Introduction
Ovarian neoplasms are the sixth most common type of
cancer in terms of affected population and the seventh leading
cause of cancer-associated mortality among all gynecological
malignancies worldwide (1,2). Advanced ovarian neoplasms may exhibit
extensive spread to the surrounding abdominal organs, including the
stomach, intestine, colon, liver, lungs and pancreas, and may also
promote ascites within the peritoneal cavity (3). Due to the rapid proliferation and spread
of ovarian cancer within the abdominal cavity, affected patients
usually undergo primary debulking surgery prior to chemotherapy
with carboplatin and taxol, which may result in a disease-free
survival time of 16–22 months; however, the 5-year survival rate is
only 27% (4). Ovarian neoplasms have
diverse morphological features and varied genetic and epigenetic
alterations; consequently, distinguishing between benign lesions
and malignant ovarian cancers is challenging (5). Certain evidence indicates that
combination therapies are incapable of improving the clinical
outcome of patients with advanced ovarian neoplasms; therefore, the
early detection of ovarian malignancy is critical for patient
survival (6).
Diagnostic imaging is employed to determine the
suitability of a patient for surgery by assessing the primary
tumor, disease volume and extent (7,8). Diffusion
weighted imaging (DWI) is a non-invasive technique based on
molecular water diffusion properties, and may be used to
characterize tissue architecture based on microstructure, cellular
density, microcirculation and cell organization (9–11). DWI has
been demonstrated to be more accurate compared with computed
tomography (CT) for characterizing the sites of implants of ovarian
cancers, including metastases to the liver, lungs, kidneys, uterus
and pancreas (12,13). In addition, the combination of DWI
with gadolinium-enhanced magnetic resonance imaging (MRI), which is
used for whole-body imaging of advanced ovarian cancers, was shown
to increase the accuracy of determining the operability of the
tumor and reduce the rate of false diagnosis, particularly for
lesions that were obscured by impeded diffusion in the spleen
(9).
Quantitative analysis of DWI is conducted by
employing the apparent diffusion coefficients (ADCs) (14). In general, high ADC values indicate
that water molecules may move freely, which suggests a low
cellularity and better organization of the tissue structure, a
typical feature of normal tissues. By contrast, low ADC values
indicate the impeded mobility of water molecules, which suggests
high cellularity, a characteristic feature of malignant lesions.
Benign lesions, including simple cysts and hemangiomas, also have
high ADC values due to the low cell density of the lesion (15–17).
Although multiple studies have described the advantages of DWI over
other techniques for the differential diagnosis of benign and
malignant tumors, other reports have indicated that DWI has a
comparable sensitivity and lower specificity compared with CT
(18,19). In order to address this issue, a
meta-analysis based on high quality published studies was conducted
in the present study in order to evaluate the diagnostic value of
DWI in discriminating between benign and malignant ovarian
neoplasms.
Materials and methods
Data sources and keywords
In order to identify relevant papers that assessed
the diagnostic value of DWI in ovarian neoplasms, a comprehensive
search of the Ovid (available from, ovidsp.ovid.com/ovidweb.cgi), PubMed (available from,
www.ncbi.nlm.nih.gov/pubmed), EBSCO
(available from, search.ebscohost.com/), Wiley (available from,
onlinelibrary.wiley.com/), Web of
Science (available from, www.webofknowledge.com/), China National Knowledge
Infrastructure (available from, www.cnki.net),
Wanfang (available from, www.wanfangdata.com.cn/) and VIP (available from, s)
databases (last updated search on September 30, 2014) was
conducted, applying selected common keywords associated with
ovarian neoplasms and DWI. The search terms for a sensitive search
strategy were as follows: ‘Diffusion weighted imaging’, ‘diffusion
magnetic resonance imaging’, ‘diffusion MRI’, ‘diffusion weighted
MRI’, ‘diffusion weighted imaging’ or ‘diffusion’ in combination
with ‘ovarian neoplasms’, ‘ovary neoplasms’, ‘ovary cancer’,
‘ovarian cancer’, ‘cancer of ovary’, ‘ovarian tumor’, ‘malignant
tumor of ovary’, ‘ovarian carcinoma’, ‘ovarian epithelial
carcinoma’ or ‘OCE’. A manual search was also employed to identify
other relevant studies.
Selection and exclusion criteria
The published studies that were selected for the
current meta-analysis fulfilled the following inclusion criteria:
i) Studies are prospective or retrospective and evaluate the
accuracy of DWI in discriminating between benign and malignant
ovarian neoplasms; ii) studies must use histopathological results
as the diagnostic gold standard; iii) studies must provide
available data to calculate the sensitivity, specificity, negative
likelihood ratio and positive likelihood ratio, and provide the
number of ovarian neoplasm lesions; iv) studies must provide
information on the type of MRI device used; and v) studies must be
Chinese or English articles. The exclusion criteria were as
follows: i) Studies do not conform to the inclusion criteria; ii)
articles are abstracts, reviews, case reports, letters,
meta-analyses or proceedings; iii) studies do not contain the
number of benign or malignant neoplasms; iv) the data lacks
integrity; v) publications are duplicates or studies contain
overlapping data; and vi) studies are conducted in a non-human
population. In addition, only the largest or most recently
published study was included in instances where the author had
published several studies based on one clinical dataset.
Data extraction and quality
assessment
A standard data extraction form was used, and
descriptive information on a range of factors was independently
collected, including first author, year of submission, state,
ethnicity, research design, number of lesions, gender, age and
pathological types; sensitivity, specificity, accuracy, true
positive, false positive, true-negative and false-negative values;
and MRI apparatus types (manufacturer) and ADC threshold.
Discrepancies during data extraction were resolved by reexamination
of all items and discussion between reviewers. In order to
determine whether the studies involved were of high quality, a
quality assessment of diagnostic accuracy studies (QUADAS) analysis
was used to evaluate the studies by two independent parties
(20). The 11 QUADAS items were: The
spectrum of patients was representative of the patients that will
receive the test in practice (QUADAS01); the selection criteria
were clearly described (QUADAS02); the time period between the
reference standard and index test was short enough to be reasonably
sure that the target condition did not change between the two tests
(QUADAS03); the whole sample or a random selection of the sample
received verification using a reference standard of diagnosis
(QUADAS04); patients received the same reference standard
regardless of the index test result (QUADAS05); the reference
standard was independent of the index test (QUADAS06); the
execution of the index test was described in sufficient detail to
permit replication of the test (QUADAS07); the execution of the
reference standard was described in sufficient detail to permit
replication (QUADAS08); the same clinical data was available at the
time test results were interpreted and at the time the test was
applied in practice (QUADAS09); uninterpretable test results were
reported (QUADAS10); and withdrawals from the study were explained
(QUADAS11).
Statistical analysis
The present meta-analysis was performed using STATA
software (version 12.0; Stata Corp, College Station, TX, USA) and
Meta-disc software (version 1.4; Meta-DiSc, Madrid, Spain). A
random effects model or a fixed effects model was applied to
calculate the diagnostic odds ratio (DOR). The multiple parameters
of specificity, sensitivity, positive likelihood ratio, negative
likelihood ratio and summary receiver operating characteristic
(SROC) curve to count the area under the curve (AUC) were used to
assess the diagnostic value of DWI in discriminating between benign
and malignant ovarian neoplasms. A heterogeneity test, that
included tests of threshold effect and non-threshold effect, was
evaluated by applying the Spearman correlation coefficients
(21) of the logarithms of
sensitivity and 1-specificity. A non-threshold effect occurred with
P>0.05 (P<0.05 was considered to indicate that a threshold
effect existed). The heterogeneity test for a non-threshold effect
was applied with an I2 test (0%, no heterogeneity; 100%,
maximal heterogeneity) (22) to
reflect the potential for heterogeneity between studies. A random
effects analysis was used for cases in which heterogeneity was
observed between studies (P<0.05 or I2>50%);
otherwise a fixed effects analysis was applied.
Data combination was conducted by applying a SROC to
calculate the AUC (23), with scores
that ranged from 0–1 in case of heterogeneity due to the threshold
effect (an AUC of 1 was considered to be the maximal diagnostic
value). If the heterogeneity was not caused by threshold effect,
the targets, including specificity, sensitivity, positive
likelihood ratio, negative likelihood ratio and DOR, were combined
to produce a forest plot. The studies were excluded in turn, in
order to analyze the effect of single study on the overall results.
Fagan's nomogram was applied to estimate the pre-test and post-test
probability of three diagnostic criteria, and a bivariate boxplot
was used to judge the heterogeneity between studies. The included
angle between the regression line in Deeks funnel plot (24) and DOR was applied to assess
publication bias.
Results
Included studies
A total of 285 articles were retrieved from the 9
databases by keyword search. Following the exclusion of duplicates
(n=89), reviews, letters or meta-analyses (n=42), non-human studies
(n=53), and studies not relevant to our research topics (n=56), the
remaining studies (n=45) were carefully reviewed. An additional 11
studies were excluded as they were not cohort or case-control
studies (n=2), not relevant to DWI (n=4), or not relevant to
ovarian neoplasms (n=5). Subsequent to additional reviewing of the
remaining 34 trials, the studies that did not supply enough
information were eliminated (n=4), and 10 high quality studies were
included in the final meta-analysis. These 10 case-control studies,
published between 2005 and 2014, provided the required information
on the diagnostic value of DWI and ovarian neoplasms (9,11,12,25–31). The
meta-analysis included a total of 1,159 ovarian tumor patients,
comprising 559 benign tumor patients and 600 malignant tumor
patients. Of the 10 studies, 6 were conducted on Asian patient
populations and 4 on Caucasian patient populations. The QUADAS of
included studies is shown in Fig. 1,
and Table I presents the baseline
characteristics of the selected study samples.
 | Table I.Baseline characteristics of
patients. |
Table I.
Baseline characteristics of
patients.
|
|
|
| Number of cases | Age, years |
|
|
|---|
|
|
|
|
|
|
|
|
|---|
| Study author | Year | Ethnicity | Total | Benign | Malignant | Benign | Malignant | All patients | QUADAS score | Ref. |
|---|
| Michielsen et
al | 2014 | Caucasian | 475 | 267 | 208 |
|
| 61.9±31.5 | 9 | (9) |
| Kierans et
al | 2013 | Caucasian | 37 | 28 |
9 |
|
| 54.0±14.0 | 8 | (25) |
| Espada et
al | 2013 | Caucasian | 34 | 26 |
8 |
|
| 53.1±11.9 | 9 | (12) |
| Zhang et
al | 2012 | Asian | 75 | 45 | 30 | 48.2±30.0 | 50.2±28.5 |
| 7 | (28) |
| Cui et
al | 2012 | Asian | 84 | 34 | 50 | 50.8±13.1 | 54.5±11.5 |
| 7 | (27) |
| Zhang et
al | 2012 | Asian | 202 | 74 | 128 |
|
| 56.5±15.3 | 8 | (26) |
| Li et
al | 2012 | Asian | 131 | 46 | 85 | 46.2±15.5 | 59.9±10.6 |
| 7 | (29) |
| Yuan et
al | 2011 | Asian | 45 | 22 | 23 |
|
| 47.8±26.5 | 7 | (30) |
| Low et
al | 2009 | Caucasian | 34 |
7 | 27 |
|
| 58.5 | 9 | (11) |
| Han et
al | 2005 | Asian | 42 | 10 | 32 |
|
| – | 8 | (31) |
Although the majority of the studies were located in
the middle region of the bivariate boxplot, two studies were
located outside of the boxplot, which indicated heterogeneity
between the studies (Fig. 2). The
result of the Spearman correlation coefficient of the sensitivity
logarithm and 1-specificity logarithm indicated that no threshold
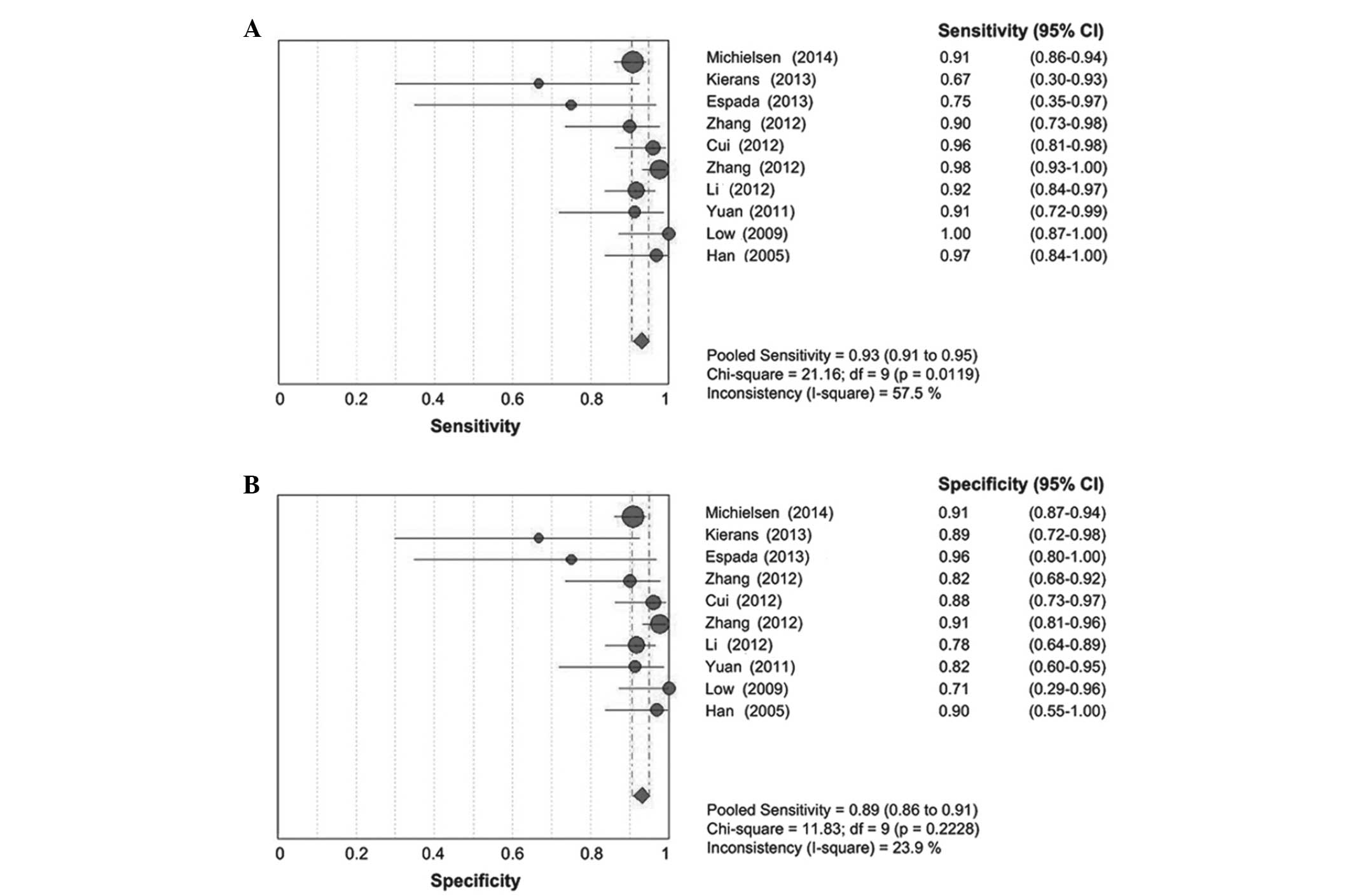
effect existed (0.345; P=0.328). The I2 values of
sensitivity (57.5%) and negative likelihood ratio (54.5%) were
>50%, which indicated statistical heterogeneity between studies,
and a random effects model was applied. However, the I2
values for specificity (23.9%) and positive likelihood ratio
(33.1%) were <50%, which suggested that no statistical
heterogeneity existed; therefore, a fixed effects model was
used.
Quantitative data synthesis
The results of the present meta-analysis
demonstrated that DWI has high diagnostic capabilities in
discriminating between benign and malignant ovarian neoplasms,
based on the outcomes of pooled sensitivity [0.93; 95% confidence
interval (CI), 0.91–0.95; Fig. 3A],
pooled specificity (0.89; 95% CI, 0.86–0.91; Fig. 3B), pooled positive likelihood ratio
(7.58; 95% CI, 6.00–9.56), pooled negative likelihood ratio (0.10;
95% CI, 0.06–0.16), pooled DOR (85.33; 95% CI, 57.15–127.40)
(Fig. 4A) and the AUC of the SROC
curve (0.95; Fig. 4B). The results of
the pooled likelihood ratio suggested a limited clinical value of
DWI for discriminating between benign and malignant ovarian
neoplasms (Fig. 5A). The results of
Fagan's nomogram indicated that the pre-test probability ratio
(20%) × positive likelihood ratio (8.00) yielded a post-test
probability ratio of 66%, while the pre-test probability ratio ×
negative likelihood ratio (0.08) yielded a post-test probability
ratio of 2% (Fig. 5B). Subgroup
analyses of ethnicity and MRI types were conducted. The results
indicated that the DORs for patients of Asian and Caucasian descent
were 86.37 (95% CI, 48.35–154.27) and 84.33 (95% CI, 48.44–146.81),
respectively (Fig. 6A), suggesting no
statistically significant differences between Asians and
Caucasians. Notably, the DORs for GE Healthcare Life Sciences and
Siemens AG MRIs were 100.76 (95% CI, 65.28–155.53) and 30.85 (95%
CI, 10.40–91.53), respectively; thus, the diagnostic value of GE
Healthcare Life Sciences machine appears to be superior to that of
the Siemens AG equipment (Fig.
6B).
Sensitivity analysis and publication
bias
A sensitivity analysis was applied in order to
estimate the stability of the present meta-analysis. The outcomes
of the sensitivity analysis revealed that none of the included
studies had an influence on the pooled DOR of the DWI for
discriminating between benign and malignant ovarian neoplasms
(Fig. 7A). The included angle between
the regression line and the DOR axis was close to 90° in Deeks,
which indicated that no publication bias was present in the current
meta-analysis (Fig. 7B).
Discussion
In order to evaluate the diagnostic value of DWI in
discriminating between benign and malignant ovarian neoplasms, a
systematic meta-analysis was conducted. The outcomes of the present
study indicated that DWI has a high diagnostic value with regard to
discriminating between benign and malignant ovarian neoplasms.
Ovarian neoplasms are the sixth most prevalent cancer and the
seventh leading cause of cancer-associated mortality among all
types of gynecological malignancy worldwide (1,2). Due to
the high frequency of peritoneal and distant metastases and the
complicated nature of ovarian neoplasms, the patient survival rate
is poor (27,32). Diagnostic imaging is used to determine
the suitability of the patient for surgery by assessing the primary
tumor or by describing the disease volume and extent (7,8). DWI, as a
functional MRI technique, provides non-invasive and high-resolution
information on tissue characteristics based on molecular water
diffusion properties, and the imaging output is useful to determine
tissue microstructure, density, microcirculation and cellular
organization (9,11,33).
Several studies describe a close association between cell density
and ADC values (16,33–35). High
ADC values are present in benign lesions, including primary
neoplasms that contain low cell densities, while low ADC values are
typically observed in malignant neoplasms, due to high cell
densities and altered membrane permeability (15,16). Based
on the results of the current meta-analysis, a major conclusion is
that DWI demonstrates an excellent diagnostic performance for
discriminating between benign and malignant ovarian neoplasms.
Subgroup analyses based on ethnicity and MRI devices
were performed. The result of the subgroup analysis on ethnicity
indicated no statistically significant differences between Asians
and Caucasians. However, the subgroup analysis on the MRI machine
type demonstrated that a better diagnostic performance of DWI was
achieved with GE Healthcare Life Sciences equipment compared with
Siemens AG machines.
The present study was subject to certain
limitations. First, the number of studies included was small, and
several relevant unpublished studies and abstracts were not
included, which may have influenced the conclusion of the current
study. A second limitation of the present study was that the sample
size was relatively small, leading to a lower confidence in
assessing the value of DWI for discriminating between benign and
malignant ovarian neoplasms. Third, only studies published in
English and Chinese were included, with studies published in other
languages excluded, which potentially eliminated important studies
that may have influenced the conclusion of the present
meta-analysis. Additional studies are required that involve a
larger sample size and diverse ethnicities for a more comprehensive
evaluation of the diagnostic performance of DWI in ovarian
neoplasms.
In conclusion, DWI demonstrates an excellent
diagnostic value for discriminating between benign and malignant
ovarian neoplasms, and may provide a reliable predictive tool for
the surgical outcome in ovarian neoplasms.
References
|
1
|
Zeng ST, Guo L, Liu SK, Wang DH, Xi J,
Huang P, Liu DT, Gao JF, Feng J and Zhang L: Egg consumption is
associated with increased risk of ovarian cancer: Evidence from a
meta-analysis of observational studies. Clin Nutr. 34:635–641.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ahmed N, Abubaker K and Findlay JK:
Ovarian cancer stem cells: Molecular concepts and relevance as
therapeutic targets. Mol Aspects Med. 39:110–125. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lengyel E: Ovarian cancer development and
metastasis. Am J Pathol. 177:1053–1064. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kipps E, Tan DS and Kaye SB: Meeting the
challenge of ascites in ovarian cancer: New avenues for therapy and
research. Nat Rev Cancer. 13:273–282. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Conic I, Dimov I, Tasic-Dimov D,
Djordjevic B and Stefanovic V: Ovarian epithelial cancer stem
cells. ScientificWorldJournal. 11:1243–1269. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bristow RE, Puri I and Chi DS:
Cytoreductive surgery for recurrent ovarian cancer: A
meta-analysis. Gynecol Oncol. 112:265–274. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Forstner R, Sala E, Kinkel K and Spencer
JA: European Society of Urogenital Radiology: ESUR guidelines:
Ovarian cancer staging and follow-up. Eur Radiol. 20:2773–2780.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kumar Dhingra V, Kand P and Basu S: Impact
of FDG-PET and -PET/CT imaging in the clinical decision-making of
ovarian carcinoma: An evidence-based approach. Women's Health (Lond
Engl). 8:191–203. 2012. View Article : Google Scholar
|
|
9
|
Michielsen K, Vergote I, de Op Beeck K,
Amant F, Leunen K, Moerman P, Deroose C, Souverijns G, Dymarkowski
S, De Keyzer F and Vandecaveye V: Whole-body MRI with
diffusion-weighted sequence for staging of patients with suspected
ovarian cancer: A clinical feasibility study in comparison to CT
and FDG-PET/CT. Eur Radiol. 24:889–901. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Low RN and Gurney J: Diffusion-weighted
MRI (DWI) in the oncology patient: Value of breathhold DWI compared
to unenhanced and gadolinium-enhanced MRI. J Magn Reson Imaging.
25:848–858. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Low RN, Sebrechts CP, Barone RM and Muller
W: Diffusion-weighted MRI of peritoneal tumors: Comparison with
conventional MRI and surgical and histopathologic findings - a
feasibility study. AJR Am J Roentgenol. 193:461–470. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Espada M, Garcia-Flores JR, Jimenez M,
Alvarez-Moreno E, De Haro M, Gonzalez-Cortijo L, Hernandez-Cortes
G, Martinez-Vega V and De La Sainz Cuesta R: Diffusion-weighted
magnetic resonance imaging evaluation of intra-abdominal sites of
implants to predict likelihood of suboptimal cytoreductive surgery
in patients with ovarian carcinoma. Eur Radiol. 23:2636–2642. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Low RN and Barone RM: Combined
diffusion-weighted and gadolinium-enhanced MRI can accurately
predict the peritoneal cancer index preoperatively in patients
being considered for cytoreductive surgical procedures. Ann Surg
Oncol. 19:1394–1401. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
White NS, McDonald CR, Farid N, Kuperman
JM, Kesari S and Dale AM: Improved conspicuity and delineation of
high-grade primary and metastatic brain tumors using ‘restriction
spectrum imaging’: Quantitative comparison with high B-value DWI
and ADC. AJNR Am J Neuroradiol. 34:958–964. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Testa ML, Chojniak R, Sene LS, Damascena
AS, Guimarães MD, Szklaruk J and Marchiori E: Is DWI/ADC a useful
tool in the characterization of focal hepatic lesions suspected of
malignancy? PLoS One. 9:e1019442014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li SP and Padhani AR: Tumor response
assessments with diffusion and perfusion MRI. J Magn Reson Imaging.
35:745–763. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Galea N, Cantisani V and Taouli B: Liver
lesion detection and characterization: Role of diffusion-weighted
imaging. J Magn Reson Imaging. 37:1260–1276. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Soussan M, Des Guetz G, Barrau V,
Aflalo-Hazan V, Pop G, Mehanna Z, Rust E, Aparicio T, Douard R,
Benamouzig R, et al: Comparison of FDG-PET/CT and MR with
diffusion-weighted imaging for assessing peritoneal carcinomatosis
from gastrointestinal malignancy. Eur Radiol. 22:1479–1487. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Satoh Y, Ichikawa T, Motosugi U, Kimura K,
Sou H, Sano K and Araki T: Diagnosis of peritoneal dissemination:
Comparison of 18F-FDG PET/CT, diffusion-weighted MRI, and
contrast-enhanced MDCT. AJR Am J Roentgenol. 196:447–453. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Whiting P, Rutjes AW, Reitsma JB, Bossuyt
PM and Kleijnen J: The development of QUADAS: A tool for the
quality assessment of studies of diagnostic accuracy included in
systematic reviews. BMC Med Res Methodol. 3:252003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pfeiffer KA, McIver KL, Dowda M, Almeida
MJ and Pate RR: Validation and calibration of the Actical
accelerometer in preschool children. Med Sci Sports Exerc.
38:152–157. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Higgins JP and Thompson SG: Quantifying
heterogeneity in a meta-analysis. Stat Med. 21:1539–1558. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Walter SD: Properties of the summary
receiver operating characteristic (SROC) curve for diagnostic test
data. Stat Med. 21:1237–1256. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Deeks JJ, Macaskill P and Irwig L: The
performance of tests of publication bias and other sample size
effects in systematic reviews of diagnostic test accuracy was
assessed. J Clin Epidemiol. 58:882–893. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kierans AS, Bennett GL, Mussi TC, Babb JS,
Rusinek H, Melamed J and Rosenkrantz AB: Characterization of
malignancy of adnexal lesions using ADC entropy: Comparison with
mean ADC and qualitative DWI assessment. J Magn Reson Imaging.
37:164–171. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang P, Cui Y, Li W, Ren G, Chu C and Wu
X: Diagnostic accuracy of diffusion-weighted imaging with
conventional MR imaging for differentiating complex solid and
cystic ovarian tumors at 1.5T. World J Surg Oncol. 10:2372012.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cui YF, Li WH, Zhu MJ, Wang DB, Zhang P,
Zhang ZY and Chu CT: Clinical application and research of diffusion
weighted MR imaging in complex ovarian tumors. Zhongguo Linchuang
Yixue Yingxiang Zazhi. 12(856): 859–869. 2012.(In Chinese).
|
|
28
|
Zhang W, Zou S, Shen DH and Tong YX: The
value of MR diffusion-weighted imaging combined with serum CA125 in
the qualitative diagnosis of ovarian occupying lesion. Yixue
Yingxiangxue Zazhi. 8:1348–1353. 2012.(In Chinese).
|
|
29
|
Li WH, Chu C, Cui Y, Zhang P and Zhu M:
Diffusion-weighted MRI: A useful technique to discriminate benign
versus malignant ovarian surface epithelial tumors with solid and
cystic components. Abdom Imaging. 37:897–903. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yuan XC, Wang X, Yao G, Zheng L and Hu Y:
The value of 3.0T MRI for the diagnosis of the ovarian tumor.
Shiyong Fangshexue Zazhi. 27(1695): 1698–1712. 2011.(In
Chinese).
|
|
31
|
Han DM, Yang XP, Wang HP and Li YX:
Comparative study of CT and MRI in the diagnosis of ovarian
neoplasms. Zhongguo Linchuang Yixue Yingxiang Zazhi. 16:441–443.
2005.(In Chinese).
|
|
32
|
Ferlay J, Parkin DM and Steliarova-Foucher
E: Estimates of cancer incidence and mortality in Europe in 2008.
Eur J Cancer. 46:765–781. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Decker G, Mürtz P, Gieseke J, Träber F,
Block W, Sprinkart AM, Leitzen C, Buchstab T, Lütter C, Schüller H,
et al: Intensity-modulated radiotherapy of the prostate: dynamic
ADC monitoring by DWI at 3.0 T. Radiother Oncol. 113:115–120. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ginat DT, Mangla R, Yeaney G, Johnson M
and Ekholm S: Diffusion-weighted imaging for differentiating benign
from malignant skull lesions and correlation with cell density. AJR
Am J Roentgenol. 198:W597–601. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Rechichi G, Galimberti S, Signorelli M,
Franzesi CT, Perego P, Valsecchi MG and Sironi S: Endometrial
cancer: Correlation of apparent diffusion coefficient with tumor
grade, depth of myometrial invasion, and presence of lymph node
metastases. AJR Am J Roentgenol. 197:256–262. 2011. View Article : Google Scholar : PubMed/NCBI
|