Introduction
Cancer, including osteosarcoma (OS), is becoming an
increasingly common disease and is characterized by uncontrolled
growth (1). OS is the most commonly
observed primary malignant bone tumor with a high morbidity in
adolescents and young adults, and it primarily originates from
mesenchymal tissue (2). OS is
additionally the third most common cancer in childhood (3). Annually, ~5.6 out of 1 million children
<15 years old suffer from OS (4).
With comprehensive treatment, the 5-year survival rate has
increased to 60–70% (5); however,
although the therapeutic management has been improved, metastasis
eventually occurs in ~80% of patients, and the lung is typically
the most susceptible organ (6). The
prognosis and curative effects of OS are closely associated with
metastasis, and the majority of clinicians agree that early
diagnosis is key to successful treatment (7).
MicroRNAs (miRNAs) belong to a family of small
non-coding RNAs that have been identified in plants and animals,
which are typically 19–24 nucleotides in length and function in
transcriptional and post-transcriptional processes (8–10). The
human genome may encode >1,000 miRNAs (11), which exist in a large number of human
cells. miRNAs are generated from their own genes or introns, and
are synthesized in the nucleus and cytosol. Generally, the
formation of mature miRNAs is a complex process that requires the
aid of a variety of biological molecules, including RNA polymerase
II/III, Drosha, Pasha, exportin-5 and Dicer (12–16).
However, only the end products of this process have biological
activity and execute biological functions (17). It has been demonstrated that miRNAs
regulate the expression of their target messenger (m)RNAs primarily
by binding the mRNA 3′ untranslated region (18–20).
Furthermore, a miRNA may target multiple mRNA targets, and a target
mRNA may be targeted by various miRNAs, which suggests that these
regulatory RNAs exert complex, post-transcriptional control of gene
expression (21,22). miRNAs have enormous potential to
regulate various crucial biological processes, including the
differentiation, progression, proliferation, apoptosis, metastasis
and invasion of tumor cells (23–26).
With improvements in medicine, increased knowledge
and technology advancement, the association between miRNAs and
cancer is being gradually revealed (27). As miRNAs are known to be implicated in
the functioning of eukaryotic cells, consequently the dysregulation
of miRNAs has been associated with various diseases (28). The first miRNAs identified to be
involved in disease were miRNA-15 and miRNA-16, which were observed
to be closely associated with chronic lymphocytic leukemia by Calin
et al (29) in 2002. As an
important member of the miRNA family, miRNA-182 is vital in tumor
development. Studies have revealed that miRNA-182 functions as an
oncogene or cancer suppressor gene in various tumors, including
triple-negative breast cancer (30),
gastric adenocarcinoma (31) and
colon cancer (32); however, it
remains to be elucidated whether miRNA-182 has similar functions in
OS. Therefore, examining the expression level of miRNA-182 in
normal osteoblast hFOB1.19 cells, as well as human OS MG-63 cells
and OS tissues, was the primary aim of the present study. In
addition, the present study was interested in controlling the
expression level of miRNA-182 in MG-63 cells and subsequently
investigating the biological activity of those cells.
Materials and methods
Cell lines and tissue specimens
The control cell line hFOB1.19 and 9 clinical
specimens were obtained from the Department of Orthopedics of the
First Hospital of China Medical University (Shenyang, China).
hFOB1.19 is an established cell line that has been described in the
literature (33). The 9 participants
in the present study ranged in age between 11 months and 16 years.
During surgery, a 2×2-cm section of each tumor was removed
following excision of the tumor by the surgeon, and each specimen
was subsequently transported on ice and preserved in liquid
nitrogen until required. The human OS MG-63 cell line was purchased
from the Culture Preservation Commission Cell Bank of the Chinese
Academy of Sciences (Shanghai, China). The cell lines were cultured
in Dulbecco's modified Eagle's medium (DMEM; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) containing 10% fetal bovine
serum (FBS; Thermo Fisher Scientific, Inc.) at 37°C in a humidified
atmosphere of 5% CO2. In total, 9 patients were enrolled
in the present study, and none of the patients had a history of
chemotherapy, radiotherapy or other treatment prior to surgery.
Furthermore, none of the patients had metastasis, as confirmed with
ultrasound, computed tomography (CT), magnetic resonance imaging,
and positron emission tomography-CT. All specimens were used only
for miRNA extraction. Written informed consent was obtained from
the parents or guardians of the patients prior to surgery. The
present study was approved by the First Affiliated Hospital of
China Medical University Medical Research Ethics Committee
(Shenyang, China).
miRNA extraction
miRNA was isolated using RNAiso Plus (Takara
Biotechnology Co., Ltd., Dalian, China) according to the
manufacturer's protocol. Briefly, 1×104-106
cultured cells and 50–200 mg of tissue were collected and washed
twice in phosphate-buffered saline (PBS; ZSGB-Bio Co., Ltd.,
Beijing, China) at 4°C, and subsequently 1 ml of RNAiso was added
per 50–200 mg or 1×104-106 cells. Following 5
min of incubation, 200 µl chloroform (Tianjin Bodi Chemical Co.,
Ltd., Tianjin, China) was added, and the cells were agitated for 15
sec. Following an additional 5 min of incubation at room
temperature, the mixture was centrifuged [Allegra X-30R; Beckman
Coulter Commercial Enterprise (China) Co., Ltd., Shanghai, China]
for 15 min at 12,000 × g at 4°C. In total, ~400 µl of the aqueous
phase was recovered and transferred to a fresh tube, to which 400
µl isopropyl alcohol (Tianjin Bodi Chemical Co., Ltd.) was added,
and the mixture was incubated for 10 min. Subsequently, the mixture
was centrifuged [Allegra X-30R; Beckman Coulter Commercial
Enterprise (China) Co., Ltd.] for 10 min at 12,000 × g at 4°C.
Following centrifugation, 1 ml 100% ethanol (Tianjin Bodi Chemical
Co., Ltd.) was added to wash the precipitate three times. Finally,
the precipitate was dried at room temperature and dissolved in 20
µl RNAse-free dH2O (Takara Biotechnology Co., Ltd.). Immediately,
the concentrations of the isolated miRNA samples were determined
using a microplate reader (Multiskan™ GO; Thermo Fisher Scientific,
Inc.).
Reverse transcription (RT)
The extracted miRNAs underwent RT to complementary
(c)DNA using the PrimeScript RT Reagent kit (Takara Biotechnology
Co., Ltd.) according to the manufacturer's protocol with minor
modifications in the cycling conditions. The RT reaction volume was
10 µl, and the mixture contained 2 µl 5X PrimeScript Buffer, 0.5 µl
PrimeScript RT Enzyme Mix I, 500 ng extracted RNA and 0.5 µl
specific miRNA primer (2 µM). The primers were obtained from
GenScript Co., Ltd (Nanjing, China) and the sequences were as
follows: miR-182-RT,
5′-GTCGTATCCAGTGCAGGGTCCGAGGTGCACTGGATACGACAGTGTGA-3′; U6-RT,
5′-GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACAAAATATGGAAC-3′. U6
served as the internal reference gene. Finally, 10 µl RNase-free
dH2O was added to the mixture. The reaction conditions for reverse
transcription were as follows: 42°C for 15 min, 85°C for 5 sec and
4°C for 60 min. The PCR apparatus (Dice® Gradient TP600)
was purchased from Takara Biotechnology Co., Ltd. The cDNA was
stored at −20°C until required.
Identification of miRNA-182 expression
in MG-63 cells and tissue specimens by quantitative PCR (qPCR)
The expression of mature miRNA-182 was detected
using the SYBR® Premix Ex Taq™ II kit (Takara
Biotechnology Co., Ltd.), with U6 as the internal reference gene.
The reaction volume was 20 µl, and the mixture contained 10 µl
SYBR® Premix Ex Taq, 2 µl cDNA, 0.4 µl (10 µM) miRNA-182
forward primer and miRNA-182 reverse primer or 0.4 µl (10 µM) U6
forward primer and U6 reverse primer, and 7.2 µl dH2O. The primers
were as follows: miRNA-182, forward 5′-TGCGGTTTGGCAATGGTAGAAC-3′
and reverse, 5′-CCAGTGCAGGGTCCGAGGT-3′; U6, forward
5′-GCTTCGGCAGCACATATACTAAAAT-3′ and reverse
5′-CGCTTCACGAATTTGCGTGTCAT-3′. The PCR amplification conditions
were as follows: 95°C for 30 sec and 40 cycles of 95°C for 5 sec,
60°C for 20 sec, 95°C for 5 sec and 65°C for 15 sec. Three
replicates were performed for each reaction. The qPCR instrument
(LightCycler® 480 II) was purchased from Roche
Diagnostics, GmbH (Mannheim, Germany). The cycle threshold (Cq)
value of the specimens in each reaction tube was recorded, and the
experimental data were analyzed using the qPCR relative
quantification method (34):
2−ΔΔCq represented the fold change in the expression
level of miRNA-182 in MG-63 cells or OS tissues compared with
normal osteoblast hFOB1.19 cells.
Cell transfection and analysis of the
expression level of miRNA-182 in MG-63 cells
Shortly prior to transfection, MG-63 cells were
seeded into 6-well plates (BD Biosciences, Franklin Lakes, NJ, USA)
at a concentration of 5–6×105 cells/ml in 2 ml per well
of DMEM containing 10% FBS. In total, 4–6 h prior to transfection,
the cells were incubated in DMEM containing 10% FBS at 37°C in a
humidified atmosphere of 5% CO2. Subsequent to the cells
adhering to the wells for 6–8 h, the culture medium was aspirated
from each well. Following aspiration, transfection complexes were
formed by mixing 250 µl culture medium without FBS, 10 µl
HiPerFect® Transfection Reagent (Qiagen, Inc., Valencia,
CA, USA) and 10 µl miRNA-182-5p inhibitor (100 nM; catalog no.,
miR20000259-1-5; Guangzhou RiboBio Co., Ltd., Guangzhou, China) or
8 µl miRNA-182-5p mimic (80 nM; catalog no., miR10000259-1-5;
Guangzhou RiboBio Co., Ltd.). The mixtures were incubated for 5–10
min at room temperature. Subsequently, the mixtures were added
dropwise to the cells, and the plates were gently swirled to ensure
uniform distribution of the transfection complexes. Non-transfected
(MG-63 cells without transfection), negative (catalog no.,
miR02101-1-5; Guangzhou RiboBio Co., Ltd.) and fluorescence
(catalog no., siN05815122149-1-5; Guangzhou RiboBio Co., Ltd.)
controls were performed at the same time. Three duplicate wells
were set up for each reaction. The cells were placed in an
incubator at 37°C with 5% CO2 for 24 h. RNAiso for Small
RNA (Takara Biotechnology Co., Ltd.) was utilized to extract the
RNA from each group of cells, and RT-qPCR was performed to detect
the expression level of miRNA-182 in each group of MG-63 cells.
Cell viability and proliferation
determined by cell counting kit-8 (CCK-8) assays
MG-63 cells that had undergone transfection were
used in this assay. A total of 24 h subsequent to transfection,
cells were treated with trypsin (Biosharp, Hefei, China) and
counted using a cell counting plate. Subsequently, the cells were
plated in 96-well plates (BD Biosciences) at a concentration of
5×104 cells/ml in 100 µl per well of DMEM with 10% FBS,
and the plates were incubated at 37°C with 5% CO2. The
culture medium was replaced every 48 h. Subsequent to 24, 48, 72
and 96 h, 10 µl CCK-8 (Dojindo Molecular Technologies, Inc.,
Kumamoto, Japan) was added to each well, and the plates were
incubated for 4 h at 37°C with 5% CO2. Subsequently, the
optical density (OD) values at 450 nm were measured with a
microplate reader (Multiskan™ GO), and a standard curve was
constructed using 2-fold serial dilutions, thereby forming a cell
concentration gradient. Each experimental group contained five
duplicated wells, and the experiment was repeated three times.
Cell apoptosis determined using flow
cytometry
Transfection of the miRNA-182 inhibitor, mimic and
negative control was performed using HiPerFect Transfection Reagent
according to the manufacturer's protocol. A total of 24 h
subsequent to transfection, the culture medium was aspirated and
the transfected cells were washed twice with PBS, treated with
trypsin and centrifuged at 878 × g for 5 min (TDZ5-WS; Shanghai
Xiangyi Centrifuge Instrument Co., Ltd., Shanghai, China).
Subsequently, the precipitates were washed 2–3 times with PBS and
centrifuged at 878 × g for 5 min. A total of 200 µl binding buffer
(NeoBioscience, Shenzhen, China) was added to each tube of cells,
and the mixtures were transferred into 1.5 ml Eppendorf tubes
(Eppendorf, Hamburg, Germany). Subsequently, 5 µl Annexin
V-fluorescein isothiocyanate (FITC; NeoBioscience) was immediately
added to each tube, and the tubes were incubated at room
temperature for 5 min. Next, 10 µl propidium iodide (PI;
NeoBioscience) was added to each tube, and the tubes were incubated
at room temperature for 10 min. Finally, 300 µl binding buffer was
added to the tubes and the cells were analyzed using flow cytometry
(BD FACSCalibur™; BD Biosciences). The BD FACSCalibur was equipped
with a dual laser (488 and 635 nm) and CellQuest version 3.0
software (BD Biosciences). The analysis speed was 1×104
cells/sec.
Cell invasion and migration analyzed
using Transwell invasion chambers
Transfection of the miRNA-182 inhibitor, mimic and
negative control was performed using HiPerFect Transfection Reagent
according to the manufacturer's protocol. A total of 24 h
subsequent to transfection, 100 µl diluted Matrigel (BD
Biosciences; 1:2 dilution, Matrigel:DMEM) was added to a 8.0 µm
pore size Transwell chamber (12/24 well chamber; Corning
Incorporated, Corning, NY, USA) and incubated for 4–5 h at 37°C
with 5% CO2. Following solidification of the Matrigel,
the cells were dissociated with trypsin and resuspended in 1 ml
DMEM containing 8% FBS. In total, 600 µl DMEM containing 15% FBS
was added to the lower chamber, and 200 µl (4–6×105
cells/ml) of the cell suspension was seeded in the upper chamber of
the Transwell apparatus. The experimental procedures for the
migration assays were the same for invasion assays, except that
Matrigel was not used. Cell invasion was observed 24 h later, and
migration was observed 5 h later. Specifically, the cells were
stained with hematoxylin (Wanlei Biotechnology Co., Ltd., Shenyang,
China), and the number of cells considered to be invasive, or those
that migrated through the polycarbonate membrane of the Transwell
chamber, were counted under a microscope (Eclipse 80i; Nikon
Corporation, Tokyo, Japan; magnification, ×200). Ten fields were
randomly observed.
Statistical analyses
GraphPad Prism 5 software (GraphPad Software, Inc.,
La Jolla, CA, USA) was used to statistically analyze the
experimental data. Student's t test was applied to compare
two groups, and analysis of variance was applied to compare two or
more sets of data followed by Student's t test for post hoc
analysis. Data is presented as the mean ± standard deviation.
P<0.05 indicated a statistically significant difference.
Results
Relative expression level of miRNA-182
in MG-63 cells and tissue specimens
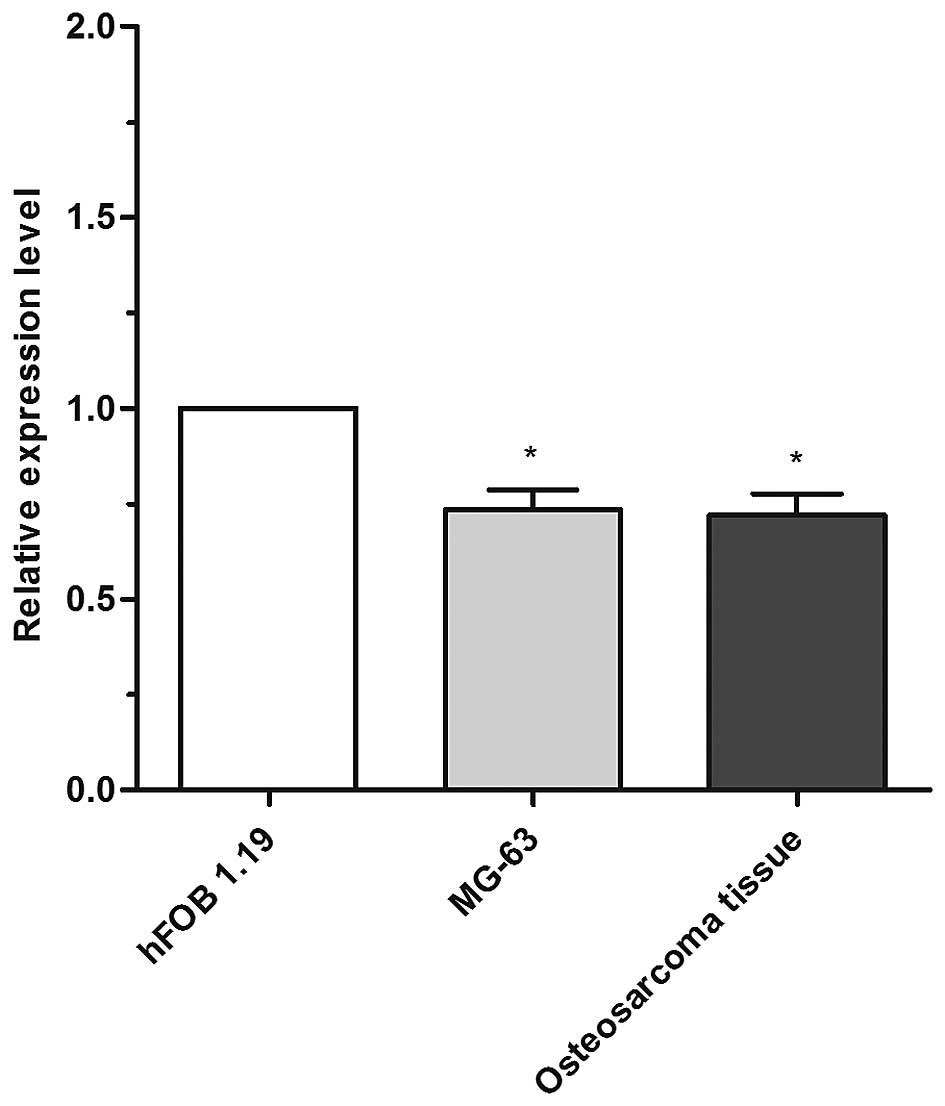
Using RT-qPCR, the present study demonstrated that
the expression level of miRNA-182 in MG-63 cells (0.73±0.09) and OS
tissues (0.72±0.09) was significantly reduced compared with the
control hFOB1.19 cell line (1.00±0.00) (Fig. 1). The OS tissue comprised 9 patient
specimens, and the basic patient characteristics are presented in
Table I. All specimens had a low
expression of miRNA-182 in comparison to the hFOB1.9 control cells.
Finally, the statistical mean of the expression level of miRNA-182
in the 9 patients was calculated (0.72±0.09).
 | Table I.Basic characteristics of 9 patients
with osteosarcoma. |
Table I.
Basic characteristics of 9 patients
with osteosarcoma.
| Patient | Gender | Age | Number of
lesions | Lesion site | Treatment |
|---|
| 1 | M | 12y | 1 | Terminal femur | None |
| 2 | F | 8y | 1 | Proximal tibia | None |
| 3 | M | 11m | 1 | Tibia
metaphyses | None |
| 4 | M | 7y | 1 | Proximal tibia | None |
| 5 | M | 16y | 1 | Femur
metaphyses | None |
| 6 | M | 13y | 1 | Tibia
metaphyses | None |
| 7 | M | 15y | 1 | Proximal tibia | None |
| 8 | F | 8y | 1 | Femur
metaphyses | None |
| 9 | F | 10y | 1 | Terminal femur | None |
Relative expression level of miRNA-182
in MG-63 cells following transfection
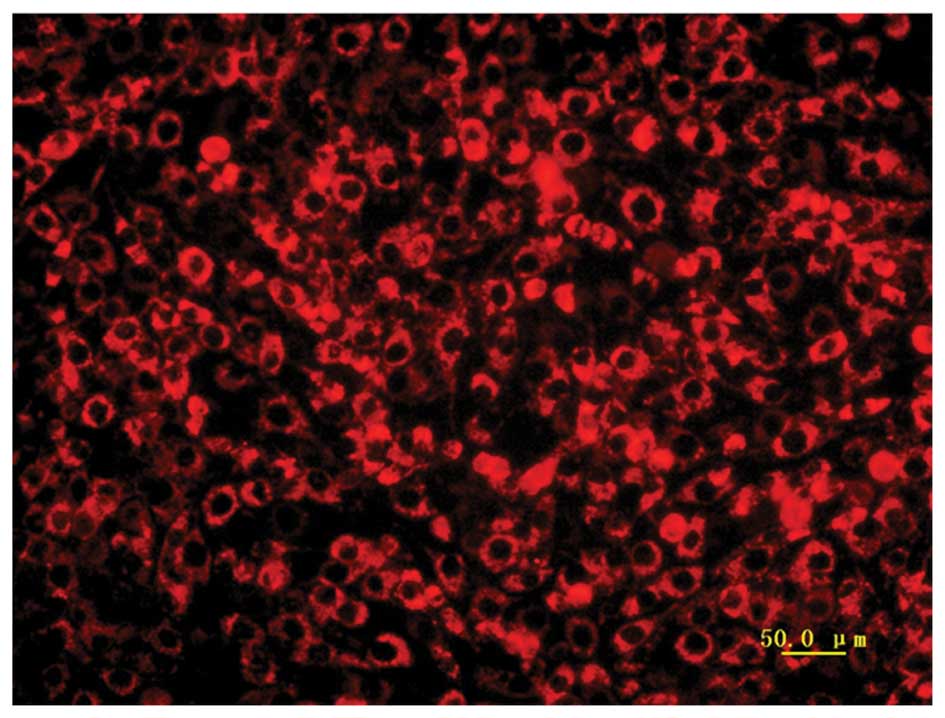
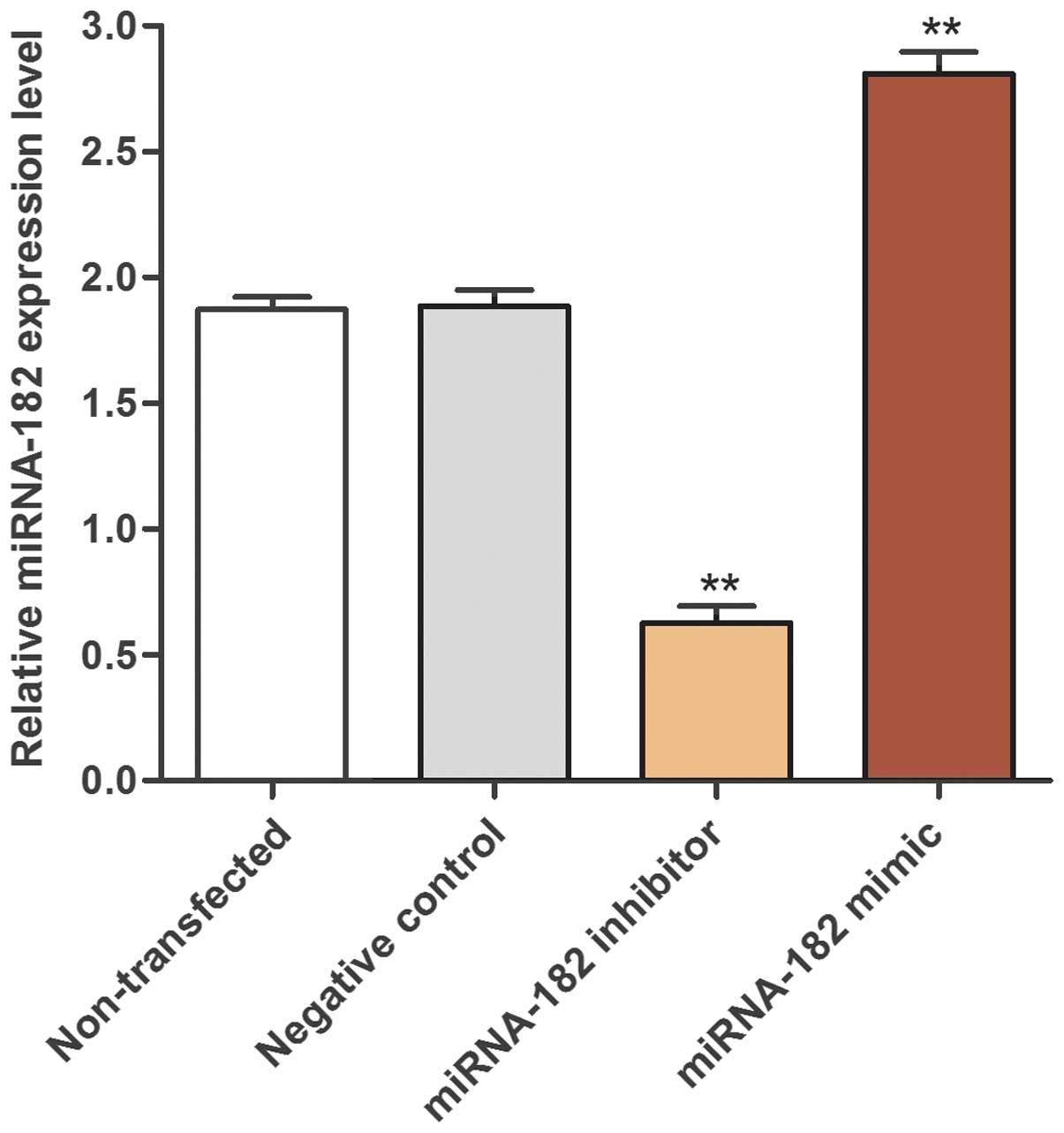
A miRNA-182 inhibitor, mimic and negative control
were transfected into MG-63 cells. The fluorescence control group
confirmed that the transfection was efficient (Fig. 2). Using RT-qPCR, the present study
identified that the expression levels of miRNA-182 in the miRNA-182
inhibitor transfection group (0.63±0.12) were significantly
decreased compared with the expression levels in the
non-transfected control MG-63 cells (1.87±0.08) and negative
control groups (1.89±0.11). Furthermore, the expression level of
miRNA-182 in the group transfected with miRNA-182 mimic (2.81±0.15)
was significantly increased compared with the control groups
(Fig. 3).
Effects of miR-182 on cell viability
and proliferation
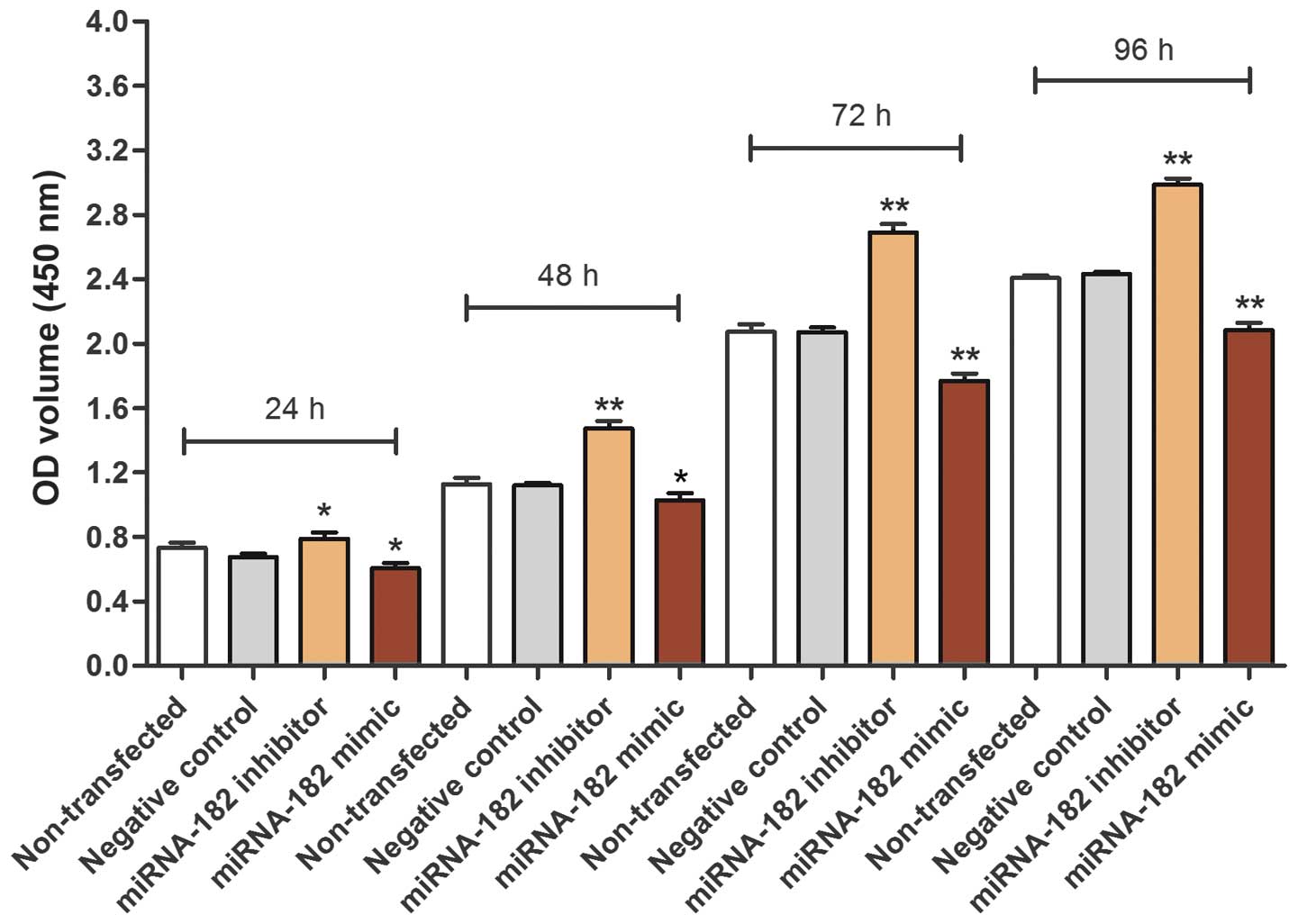
Using a CCK-8 assay, it was demonstrated that the
cell viability of cells transfected with the miRNA-182 inhibitor
was increased compared with the non-transfected control and
negative control transfected cell groups. In addition, the
viability of the group transfected with miRNA-182 mimic was
decreased compared with the control groups, suggesting that
upregulation of miRNA-182 expression reduces MG-63 cell viability
(Fig. 4). A cell proliferation curve
was plotted according to the standard curve, which was obtained
from OD values (Fig. 5). The results
were similar to the results observed for cell viability and were
statistically significant; cell proliferation was increased in
cells transfected with the miRNA-182 inhibitor and decreased in
cells transfected with the miRNA-182 mimic compared with the
control groups.
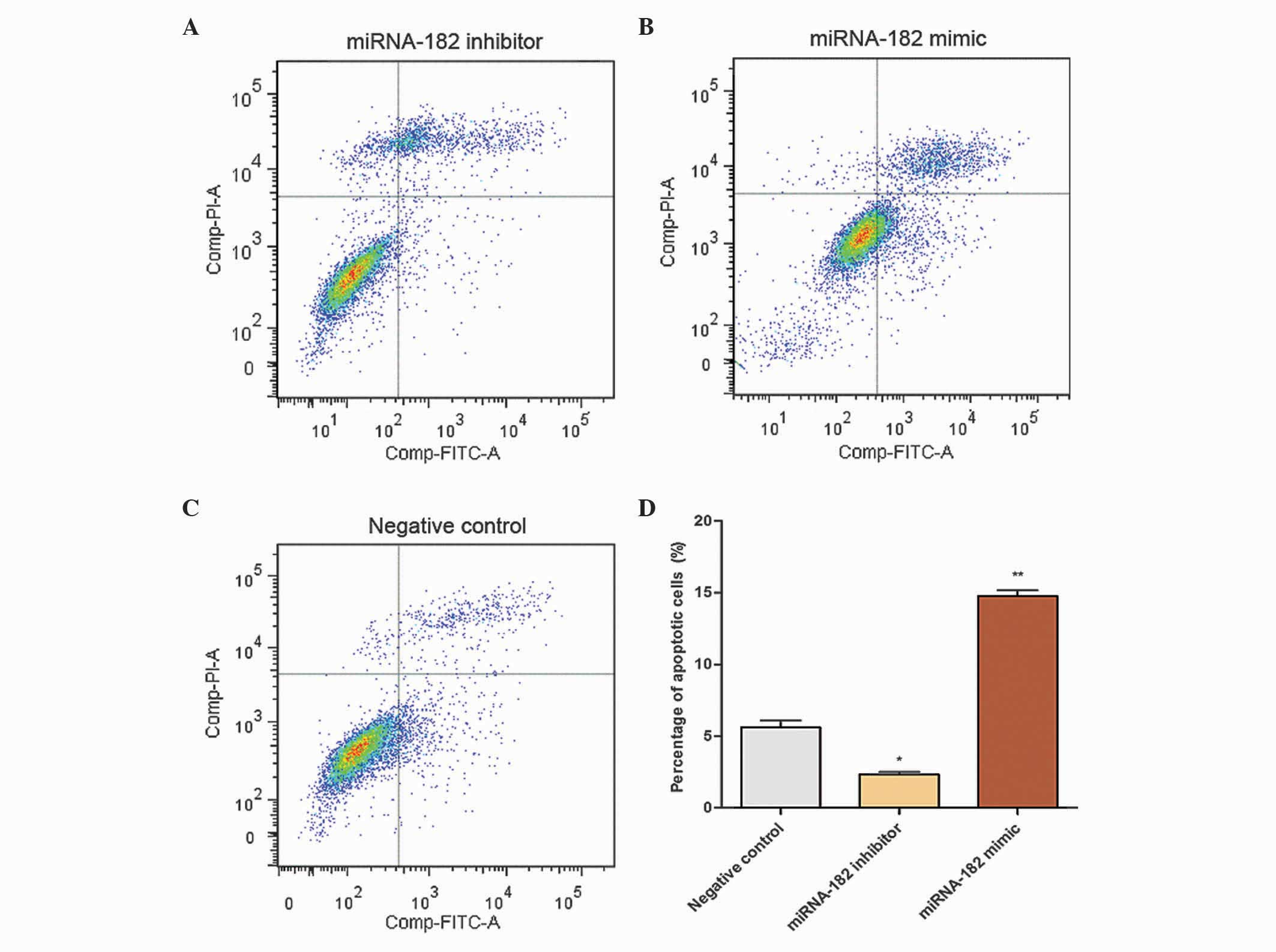
Effects of miRNA-182 on cell
apoptosis
A total of 24 h subsequent to transfection, Annexin
V-FITC and PI were used for cell staining. The single-FITC stained
cells were analyzed statistically as the ratio of early apoptotic
cells. When cells were transfected with the miRNA-182 inhibitor,
mimic or negative control, the percentage of apoptotic cells was
2.33±0.27, 14.76±0.72 and 5.62±0.84%, respectively. Flow cytometric
analysis indicated that the percentage of apoptotic cells was
significantly increased in the group transfected with the miRNA-182
mimic and reduced in the group transfected with the miRNA-182
inhibitor compared with the negative control group (Fig. 6). These results suggest that
upregulation of miRNA-182 expression promotes apoptosis of MG-63
cells.
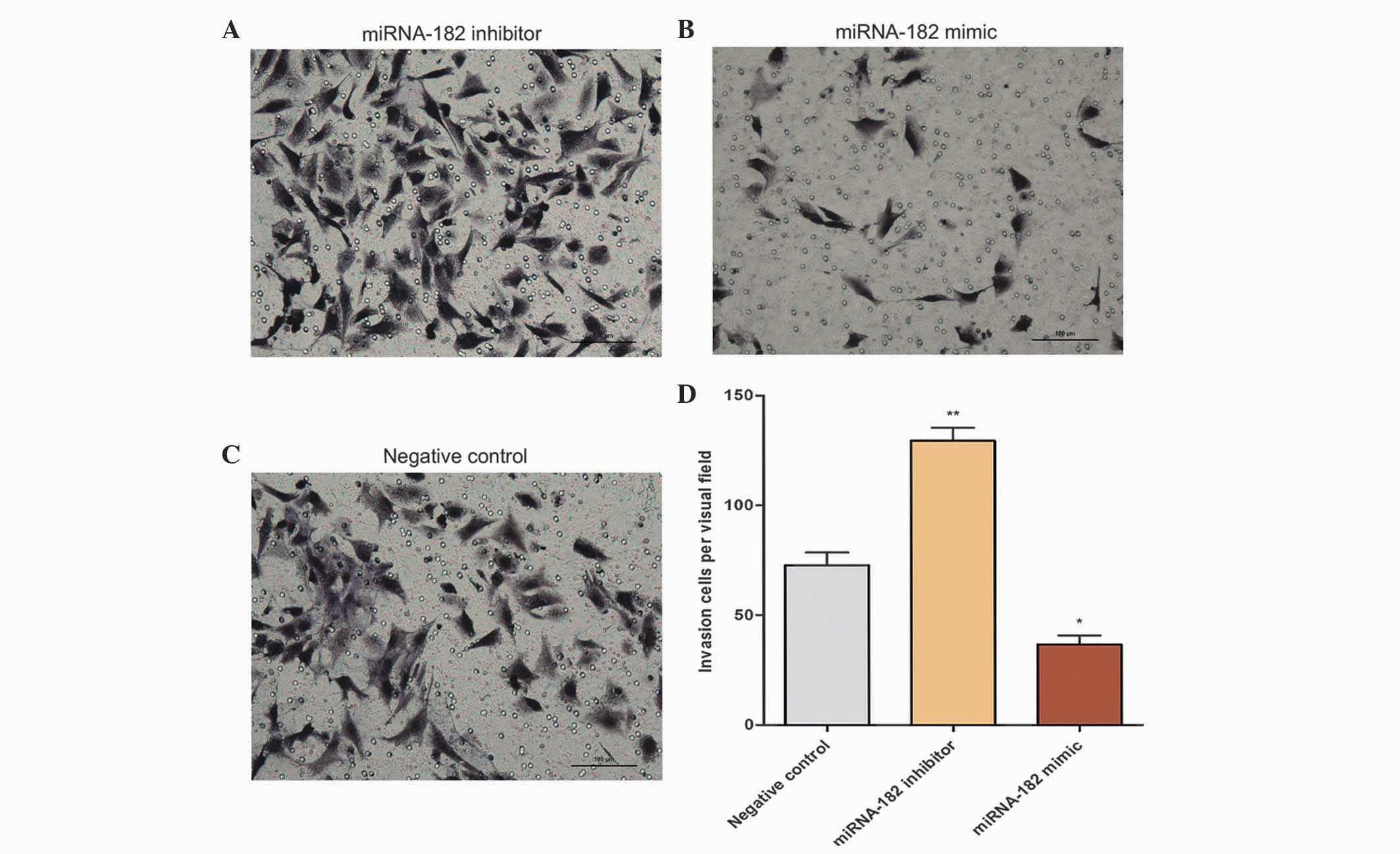
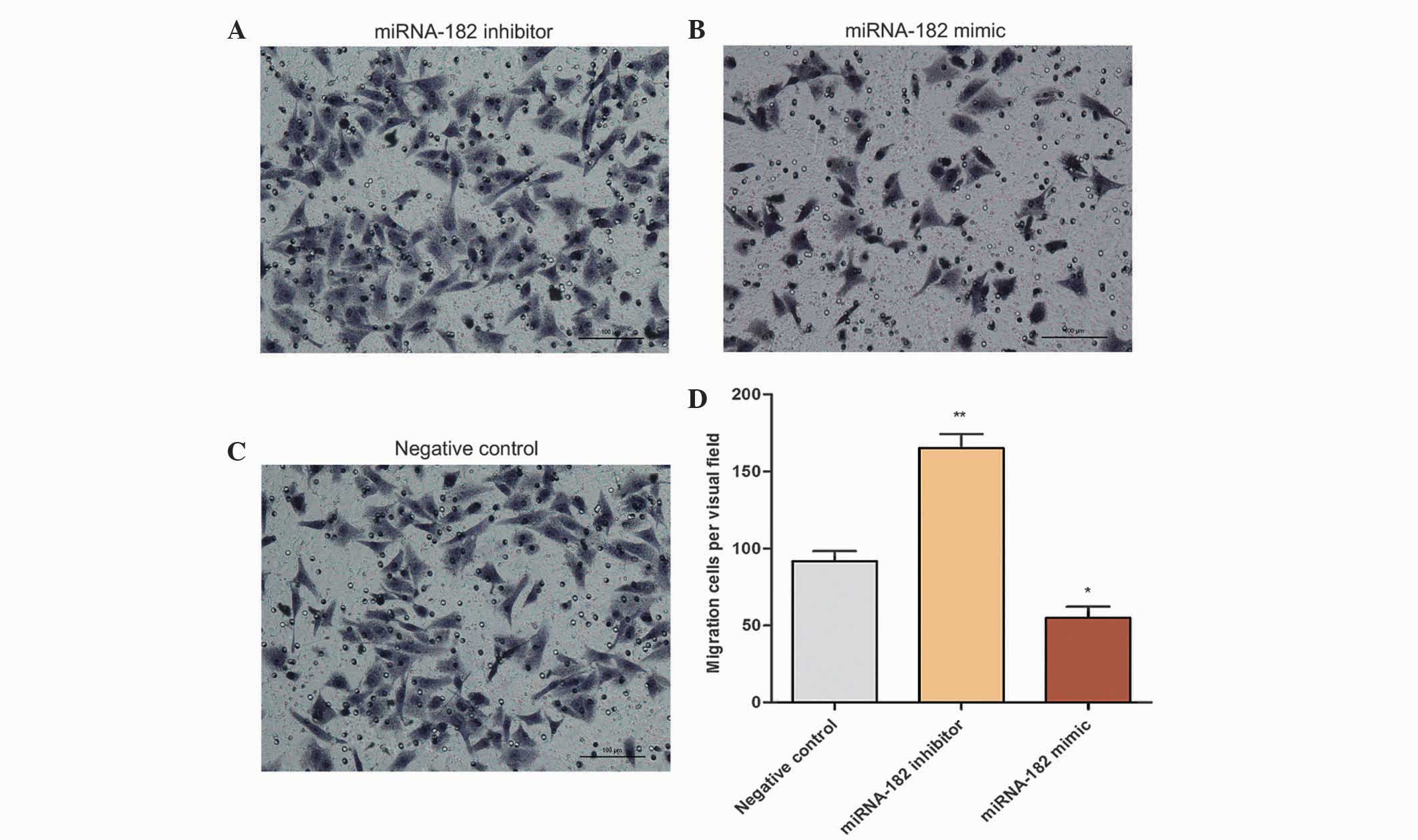
Effects of miRNA-182 on cell invasion
and migration
Following solidification, the number of cells
passing through the Matrigel was counted following an incubation
period of 24 h for invasive cells and 5 h for migrating cells. The
numbers of cells in the inhibitor, mimic and negative control
groups were 129±11, 36±7 and 72±10, respectively, per visual field
following 24 h of incubation. The number of MG-63 cells in the
inhibitor group that passed through the Transwell chamber was
significantly increased (Fig. 7),
which suggests that transfection of the miRNA-182 inhibitor
promoted cell invasiveness. Following incubation in Transwell
chambers for 5 h, the number of cells passing through the Matrigel
was counted. The number of migrating cells in the inhibitor, mimic
and negative groups were 165±9, 55±8 and 91±7, respectively, per
visual field. The number of MG-63 cells in the inhibitor group that
passed through the Transwell chamber was significantly increased
(Fig. 8), revealing that inhibition
of miRNA-182 expression promoted cell migration.
Discussion
One of the significant challenges in cancer research
is the identification of novel molecular biomarkers (35). miRNAs regulate complex networks of
gene expression, and an altered expression of miRNA genes has been
associated with the development of multiple types of human cancer,
including OS (36,37). However, the role of miRNAs in cancer
biology is not well understood. Previously, it has been observed
that there is a complex association between miRNA-182 and
neoplasms; upregulation of miRNA-182 has been associated with
cervical cancer (38), while its
significant downregulation has been reported in human uveal
melanoma (39). The results of the
present study revealed that miRNA-182 expression levels were
decreased in OS MG-6 cells and OS tissues compared with normal
osteoblast hFOB1.19 cells and are similar to the results of
previous reports, which describe the association of lower levels of
miRNA-182 with colon cancer and uveal melanoma (32,39). In
the present study, miRNA-182 is referred to as a tumor suppressor
miRNA, as its functions are similar to those of tumor suppressor
genes. As a tumor suppressor, miRNA-182 is important in the
biological behavior of OS.
The present study revealed that an upregulation of
miRNA-182 promotes cell apoptosis and inhibits cell viability,
proliferation, invasion and migration. To the best of our
knowledge, >20 articles concerning miRNA-182 have been published
in various journals. The majority of these studies have
demonstrated that miRNA-182 is an oncogene, and a small number of
studies have confirmed that miRNA-182 is a tumor suppressor gene.
Ning et al (40) confirmed
that the expression level of miRNA-182 in human lung cancer A549
cells was significantly increased compared with the expression
level in the human bronchial epithelial NHBE cell line.
Furthermore, it was identified that an increase in programmed cell
death 4 (PDCD4) protein levels occurred following transfection of a
miRNA-182 inhibitor using western blot analysis (40). The results from this study demonstrate
that overexpression of miRNA-182 may be involved in the
chemoresistance of non-small cell lung cancer cells to cisplatin by
downregulating PDCD4 (40). Yang
et al (41) revealed that
miRNA-182 expression was increased in colorectal cancer (CRC) cells
originating from metastatic foci and human primary CRC tissues with
lymph node metastases. The results of this previous study
illustrated that the upregulation of miRNA-182 is pivotal in CRC
tumorigenesis and metastasis (41).
Guo et al (42) demonstrated
that miRNA-182 was significantly upregulated in an embryonal
carcinoma (EC) cell line compared with nonendometrioid carcinoma.
miRNA-182 serves as an oncogenic miRNA in EC as it promotes cell
proliferation by targeting the tumor suppressor gene transcription
elongation factor A (SII)-like 7 and modulating the activity of its
downstream effectors v-myc avian myelocytomatosis viral oncogene
homolog, cyclin D1 and NFκB (42).
Liu et al (43) reported that
miRNA-182 expression was significantly upregulated in prostate
cancer tissues and four prostate cancer cell lines compared with
benign prostatic hyperplasia tissues and normal prostatic
epithelial RWPE-1 cells. A novel mechanism by which n-myc
downstream regulated 1 is epigenetically regulated was revealed
(43).
Notably, the results of Cekaite et al
(32) contradicted those of Yang
et al (41), and demonstrated
that deregulation of miRNA-182 promoted colon cancer cell
proliferation. In these previous studies, the majority of the
tumors used originated from epithelial tissue. By contrast, in the
current study, OS was the source of the mesenchymal tumors. The
same miRNA may be expressed at various levels in tumors with
various tissue origins (44–46). The majority of experimental studies
focus on RNA levels and a small percentage investigate the
regulation of proteins by miRNA. As regulation by miRNAs is
extremely extensive, these RNAs may regulate numerous proteins in a
variety of ways (32,41), which resolves the former contradiction
that certain studies have demonstrated that miRNA-182 is an
onco-gene, and a small number of studies have confirmed that
miRNA-182 is a tumor suppressor gene. miRNA-182 regulates various
proteins that in turn regulate the biological behavior of tumor
cells.
The results of the present study indicate that
miRNA-182 is a potential tumor suppressor gene for OS, and that
upregulation of miRNA-182 expression inhibits cell viability,
proliferation, invasion and migration. Furthermore, miRNAs have
been proposed to be used in treatment strategies that may
potentially achieve improved clinical outcomes in patients with
cancer. In future research, the regulatory pathways and target
proteins of miRNA-182 may be determined, and studies may be
performed in vivo in animal models.
Acknowledgements
The authors are grateful to the Department of
Orthopedics of the First Affiliated Hospital of China Medical
University (Shenyang, China).
References
|
1
|
Bramwell VH: Osteosarcomas and other
cancers of bone. Curr Opin Oncol. 12:330–336. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kobayashi E, Hornicek FJ and Duan Z:
MicroRNA involvement in osteosarcoma. Sarcoma. 2012:3597392012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Longhi A, Errani C, De Paolis M, Mercuri M
and Bacci G: Primary bone osteosarcoma in the pediatric age: State
of the art. Cancer Treat Rev. 32:423–436. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hartford CM, Wodowski KS, Rao BN, Khoury
JD, Neel MD and Daw NC: Osteosarcoma among children aged 5 years or
younger: The St. Jude children's research hospital experience. J
Pediatr Hematol Oncol. 28:43–47. 2006.PubMed/NCBI
|
|
5
|
Bielack SS, Kempf-Bielack B, Delling G,
Exner GU, Flege S, Helmke K, Kotz R, Salzer-Kuntschik M, Werner M,
Winkelmann W, et al: Prognosticfactors in high-grade osteosarcoma
of the extremities or trunk: An analysis of 1,702 patients treated
on neoadjuvant cooperative osteosarcoma study group protocols. J
Clin Oncol. 20:776–790. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Marina N, Gebhardt M, Teot L and Gorlick
R: Biology and therapeutic advances for pediatric osteosarcoma.
Oncologist. 9:422–441. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Endicott M: Principles of treatment for
osteosarcoma. Clin Tech Small Anim Pract. 18:110–114. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Szcześniak MW, Owczarkowska E, Gapski J
and Makałowska I: MicroRNA databases. Postepy Biochem. 58:91–99.
2012.(In Polish). PubMed/NCBI
|
|
9
|
Iorio MV and Croce CM: MicroRNA
involvement in human cancer. Carcinogenesis. 33:1126–1133. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nikitina EG, Urazova LN and Stegny VN:
MicroRNAs and human cancer. Exp Oncol. 34:2–8. 2012.PubMed/NCBI
|
|
11
|
Bentwich I, Avniel A, Karov Y, Aharonov R,
Gilad S, Barad O, Barzilai A, Einat P, Einav U, Meiri E, et al:
Identification of hundreds of conserved and nonconserved human
microRNAs. Nat Genet. 37:766–770. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lee Y, Kim M, Han J, Yeom KH, Lee S, Baek
SH and Kim VN: MicroRNA genes are transcribed by RNA polymerase II.
EMBO J. 23:4051–4060. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cai X, Hagedorn CH and Cullen BR: Human
microRNAs are processed from capped, polyadenylated transcripts
that can also function as mRNAs. RNA. 10:1957–1966. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Faller M and Guo F: MicroRNAbiogenesis:
There's more than one way to skin a cat. Biochim Biophys Acta.
1779:663–667. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Murchison EP and Hannon GJ: MiRNAson the
move: MiRNA biogenesis and the RNAi machinery. Curr Opin Cell Biol.
16:223–229. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lund E and Dahlberg JE: Substrate
selectivity of exportin 5 and Dicer in the biogenesis of microRNAs.
Cold Spring Harb Symp Quant Biol. 71:59–66. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang XJ, Reyes JL, Chua NH and Gaasterland
T: Prediction and identification of Arabidopsis thaliana microRNAs
and their mRNA targets. Genome Biol. 5:R652004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shukla GC, Singh J and Barik S: MicroRNAs:
Processing, maturation, target recognition and regulatory
functions. Mol Cell Pharmacol. 3:83–92. 2011.PubMed/NCBI
|
|
20
|
Wang Z, Yao H, Lin S, Zhu X, Shen Z, Lu G,
Poon WS, Xie D, Lin MC and Kung HF: Transcriptional and epigenetic
regulation of human microRNAs. Cancer Lett. 331:1–10. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rajewsky N: MicroRNA target predictions in
animals. Nat Genet. 38:S8–S13. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Krek A, Grün D, Poy MN, Wolf R, Rosenberg
L, Epstein EJ, MacMenamin P, da Piedade I, Gunsalus KC, Stoffel M
and Rajewsky N: Combinatorial microRNA target predictions. Nat
Genet. 37:495–500. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jovanovic M and Hengartner MO: MiRNAs and
apoptosis: RNAs to die for. Oncogene. 25:6176–6187. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Babar IA, Slack FJ and Weidhaas JB: MiRNA
modulation of the cellular stress response. Future Oncol.
4:289–298. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Akçakaya P, Ekelund S, Kolosenko I,
Caramuta S, Ozata DM, Xie H, Lindforss U, Olivecrona H and Lui WO:
MiR-185 and miR-133b deregulation is associated with overall
survival and metastasis in colorectal cancer. Int J Oncol.
39:311–318. 2011.PubMed/NCBI
|
|
26
|
Wu H and Mo YY: Targeting miR-205 in
breast cancer. Expert Opin Ther Targets. 13:1439–1448. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kiselev FL: MicroRNA and cancer. Mol Biol
(Mosk). 48:232–42. 2014.(In Russian). PubMed/NCBI
|
|
28
|
Mraz M and Pospisilova S: MicroRNAs in
chronic lymphocytic leukemia: From causality to associations and
back. Expert Rev Hematol. 5:579–581. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Calin GA, Dumitru CD, Shimizu M, et al:
Frequent deletions and down-regulation of micro-RNA genes miR15 and
miR16 at 13q14 in chronic lymphocytic leukemia. Proc Natl Acad Sci
USA. 99:15524–15529. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu H, Wang Y, Li X, Zhang YJ, Li J, Zheng
YQ, Liu M, Song X and Li XR: Expression and regulatory function of
miRNA-182 in triple-negative breast cancer cells through its
targeting of profilin 1. Tumour Biol. 34:1713–1722. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kong WQ, Bai R, Liu T, Cai CL, Liu M, Li X
and Tang H: MicroRNA-182 targets cAMP-responsive element-binding
protein 1 and suppresses cell growth in human gastric
adenocarcinoma. FEBS J. 279:1252–1260. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cekaite L, Rantala JK, Bruun J, Guriby M,
Agesen TH, Danielsen SA, Lind GE, Nesbakken A, Kallioniemi O, Lothe
RA, et al: MiR-9, −31 and −182 deregulation promote proliferation
and tumor cell survival in colon cancer. Neoplasia. 14:868–879.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Guo D, Li Q, Lv Q, Wei Q, Cao S and Gu J:
MiR-27a targets sFRP1 in hFOB cells to regulate proliferation,
apoptosis and differentiation. PLoS One. 9:e913542014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real time quantitative PCR and
the 2(Delta Delta C(T)) Method. Methods. 25:402 4082001. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Duffy MJ, O'Donovan N and Crown J: Use of
molecular markers for predicting therapy response in cancer
patients. Cancer Treat Rev. 37:151–159. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Guled M and Knuutila S: MicroRNAs and
cancer. Duodecim. 129:1661–1669. 2013.(In Finnish). PubMed/NCBI
|
|
37
|
Ell B and Kang Y: MicroRNAs as regulators
of bone homeostasis and bone metastasis. Bonekey Rep. 3:5492014.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Tang T, Wong HK, Gu W, Yu MY, To KF, Wang
CC, Wong YF, Cheung TH, Chung TK and Choy KW: MicroRNA-182 plays an
onco-miRNA role in cervical cancer. Gynecol Oncol. 129:199–208.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yan D, Dong XD, Chen X, Yao S, Wang L,
Wang J, Wang C, Hu DN, Qu J and Tu L: Role of microRNA-182 in
posterior uveal melanoma: Regulation of tumor development through
MITF, BCL2 and cyclin D2. PLOS One. 7:e409672012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ning FL, Wang F, Li ML, Yu ZS, Hao YZ and
Chen SS: MicroRNA-182 modulates chemosensitivity of human non-small
cell lung cancer to cisplatin by targeting PDCD4. Diagn Pathol.
9:1432014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yang MH, Yu J, Jiang DM, Li WL, Wang S and
Ding YQ: MicroRNA-182 targets special AT-rich sequence-binding
protein 2 to promote colorectal cancer proliferation and
metastasis. J Transl Med. 12:1092014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Guo Y, Liao Y, Jia C, Ren J, Wang J and Li
T: MicroRNA-182 promotes tumor cell growth by targeting
transcription elongation factor A-like 7 in endometrial carcinoma.
Cell Physiol Biochem. 32:581–590. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Liu R, Li J, Teng Z, Zhang Z and Xu Y:
Overexpressed microRNA-182 promotes proliferation and invasion in
prostate cancer PC-3 cells by down-regulating N-myc downstream
regulated gene 1 (NDRG1). PLOS One. 8:e689822013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Hu X, Chen D, Cui Y, Li Z and Huang J:
Targeting microRNA-23a to inhibit glioma cell invasion via HOXD10.
Sci Rep. 3:34232013.PubMed/NCBI
|
|
45
|
He Y, Meng C, Shao Z, Wang H and Yang S:
MiR-23a functions as a tumor suppressor in osteosarcoma. Cell
Physiol Biochem. 34:1485–1496. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Perng DW, Yang DM, Hsiao YH, Lo T, Lee OK,
Wu MT, Wu YC and Lee YC: miRNA-146a expression positively regulates
tumor necrosis factor-α-induced interleukin-8 production in
mesenchymal stem cells and differentiated lung epithelial-like
cells. Tissue Eng Part A. 18:2259–67. 2012. View Article : Google Scholar : PubMed/NCBI
|