Introduction
Gastrointestinal stromal tumors (GISTs) are common
mesenchymal tumors of the gastrointestinal tract that are
categorized as being borderline benign and malignant tumors
(1). GISTs arise predominantly in the
stomach (60%), small intestine (30%) and colorectum (10%) (2). Previous studies have elucidated that the
majority of GISTs are caused by a mutation of the receptor tyrosine
kinase KIT [also known as cluster of differentiation (CD)117] or
platelet-derived growth factor receptor-α (PDGFRA) (3–5).
Immunohistochemistry demonstrates that the majority of GISTs
express CD117 (6). In addition,
60–80% of GISTs diffusely express CD34 (7).
The synchronous or metachronous coexistence of GISTs
and other malignancies, including liver cancer, pancreatic tumors
and lymphoma, has been extensively reported (8–10).
However, to the best of our knowledge, only 1 case of the
synchronous development of GIST and acute myeloid leukemia (AML)
has been reported in the literature (11). The present study reports the case of a
patient diagnosed by GIST and AML. To the best of our knowledge,
the present study is the first to report the synchronous
development of a CD117-positive GIST and AML in China.
Case report
A 69-year-old man was admitted to the Binzhou
Medical University Hospital (Binzhou, Shandong, China) in November
2013 due to heart palpitations, dizziness and general fatigue that
had lasted for 2 months. The patient had suffered from hypertension
and diabetes mellitus for ~10 years. A physical examination on
admission revealed that the patient had anemia. Neither multiple
superficial lymphadenopathies nor hepatosplenomegaly were evident.
Preliminary investigations revealed a hemoglobin level of 76 g/dl
(normal range, 110–160 g/dl), platelet count of 237,000
platelets/µl (normal range, 100,000–300,000 platelets), white blood
cell count of 32,400 cells/µl (normal range, 4,000–10,000 cells/µl)
and myeloblast level of 10%. No abnormalities in the liver function
or routine blood biochemical examination were evident. The results
of the blood coagulation tests and urine analysis were not notable.
The patient did not express the human immunodeficiency virus
antibody.
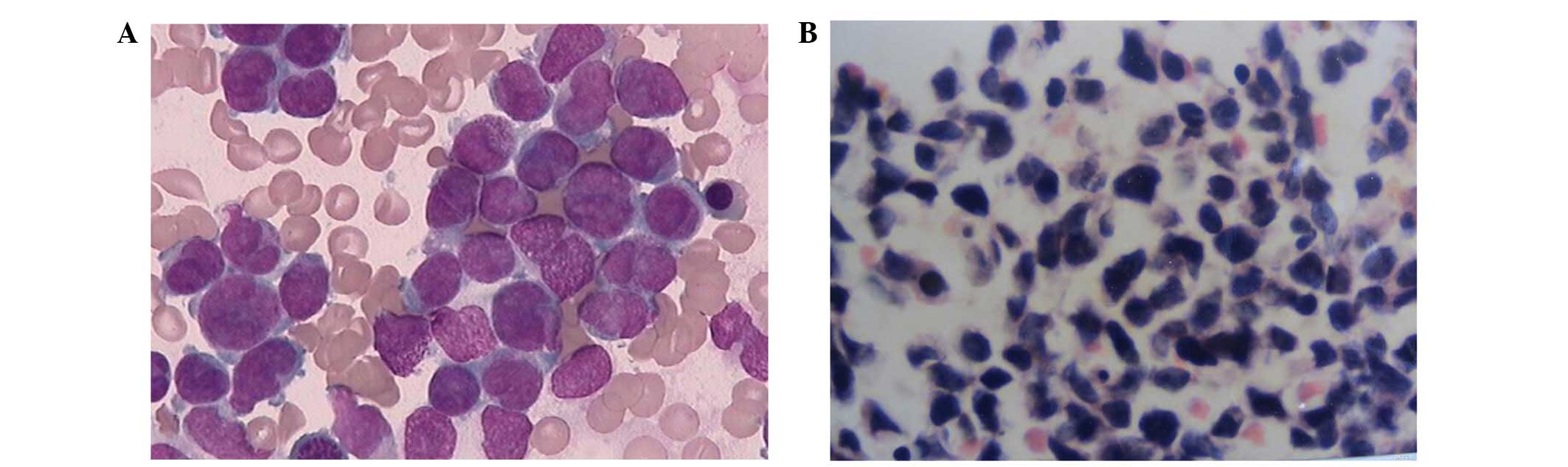
Bone marrow aspiration revealed that blasts
comprised 92.5% of the myeloid cells. The blasts were small in size
and possessed pseudopodia and processes. Bone marrow biopsy
revealed that the patient had AML (Fig.
1). Flow cytometry analysis of the bone marrow revealed that
the cellular characteristics were as follows: CD34+;
CD117+; human leukocyte antigen-DR+; and
CD33+ (Fig. 2).
Cytogenetic analysis revealed an abnormal karyotype
47,XY,+8[2]/46,XY[18] (Fig. 3). The
promyelocytic leukemia (PML)/retinoic acid receptor α and the
AML/eight-twenty one fusion gene were not expressed. From the
aforementioned data, a diagnosis of AML was eventually made.
 | Figure 2.Flow cytometric analysis revealed that
abnormal bone marrow cells expressed CD117, CD34, HLA-DR and CD33.
The red signals indicate the abnormal cells. CD, cluster of
differentiation; PECYS, phycoerythrin-cyanine 5; ECD,
phycoerythrin-Texas Red conjugate; PE, phycoerythrin; HLA-DR, major
histocompatibility complex, class II, DR; FITC, fluorescein
isothiocyanate. |
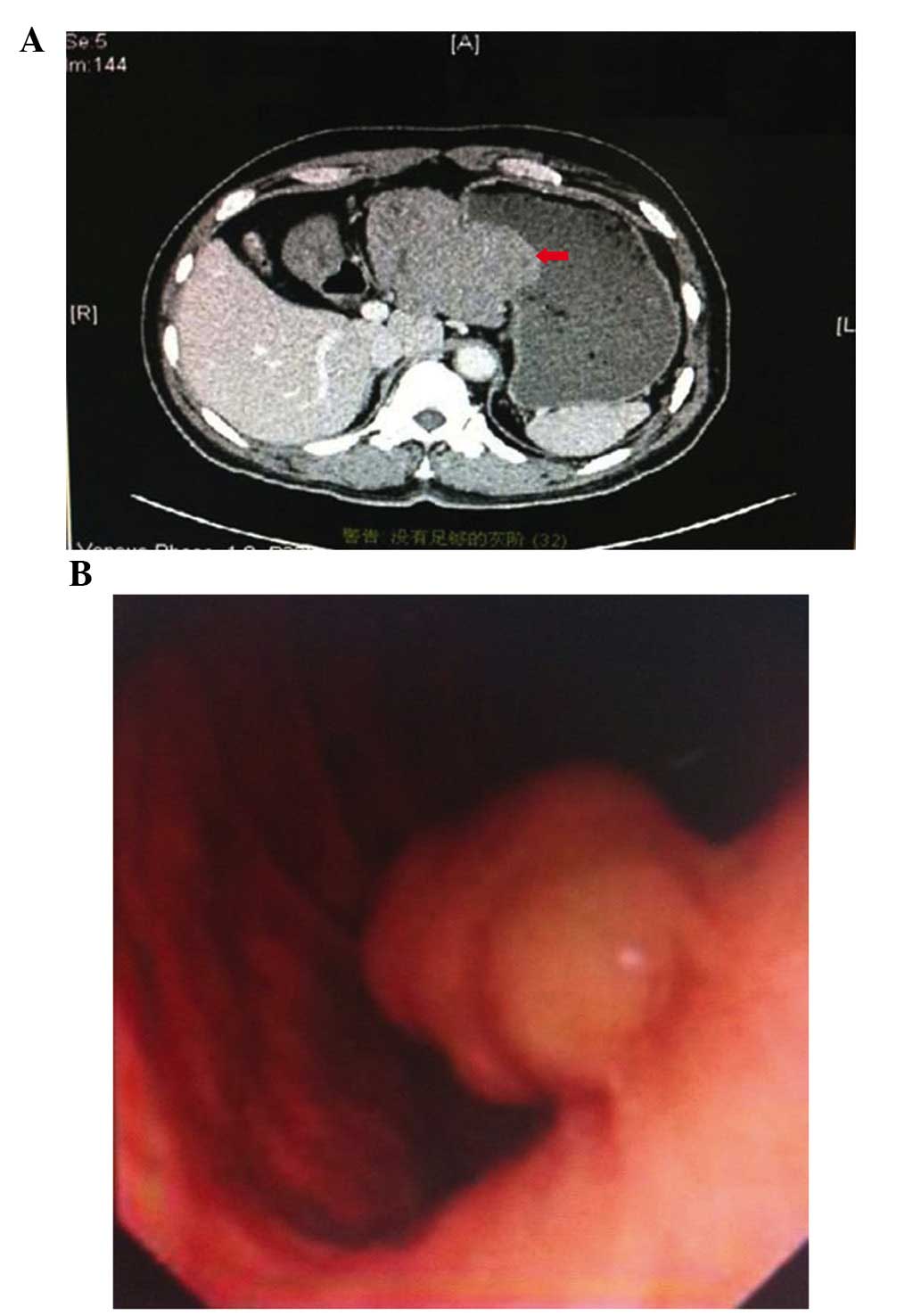
A comprehensive physical examination of the patient
was conducted prior to the implementation of chemotherapy. A
computed tomography scan of the abdomen revealed a lesion measuring
~10×9×8 cm in size that was located in the lesser curvature of the
stomach and was suspected to be a GIST (Fig. 4A). Gastroscopy revealed the presence
of GISTs and bleeding in the digestive tract (Fig. 4B).
The patient received the CAG chemotherapy regimen
(20 mg aclacinomycin, intravenous drip, days 1–4; 25 mg cytosine
arabinoside, administered subcutaneously, days 1–14; 300 µg
granulocyte-colony stimulating factor, administered subcutaneously,
days 1–14) in combination with imatinib (400 mg, oral, days 1–14)
to treat the AML, as the CAG scheme is the first choice of
treatment for the elderly in China (12). The patient demonstrated complete
remission following 2 courses of the Confidentiality Advisory Group
scheme. In order to avoid a severe digestive tract massive
hemorrhage, a subtotal gastrectomy was implemented and the patient
recovered well from the procedure. The postoperative pathology
suggested that the mass was a GIST lesion. The immunohistochemical
staining revealed CD117, CD34 and discovered on GIST-1 (DOG1)
expression, therefore also indicating that the lesion was a GIST
(Fig. 5).
The patient was prescribed imatinib (400 mg, oral,
continuous) for consolidation chemotherapy, and has regularly
received blood routine and liver function tests. On the basis of
the follow-up results, a reduction or temporary discontinuation of
the chemotherapy may be decided in the future.
Discussion
GISTs account for the majority of gastrointestinal
mesenchymal tumors, which are hypothesized to originate from the
interstitial cells of Cajal (13).
GISTs mainly occur in older patients and there is no significant
difference in the incidence of GISTs between males and females
(14). The malignant risk of a GIST
may be determined based on the mitotic index, size and location of
the lesion (15). Previous studies
indicate that certain GISTs that are small in size and possess a
low mitotic index may adopt the features of metastasis; therefore,
the concept of benign GISTs should be abandoned, as GISTs
demonstrate malignant potential (16,17).
The coexistence of GISTs with other malignancies has
been widely reported in the literature (10). However, the synchronous occurrence of
GISTs and AML has rarely been reported. Yoshioka et al
reported a case of acute PML that was diagnosed following a jejunal
GIST (18). PML was considered to be
the second malignancy in this study, and developed subsequent to
chemotherapy or radiotherapy for GIST. In the present study, the
case of a patient with synchronous occurrence of AML and GIST has
been reported. To the best of our knowledge, the present study is
the first to report the case of a patient that demonstrated the
synchronous development of CD117-positive GIST and AML in
China.
The pathogenesis of GISTs is hypothesized to be
associated with the mutational activation of CD117 or PDGFRA
(6). CD117, also termed KIT, has been
highly conserved throughout evolution. Previous studies have
indicated that the abnormal expression of genes and products caused
by CD117 mutations is the major cause of GIST development (3,19–21). Imatinib mesylate is important for the
treatment of GIST, as it may alleviate symptoms, decrease
post-surgery recurrence rates and prolong survival periods
(1).
The immunohistochemical features of GISTs include
the expression of CD117, which is widely expressed in the cytoplasm
and cytomembrane of tumor cells (95%). CD117 is widely distributed
in hematopoietic cells and other tissue cells. The detection of
CD117 expression is an effective method to distinguish between
GISTs and other mesenchymal tumors. CD117 is recognized as a highly
sensitive and specific marker for GISTs, and is also an important
pathogenetic factor in AML. Similarly to GISTs, hematopoietic
progenitor cells are dependent on the CD117-signaling pathway, and
numerous myeloid leukemias, including AML, with t(8,21) and
inv(16) also have CD117-activating
mutations. In the present study, t(8,21) was
detected using conventional cytogenetic analysis. CD117 signaling
is important for the regulation of red blood cell production,
lymphocyte proliferation and mast cell development and function.
Previous studies have demonstrated that CD117 is expressed in 68%
of patients with AML and 80% of patients with chronic myelogenous
leukemia in the blast phase, but in only 2% of patients with acute
lymphoid leukemia (22). Additional
studies indicate that AML patients demonstrating CD117 expression
have lower complete remission rates and poorer prognoses compared
with AML patients that do not express CD117 (23). Common types of CD117 receptor gene
mutation include exon 9 (73%), exon 11 (10%), exon 13 (3%) and exon
17 (1%) (24). CD117 receptor gene
mutations in exon 17 are closely associated with a poor prognosis.
Therefore, the CD117 receptor is important for AML complete
remission and recurrence. Stem cell factor, the ligand for CD117,
is a hematopoietic cytokine that has been detected in GIST
patients, and is important for maintaining the survival of
hematopoietic cells, which may lead to myeloproliferation (25), differentiation and ultimately to the
occurrence of leukemia. In addition, the primitive hematopoietic
tissue differentiation antigen CD34 is diffusely expressed in
60–80% GISTs. CD117 and CD34 are important in GISTs and AML. In
addition to CD117 and CD34, DOG1 is often expressed in GIST
patients and is particularly important in the diagnosis of patients
without CD117 expression.
The results of an epidemiological analysis in a
previous study indicated a significant association between AML and
GIST (26). This study indicated that
AML developed 1.7–21.0 years subsequent to GIST (median interval, 6
years), and that the risk of AML was significantly higher for
female patients with GIST compared to male patients with GIST. The
frequency of this non-random association and the spectrum of
neoplasms involved have not been sufficiently analyzed at present.
The potential for a non-random and causal association between GIST
and other neoplasms remains to be investigated.
References
|
1
|
Iorio N, Sawaya RA and Friedenberg FK:
Review article: The biology, diagnosis and management of
gastrointestinal stromal tumours. Aliment Pharmacol Ther.
39:1376–1386. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen HL, Xia JG and Ding HB: Imaging
features and differential diagnosis of gastrointestinal stromal
tumor. Yixue Lilun Yu Shijian. 17:2337–2339. 2013.(In Chinese).
|
|
3
|
Bello DM, Dematteo RP and Ariyan CE: The
GIST of targeted therapy for malignant melanoma. Ann Surg Oncol.
21:2059–2067. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Voltaggio L, Murray R, Lasota J and
Miettinen M: Gastric schwannoma: A clinicopathologic study of 51
cases and critical review of the literature. Hum Pathol.
43:650–659. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Corless CL, Barnett CM and Heinrich MC:
Gastrointestinal stromal tumours: Origin and molecular oncology.
Nat Rev Cancer. 11:865–878. 2011.PubMed/NCBI
|
|
6
|
Miettinen M and Lasota J: Histopathology
of gastrointestinal stromal tumor. J Surg Oncol. 104:865–873. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fan CHL and Li ZHQ: Clinical pathological
and immunohistochemical characteristics of 78 cases of
gastrointestinal stromal tumor. Shi Yong Zhong Liu Za Zhi.
25:581–583. 2010.(In Chinese).
|
|
8
|
Liu SW, Chen GH and Hsieh PP: Collision
tumor of the stomach: A case report of mixed gastrointestinal
stromal tumor and adeno-carcinoma. J Clin Gastroenterol.
35:332–334. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Herbers AH and Keuning JJ: Staging for
CLL-type non-Hodgkin lymphoma reveals a gastrointestinal stromal
tumour. Neth J Med. 63:74–75. 2005.PubMed/NCBI
|
|
10
|
Agaimy A, Wünsch PH, Sobin LH, Lasota J
and Miettinen M: Occurrence of other malignancies in patients with
gastro-intestinal stromal tumors. Semin Diagn Pathol. 23:120–129.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Joo YB, Choi SH, Kim SK, Shim B, Kim MS
and Kim YJ: Synchronous development of KIT positive acute myeloid
leukemia in a patient with gastrointestinal stromal tumor. Korean J
Hematol. 45:66–69. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hematology Society, Chinese Medical
Association: Adult acute myeloid leukemia (non acute early myeloid
leukemia) Chinese guidelines for diagnosis and treatment. (2011
Edition). Chinese Journal of Hematology. 32:1–4. 2011.(In Chinese).
PubMed/NCBI
|
|
13
|
Sonmez M, Arslan M, Cobanoglu U, Kavgaci
H, Ozbas HM, Aydin F, Ovali E and Omay SB: Association of
gastrointestinal stromal tumor and acute myeloid leukemia preceded
by myelodysplastic syndrome with refractory anemia. Tumori.
95:240–242. 2009.PubMed/NCBI
|
|
14
|
Kim MN, Kang SJ, Kim SG, Im JP, Kim JS,
Jung HC and Song IS: Prediction of risk of malignancy of
gastrointestinal stromal tumors by endoscopic ultrasonography. Gut
Liver. 7:642–647. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lewin KJ, Riddell RH and Weinstein WM:
Gastrointestinal Pathology and Its Clinical Implications.
Igaku-Shoin. New York, NY: 284–341. 1992.
|
|
16
|
Corless CL, Fletcher JA and Heinrich MC:
Biology of gastrointestinal stromal tumors. J Clin Oncol.
22:3813–3825. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Joensuu H: Gastrointestinal stromal tumor
(GIST). Ann Oncol. 7(Suppl 10×): 280–x286. 2006. View Article : Google Scholar
|
|
18
|
Yoshioka K, Yamaguchi M, Kasamatsu Y,
Ashida K, Yokoh S, Tatebe A, Yoshida T and Kondo M: Acute
promyoelocytic leukemia following leiomyosarcoma of the jejunum.
Arch Intern Med. 157:1392–1393. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Antonescu CR: The GIST paradigm: Lessons
for other kinase-driven cancers. J Pathol. 223:251–261. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yamanoi K, Higuchi K, Kishimoto H, Nishida
Y, Nakamura M, Sudoh M and Hirota S: Multiple gastrointestinal
stromal tumors with novel germline c-kit gene mutation, K642T, at
exon 13. Hum Pathol. 45:884–888. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Conca E, Miranda C, Dal Col V, Fumagalli
E, Pelosi G, Mazzoni M, Fermeglia M, Laurini E, Pierotti MA,
Pilotti S, et al: Are two better than one? A novel double-mutant
KIT in GIST that responds to Imatinib. Mol Oncol. 7:756–762. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shen HQ, Tang YM, Yang SL, Qian BQ, Song
H, Shi SW and Xu WQ: Analysis of CD117 expression on leukemia
cells. Zhonghua Xue Ye Xue Za Zhi. 24:228–230. 2003.(In Chinese).
PubMed/NCBI
|
|
23
|
Tsao AS, Kantarjian H, Thomas D, Giles F,
Cortes J, Garcia-Manero G, Huh Y, Yang Y, Shen Y, Albitar M, et al:
C-kit receptor expression in acute leukemias-association with
patient and disease characteristics and with outcome. Leuk Res.
28:373–378. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang WL, Conley A, Reynoso D, Nolden L,
Lazar AJ, George S and Trent JC: Mechanisms of resistance to
imatinib and sunitinib in gastrointestinal stromal tumor. Cancer
Chemother Pharmacol. 67(Suppl 1): S15–S24. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen SQ and Xiong AQ: The progress and
implication of stem cell factor. Basic Med Sci Clin. 22:385–390.
2002.
|
|
26
|
Miettinen M, Kraszewska E, Sobin LH and
Lasota J: A nonrandom association between gastrointestinal stromal
tumors and myeloid leukemia. Cancer. 112:645–649. 2008. View Article : Google Scholar : PubMed/NCBI
|