantation of the tumor was performed. Briefly, following
anesthetizing of the mice with 2.5% Avertin (Sigma-Aldrich, St.
Louis, MO, USA), the stomach was exteriorized. The small sections
of cancer tissue were sewn onto the gastric wall and the laparotomy
was closed. When the animals become moribund during the observation
period (2 months later), the mice were sacrificed via cervical
dislocation., organs were excised, and metastases were determined
by observation and further confirmation by hematoxylin and eosin
staining.
Reverse transcription- quantitative
polymerase chain reaction (RT-qPCR) assay
RT-qPCR was used to quantitatively measure the mRNA
expression level. Total RNA from the mouse orthotopic gastric tumor
was extracted by using TRIzol reagent according to the
manufacturer's protocol. During RNA extraction, DNase I (1 u/µg;
Thermo Fisher Scientific Inc.) was added to the sample following
the manufacturer's protocol. Subsequently, 2 µg of total RNA was
reverse-transcribed with Moloney Murine Leukemia Virus reverse
transcriptase (Thermo Fisher Scientific, Inc.) to synthesize
complementary DNA, and RT-qPCR was performed with SsoAdvanced™
Universal SYBR Green Supermix (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) according to the manufacturer's protocol. YAP,
VEGF and FGF-2 genes were amplified using specific primers and the
results were normalized against the human GAPDH gene. A negative
control was performed with no RNA added and generated no
amplification. The sequences of the primers and the size of the
products are listed in Table I. PCR
was performed using the CFX Connect™ Real-Time PCR Detection system
(Bio-Rad Laboratories, Inc.). The conditions of the RT-qPCR were as
follows: 1 cycle of denaturation at 94°C for 5 min, followed by 30
cycles at 94°C for 1 min, 60°C for 1 min and 72°C for 1 min, and a
final extension step at 72°C for 10 min. Data were normalized using
the comparative Cq method (2−∆∆Cq) (12). A total of 3 parallel RT-qPCR
experiments were performed for each group.
 | Table I.Sequences of primers. |
Table I.
Sequences of primers.
| Primer | Sequence | Size of product,
bp |
|---|
| YAP forward |
CCTGATGGATGGGAACAAGC | 134 |
| YAP reverse |
GCACTCTGACTGATTCTCTGG |
|
| VEGF forward |
GCTTACTCTCACCTGCTTCTG | 89 |
| VEGF reverse |
GGCTGCTTCTTCCAACAATG |
|
| FGF-2 forward |
ATCAAAGGAGTGTGTGCTAACC | 178 |
| FGF-2 reverse |
ACTGCCCAGTTCGTTTCAGTG |
|
| GAPDH forward |
TGACTTCAACAGCGACACCCA | 121 |
| GAPDH reverse |
CACCCTGTTGCTGTAGCCAAA |
|
Western blotting
To quantitatively determine the protein expression
level, western blot analysis was performed. Gastric tumor tissue
was cut into small pieces and milled in a mortar with liquid
nitrogen. Subsequently, tissue extracts were prepared with
radioimmunoprecipitation assay lysis buffer (100 mM Tris-HCl
buffer, 4% sodium dodecyl sulfate (SDS), 20% glycerol, 2%
β-mercaptoethanol; pH 6.8). 10% SDS-polyacrylamide gel
electrophoresis was used to separate the samples and the protein
bands were transferred onto nitrocellulose membranes. The membranes
were blocked with 5% skim milk for 1 h at room temperature,
followed by hybridization with primary antibodies [YAP, VEGF,
FGF-2, TEAD, cyclinD1, GAPDH; dilution, 1:500] at 4°C overnight.
Following three washes in Tris-buffered saline and Tween 20 (TBST;
150 mM NaCl, 0.05% Tween 20, 50 mM TrisHCl; pH 7.6), the membranes
were treated with horseradish peroxidase (HRP)-conjugated goat
anti-rabbit secondary antibody (dilution, 1:3,000; ab205718; Abcam,
Cambridge, UK) for 2 h at room temperature. Subsequently, the
membranes were washed with TBST three times and the immunoreactive
bands were visualized using the ECL Western Blotting Substrate
(Thermo Fisher Scientific, Inc.) according to the manufacturer's
protocol. The relative protein expression in various cell lines was
normalized to GAPDH expression levels. The experiments were
performed three times.
Hematoxylin and eosin (H&E)
staining
To study tumor morphology, H&E staining was
used. Briefly, tumor samples were fixed with paraformaldehyde and
embedded with paraffin. The paraffin blocks were sliced into 5-µm
thick sections and mounted onto glass microscope slides.
Subsequently, the slides were deparaffinized using xylene and
graded alcohols prior to being stained with H&E. A total of 5
randomly selected microscopic fields from each slide were examined
by two pathologists with no prior information about the samples
using a microscope (BA410; Motic Incoporation, Ltd., Causeway Bay,
Hong Kong).
Immunohistochemistry (IHC)
Tumor specimens were prepared as described above.
Subsequently, the tissue sections were deparaffinized and placed in
antigen retrieval solution (Abcam) for 15 min at 100°C. Following
incubation in 1% bovine serum albumin (Sigma-Aldrich) for 30 min,
primary antibodies [YAP, CD31, cyclinA, cyclinD1, cyclinE;
dilution, 1:200] were applied to the slides for 2 h at 37°C.
Following washing with PBS, the sections were additionally
incubated with the HRP-conjugated donkey anti-goat secondary
antibody (dilution, 1:2,000; ab205723; Abcam) and avidin-conjugated
horseradish peroxidase (43–4423; Thermo Fisher Scientific, Inc.).
Finally, the sections were treated with 3,3′-diaminobenzidine, and
counterstained with hematoxylin, and dehydrated. A negative control
was performed by replacement of primary antibody with PBS. Two
pathologists reviewed the results, with IHC staining intensity
determined by Image-Pro Plus 6.0 (Media Cybernetics, Inc.,
Rockville, MD, USA).
Proliferation assay and apoptosis
assay
The cell proliferation assay procedure was performed
in same manner as the aforementioned IHC procedure, except
anti-Ki-67 primary antibody was used to measure cancer cell
proliferation ability. Terminal deoxynucleotidyl transferase dUTP
nick end labeling (TUNEL) assay was used to measure the cancer cell
apoptosis rate, and was performed according to manufacturer's
protocol. The proliferation rate and apoptotic rate were determined
quantitatively by counting the number of positively stained cells
in 5 fields at magnification, x200.
Statistical analysis
Statistically significant differences between groups
were determined with SPSS version 15.0 (SPSS, Inc., Chicago, IL,
USA). The differences between each group were tested for
significance using the Student's two-sided t-test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Silencing of the YAP gene inhibits
orthotopic gastric tumor growth
The present study analyzed the role of YAP-shRNA in
tumor growth in vivo. As shown in Fig. 1, the average tumor weight was
significantly reduced in the YAP-shRNA group compared with the NC
and CON groups (YAP-shRNA, 0.292±0.029 g vs. NC, 0.657±0.038 g;
P<0.01; YAP-shRNA, 0.292±0.029 g vs. CON, 0.715±0.054 g;
P<0.01). No marked difference was observed between the NC group
and the CON group. It was concluded that silencing of the YAP gene
in SGC7901 cells may inhibit orthotopic tumor growth (Fig. 1).
Silencing of the YAP gene reduces
metastatic tumor formation
To elucidate the effect of YAP knockdown on gastric
cancer metastasis, a gastric cancer orthotopic implantation model
was constructed. A total of two months later, all mice were
sacrificed due to severe cachexia. Tumors from the orthotopic
implantation and metastasis in other organs were dissected and
examined by H&E staining (Fig.
2). Histological analysis of tumor metastases revealed the
typical structure of metastatic adenocarcinoma. Table II summarizes the differences between
distant metastasis in the three groups. The incidence of liver,
diaphragm, lymph node and mesentery metastasis, as well as ascites
in the NC group was 75, 37.5, 50, 87.5 and 75%, respectively. In
the CON group, the incidence of liver, diaphragm, lymph node and
mesentery metastasis, as well as ascites was 50, 25, 62.5, 75 and
62.5%, respectively. By contrast, in the YAP-shRNA group, 12.5% had
lymph node metastasis, 25% had mesentery metastasis and 12.5% had
ascites. No detectable tumors in the liver and diaphragm were
identified. These results indicated that YAP knockdown inhibited
tumor metastasis in the gastric orthotopic implantation mouse
model.
 | Table II.Cases of gastric cancer metastasis
and ascites in severe combined immunodeficiency mice following
orthotopic implantation. |
Table II.
Cases of gastric cancer metastasis
and ascites in severe combined immunodeficiency mice following
orthotopic implantation.
|
|
| Metastasis |
|
|---|
|
|
|
|
|
|---|
| Group | n | Liver | Diaphragm | Lymph node | Mesentery | Ascites |
|---|
| CON | 8 | 6 | 3 | 4 | 7 | 6 |
| NC | 8 | 4 | 2 | 5 | 6 | 5 |
| YAP-shRNA | 8 | 0 | 0 | 1 | 2 | 1 |
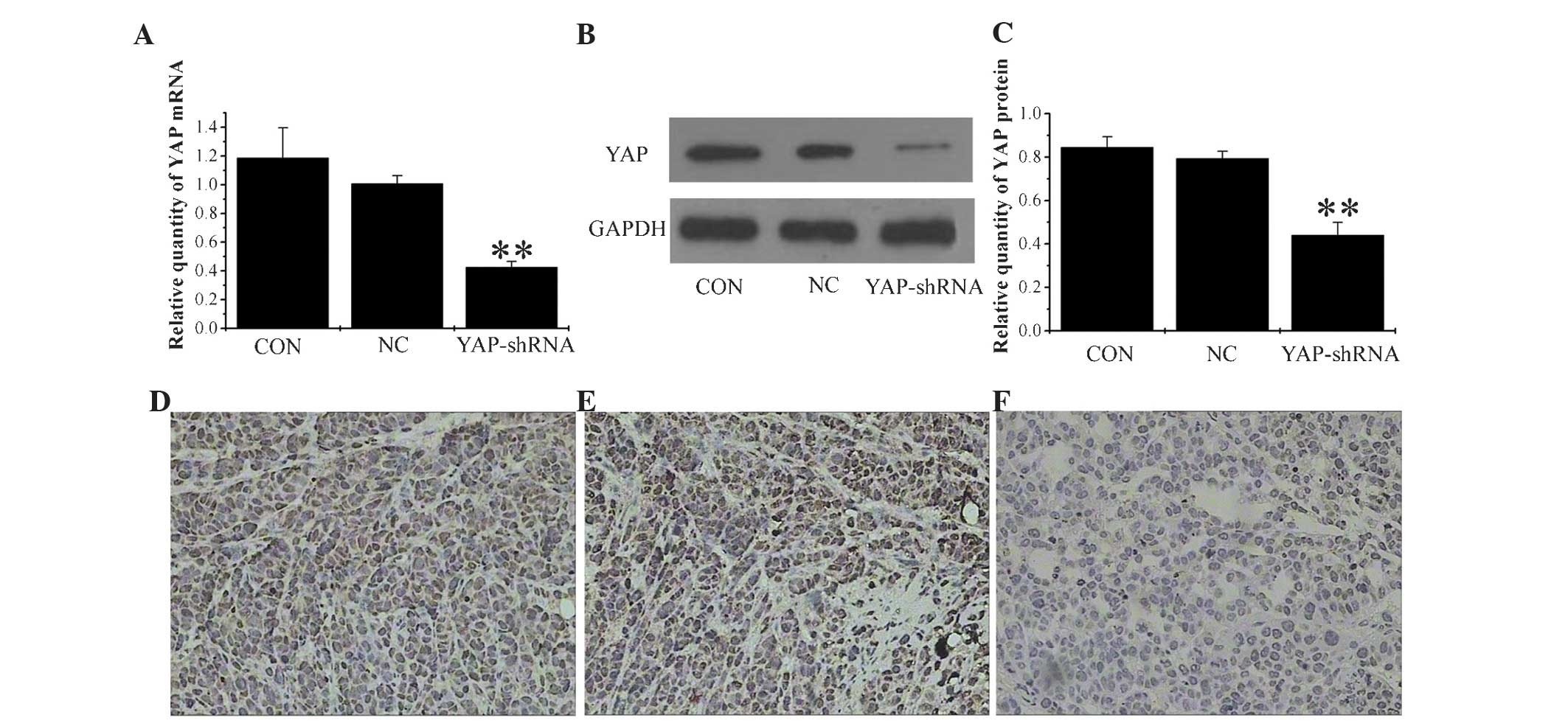
YAP-shRNA reduces YAP expression in
orthotopic implantation models
The stability of YAP-shRNA in orthotopic gastric
tumors was confirmed by RT-qPCR and western blotting. As shown in
Fig. 3A, B and C, a marked inhibition
of YAP mRNA and protein expression was observed in the YAP-shRNA
group compared with the NC and CON groups (mRNA YAP-shRNA,
0.425±0.04 vs. NC, 1.01±0.056; P<0.01; YAP-shRNA, 0.425±0.04 vs.
CON, 1.186±0.21; P<0.01; protein YAP-shRNA, 0.44±0.059 vs. NC,
0.794±0.033; P<0.01; YAP-shRNA, 0.44±0.059 vs. CON, 0.845±0.049;
P<0.01). Furthermore, following IHC, strong staining for YAP was
observed in the NC and CON groups, whereas in the YAP-shRNA group,
the tissues displayed only moderate staining (Fig. 3D, E and F). Therefore, this confirmed
that the efficiency of YAP-shRNA silencing is not altered in
vivo compared with its performance in vitro in cell
lines.
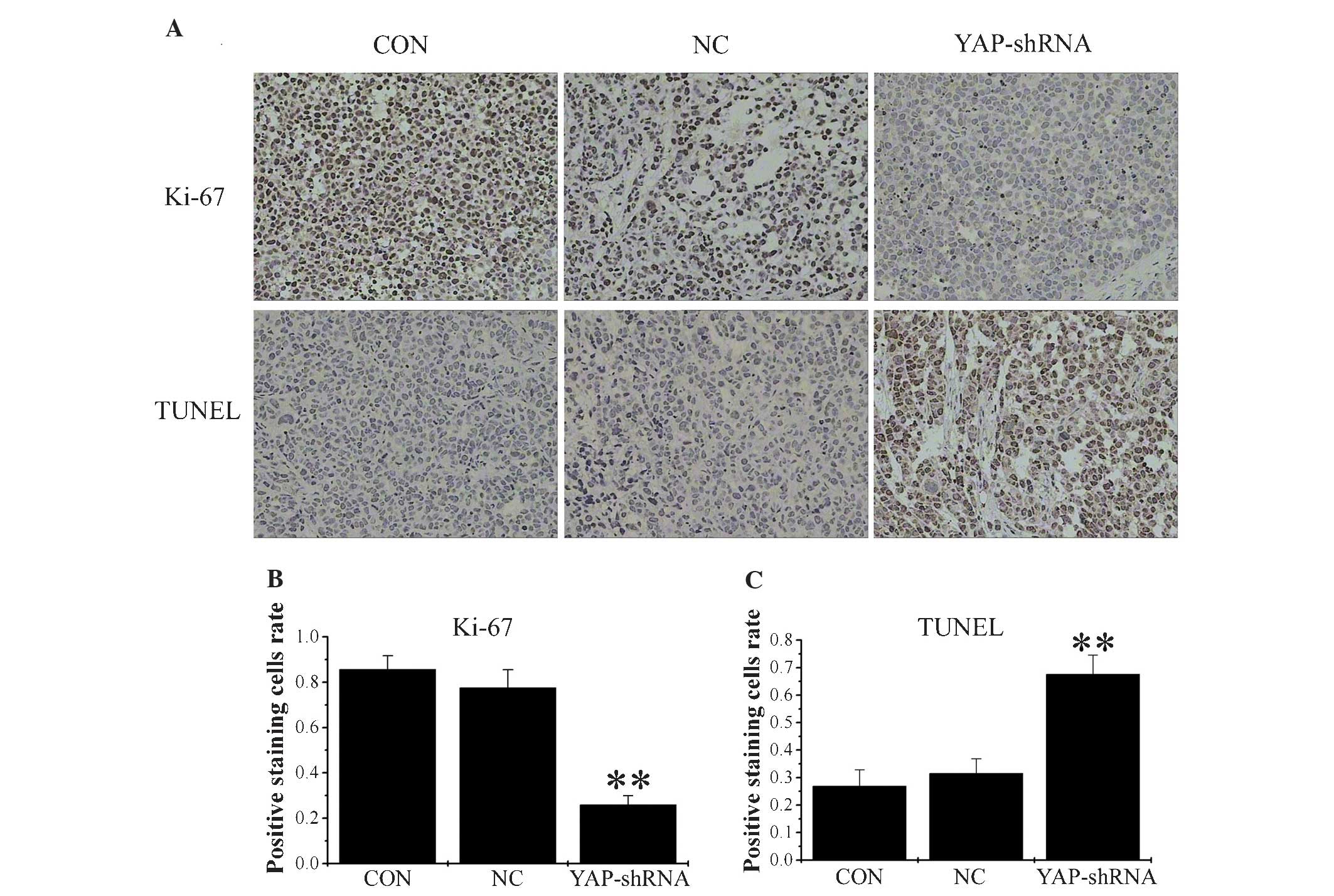
Silencing of YAP reduces proliferation
and promotes apoptosis
To examine proliferative ability, sections from
orthotopic gastric tumor were stained with anti-Ki-67 antibody, and
the percentage of cells positive for Ki-67 expression was
calculated. The results of the present study demonstrated that
there were fewer positive cells in the YAP-shRNA group compared
with the other two groups (YAP-shRNA, 0.259±0.04 vs. NC,
0.775±0.08; P<0.01; YAP-shRNA, 0.259±0.04 vs. CON, 0.875±0.06;
P<0.01). No significant differences were observed between the NC
and CON groups (Fig. 4). As Ki-67 is
a widely used proliferation biomarker and its expression indicates
active cell growth, these findings demonstrate that YAP-shRNA
reduced cancer cell proliferation in the mouse gastric cancer model
(13).
To analyze tumor cell apoptosis, the tumor sections
from orthotopic gastric tumor were stained for apoptotic markers
using a TUNEL staining assay. The number of apoptotic cells was
significantly increased in the YAP-shRNA group compared with the
other two groups (YAP-shRNA, 0.676±0.07 vs. NC, 0.315±0.05;
P<0.01; YAP-shRNA, 0.676±0.07 vs. CON, 0.269±0.06;
P<0.01). No significant difference was observed between the NC
and CON groups (Fig. 4). Therefore,
YAP-shRNA promotes gastric cancer cell apoptosis in the mouse
gastric cancer model.
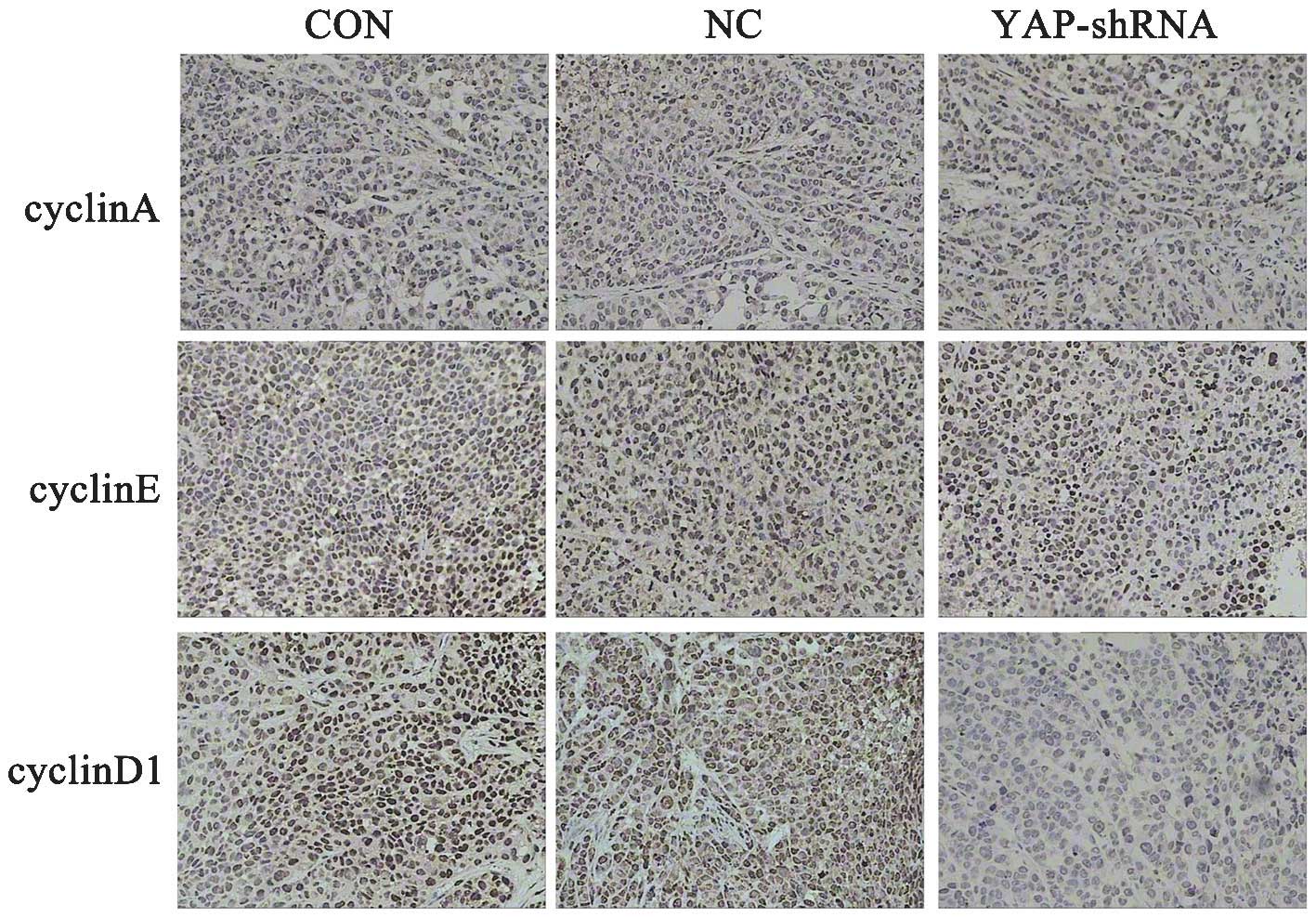
Silencing of the YAP gene affects the
expression of cyclinD1
A previous study by the present authors revealed
that cells are arrested in G1 phase in the YAP-shRNA group in
vitro (11). Therefore, the
present study examined the expression of cyclinA, cyclinD1 and
cyclinE in the orthotopic implantation gastric tumor by
immunohistochemical assay. As shown in Fig. 5, tumor cells transfected with
YAP-shRNA demonstrated lower expression of cyclinD1 compared with
that the NC and CON groups, while no significant difference in
expression was observed for cyclinA and cyclinE.
CycinD1 is a regulatory factor that regulates cell
cycle transition from G0 to G1 (14).
Our previous in vitro results are consistent with our in
vivo results (10). It is
well-known that YAP has a significant role in cancer cell
proliferation and is an important transcription factor for
regulating the cell cycle (15). A
previous study demonstrated that YAP regulates cyclinD1
transcription directly in cooperation with TEAD in malignant
mesothelioma cells (16). However, to
the best of our knowledge, no such study has been performed for
gastric cancer; therefore, the present study examined the effect of
knockdown of YAP on the expression of TEAD and cyclinD1 in
vivo. As shown in Fig. 6, the
expression of these factors was significantly decreased when YAP
gene expression was silenced, which indicates that
YAP/TEAD/cyclinD1 signaling may be involved in YAP
silencing-induced growth inhibition of gastric cancer.
Silencing of the YAP gene inhibits
angiogenesis, as well as VEGF and FGF-2 expression in vivo
To quantify the in vivo angiogenesis ability,
sections of orthotopic gastric cancer from each group were stained
for endothelial cell specific CD31. The number of vessels within
each section was quantified in five different fields at
magnification, x200. Tumors derived from the YAP-shRNA group had
reduced intratumoral microvessel density compared with the NC and
CON groups (YAP-shRNA, 6.167±1.47 vs. NC, 19.83±3.31; P<0.01;
YAP-shRNA, 6.167±1.47 vs. CON, 24.33±3.93; P<0.01; Fig. 7A, B, C and D).
VEGF and FGF-2 are potent mitogens for vascular
endothelial cells and have a significant role in gastric cancer
neovascularization (17). To
additionally explore the effects of YAP downregulation on
angiogenesis in vivo, the present study examined the
expression of VEGF and FGF-2. RT-qPCR and western blotting revealed
that YAP-shRNA-transfected cells constitutively expressed low
levels of VEGF and FGF-2 compared with the NC and CON groups
(Fig. 7E, F and G). Infiltration of
blood vessels is a sign of hematogenous metastasis (17). These results indicate that YAP
expression affects gastric cancer angiogenesis in vivo.
Discussion
The Hippo signaling pathway comprises a series of
cytoplasmic tumor suppressor proteins, including merlin, large
tumor suppressor kinase 1/2 and macrophage stimulating 1/2, and is
thought to play a critical role in determining the size of organs
and tissues (15). When activated,
the Hippo signaling pathway maintains the transcriptional activator
YAP in phosphorylated form in the cytoplasm and prevents cell
proliferation (18). When the Hippo
signaling pathway is blocked, YAP is translocated to the nucleus
and induces the expression of a variety of proteins that are
associated with a malignant cell phenotype (18).
YAP is the central player within the Hippo signaling
pathway, which has been considered an effective target for cancer
therapy (19). The role of YAP in
oncogenesis has gained significant attention. Increasing evidence
has demonstrated that YAP is involved in the development and
progression of cancer (15). However,
whether YAP is associated with cancer metastasis remains unclear.
In our previous studies, it was demonstrated that targeting the YAP
gene using RNAi inhibited the migration, invasion,
anchorage-independent growth and angiogenesis ability of SGC-7901
cells (10,11). Furthermore, in our previous study, a
lentivirus plasmid vector expressing shRNAs directed against the
YAP gene was developed, and it was demonstrated that RNAi driven by
these shRNA-based lentiviruses was able to effectively silence YAP
gene expression at the mRNA and protein level in vitro
(11). Furthermore, functional
analyses revealed that abrogation of YAP expression not only
suppressed growth and induced apoptosis of cancer cells, but
additionally inhibited cancer cell metastasis in vitro
(11). These studies indicated that
YAP may be important for cancer metastasis at a cellular level.
Therefore, in the present study a tumor model was developed in SCID
mice to further study the YAP gene.
The present study demonstrated that silencing of YAP
caused an inhibition of gastric cancer growth in an orthotopic
gastric cancer model. A surgical orthotopic implantation model
involves xenotransplantation of fresh solid tumor into
immunodeficient mice (20,21), and this model was utilized in the
present experiments. Silencing of YAP is associated with a
significant reduction of the incidence of metastasis and ascites.
For gastric cancer patients, metastasis is an indicator of poor
prognosis (22). Silencing of YAP is
associated with decreased cell migration and invasion (Table II and Fig.
7). This suggests that YAP not only regulates gastric cancer
cell proliferation, but additionally has an impact on cancer
metastasis.
Apoptosis is an important mechanism for elimination
of potentially tumorigenic cells (23). Quantification of apoptotic cells by
TUNEL assay was performed in vivo. Experimental data
demonstrated that apoptosis of cells transfected with YAP-shRNA
vectors was significantly increased compared with control groups.
The effect on cancer cell proliferation was investigated by Ki-67
assay, which is the most widely used proliferation biomarker
(24). It was demonstrated that YAP
downregulation reduces the expression levels of Ki-67. Therefore,
it appears reasonable to hypothesize that the YAP gene may promote
proliferation and inhibit apoptosis in gastric cancer.
Cell cycle factors have a significant role in cell
cycle progression (25). The present
study additionally examined the expression of cell cycle associated
proteins cyclinA, cyclinD1 and cyclinE in the three groups
following YAP inhibition. It was observed that the expression level
of cyclinD1 is significantly decreased when YAP expression is
silenced, while the expression of cyclinA and cyclinE is not
significantly changed. CyclinD1 has a significant role in
regulating cell cycle progression (14). CyclinD1 mRNA and protein are
overexpressed in several types of human cancer (26).
YAP is not able to bind the cyclinD1 gene directly,
as it possesses no DNA binding sites; YAP activates its targets by
binding transcription factors (27).
Consistent with previous results, YAP and TEAD promoted cyclinD1
expression in gastric cancer (16).
Increased angiogenesis provides a route of
dissemination from the primary tumor and may contribute to the
growth of the metastatic tumor (28).
Therefore, primary tumors that are more heavily vascularized are
more aggressive and demonstrate increased metastatic potential
(29). Antitumor efficacy of
anti-angiogenic drugs could be judged by reduced intratumoral
microvessel density (30). Although
few studies have demonstrated the association between YAP
expression and angiogenesis, the results of the present study
demonstrated a significant downregulation of tumor capillary
number, as well as VEGF and FGF-2 expression levels, following
stable YAP gene silencing.
Previous studies demonstrated that silencing of the
YAP gene inhibited gastric cancer cell proliferation, migration and
invasion in vitro (10,11). The
results of the present study demonstrate that knockdown of the YAP
gene significantly reduces tumor growth and suppresses cancer
metastasis to other organs in vivo in the orthotopic gastric
cancer models. The mechanism of this anti-tumor effect appears to
include activation of cancer cell apoptosis, and inhibition of
cancer cell proliferation and migration. Therefore, the anti-tumor
effects of YAP-shRNA on gastric cancer are not only limited to
exist in vitro, but also exist in vivo. Thus, the
results of the present study suggest that YAP may be a potential
molecular target for gastric cancer therapy.
In conclusion, the present study indicated that YAP
may exhibit an important role in the metastasis of gastric cancer,
and that downregulation of YAP gene expression may present a useful
therapeutic modality for gastric cancer treatment.
References
|
1
|
PDQ Screening Prevention Editorial Board:
Stomach (Gastric) Cancer Prevention (PDQ): Health Professional
Version. http://www.cancer.gov/types/stomach/hp/stomach-prevention-pdqAccessed.
February 05–2016
|
|
2
|
Guo HQ, Guan P, Shi HL, Zhang X, Zhou BS
and Yuan Y: Prospective cohort study of comprehensive prevention to
gastric cancer. World J Gastroenterol. 9:432–436. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Guo X and Zhao B: Integration of
mechanical and chemical signals by YAP and TAZ transcription
coactivators. Cell Biosci. 3:332013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hong W: Angiomotin'g YAP into the nucleus
for cell proliferation and cancer development. Sci Signal.
6:pe272013.PubMed/NCBI
|
|
5
|
Overholtzer M, Zhang J, Smolen GA, Muir B,
Li W, Sgroi DC, Deng CX, Brugge JS and Haber DA: Transforming
properties of YAP, a candidate oncogene on the chromosome 11q22
amplicon. Proc Natl Acad Sci USA. 103:12405–12410. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Xiao W, Wang J, Ou C, Zhang Y, Ma L, Weng
W, Pan Q and Sun F: Mutual interaction between YAP and c-Myc is
critical for carcinogenesis in liver cancer. Biochem Biophys Res
Commun. 439:167–172. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu-Chittenden Y, Huang B, Shim JS, Chen
Q, Lee SJ, Anders RA, Liu JO and Pan D: Genetic and pharmacological
disruption of the TEAD-YAP complex suppresses the oncogenic
activity of YAP. Genes Dev. 26:1300–1305. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Felley-Bosco E and Stahel R: Hippo/YAP
pathway for targeted therapy. Transl Lung Cancer Res. 3:75–83.
2014.PubMed/NCBI
|
|
9
|
Okamoto K and Murawaki Y: The therapeutic
potential of RNA interference: Novel approaches for cancer
treatment. Curr Pharm Biotechnol. 13:2235–2247. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhou Z, Zhu JS and Xu ZP: RNA interference
mediated YAP gene silencing inhibits invasion and metastasis of
human gastric cancer cell line SGC-7901. Hepatogastroenterology.
58:2156–2161. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhou Z, Zhu JS, Xu ZP and Zhang Q:
Lentiviral vector-mediated siRNA knockdown of the YAP gene inhibits
growth and induces apoptosis in the SGC7901 gastric cancer cell
line. Mol Med Rep. 4:1075–1082. 2011.PubMed/NCBI
|
|
12
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li LT, Jiang G, Chen Q and Zheng JN: Ki67
is a promising molecular target in the diagnosis of cancer
(review). Mol Med Rep. 11:1566–1572. 2015.PubMed/NCBI
|
|
14
|
Pestell RG: New roles of cyclin D1. Am J
Pathol. 183:3–9. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Moroishi T, Hansen CG and Guan KL: The
emerging roles of YAP and TAZ in cancer. Nat Rev Cancer. 15:73–79.
2015. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mizuno T, Murakami H, Fujii M, Ishiguro F,
Tanaka I, Kondo Y, Akatsuka S, Toyokuni S, Yokoi K, Osada H and
Sekido Y: YAP induces malignant mesothelioma cell proliferation by
upregulating transcription of cell cycle-promoting genes. Oncogene.
31:5117–5122. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cao R, Ji H, Feng N, Zhang Y, Yang X,
Andersson P, Sun Y, Tritsaris K, Hansen AJ, Dissing S and Cao Y:
Collaborative interplay between FGF-2 and VEGF-C promotes
lymphangiogenesis and metastasis. Proc Natl Acad Sci USA.
109:15894–15899. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hong W and Guan KL: The YAP and TAZ
transcription co-activators: Key downstream effectors of the
mammalian Hippo pathway. Semin Cell Dev Biol. 23:785–793. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kang W, Tong JH, Chan AW, Lee TL, Lung RW,
Leung PP, So KK, Wu K, Fan D, Yu J, et al: Yes-associated protein 1
exhibits oncogenic property in gastric cancer and its nuclear
accumulation associates with poor prognosis. Clin Cancer Res.
17:2130–2139. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bai F, Guo X, Yang L, Wang J, Shi Y, Zhang
F, Zhai H, Lu Y, Xie H, Wu K and Fan D: Establishment and
characterization of a high metastatic potential in the peritoneum
for human gastric cancer by orthotopic tumor cell implantation. Dig
Dis Sci. 52:1571–1578. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Thalheimer A, Otto C, Bueter M, Illert B,
Gattenlohner S, Gasser M, Fein M, Germer CT and Waaga-Gasser AM:
Tumor cell dissemination in a human colon cancer animal model:
Orthotopic implantation or intraportal injection? Eur Surg Res.
42:195–200. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lehnert T, Rudek B, Buhl K and Golling M:
Surgical therapy for loco-regional recurrence and distant
metastasis of gastric cancer. Eur J Surg Oncol. 28:455–461. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jamali M and Chetty R: Predicting
prognosis in gastroentero-pancreatic neuroendocrine tumors: An
overview and the value of Ki-67 immunostaining. Endocr Pathol.
19:282–288. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Duchrow M, Schlüter C, Key G, Kubbutat MH,
Wohlenberg C, Flad HD and Gerdes J: Cell proliferation-associated
nuclear antigen defined by antibody Ki-67: A new kind of cell
cycle-maintaining proteins. Arch Immunol Ther Exp (Warsz).
43:117–121. 1995.PubMed/NCBI
|
|
25
|
Amoedo ND, El-Bacha T, Rodrigues MF and
Rumjanek FD: Cell cycle and energy metabolism in tumor cells:
Strategies for drug therapy. Recent Pat Anticancer Drug Discov.
6:15–25. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Umekita Y, Ohi Y, Sagara Y and Yoshida H:
Overexpression of cyclinD1 predicts for poor prognosis in estrogen
receptor-negative breast cancer patients. Int J Cancer. 98:415–418.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lamar JM, Stern P, Liu H, Schindler JW,
Jiang ZG and Hynes RO: The Hippo pathway target, YAP, promotes
metastasis through its TEAD-interaction domain. Proc Natl Acad Sci
USA. 109:E2441–E2450. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Singh S, Sadanandam A and Singh RK:
Chemokines in tumor angiogenesis and metastasis. Cancer Metastasis
Rev. 26:453–467. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Barzi A and Thara E: Angiogenesis in
esophageal and gastric cancer: a paradigm shift in treatment.
Expert Opin Biol Ther. 14:1319–1332. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tuettenberg J, Friedel C and Vajkoczy P:
Angiogenesis in malignant glioma - a target for antitumor therapy?
Crit Rev Oncol Hematol. 59:181–193. 2006. View Article : Google Scholar : PubMed/NCBI
|