Introduction
Breast cancer is the most frequently diagnosed
cancer and the leading cause of cancer-related mortality among
females, accounting for 23% of all cancer cases and 14% of
cancer-related mortalities (1).
Metastasis involves a multistep process including the detachment of
cancer cells from a primary site, the invasion of surrounding
tissue, and spreading through the circulation, and represents one
of the main causes of mortality in breast cancer patients. In
recent years, disease-related mortality and metastasis have
declined as a result of adjuvant therapy. Comprehensive therapy
(including chemotherapy and endocrine therapy) facilitates the
suppression of the metastatic dissemination of local tumors
(2). However, these treatments target
the tumor cells and disregard the basement membrane, which
represents a critical barrier during breast cancer progression.
With breast cancer progression, tumor cells invade surrounding
tissues and spread to distant organs, eventually leading to
metastasis by inducing disruption of the underlying basement
membrane (3), as shown in Fig. 1. Basement membranes play a key role in
tumor progression (4). However, these
signposts of tumor progression have so far only been evaluated by
histological examination of previously excised specimens (5,6). It would
therefore be useful to have a tool that allows label-free in
situ imaging of these signposts.
The basement membrane is located between the
epithelium and stroma. Since epithelial cells are able to generate
a two-photon excited fluorescence (TPF) signal and the stroma is
composed primarily of collagen that is capable of emitting a strong
second harmonic generation (SHG) signal (7–10),
multiphoton microscopy (MPM) based on TPF and SHG signals may be
useful for visualizing the outline of basement membranes that are
not detectable by other imaging modalities.
As a nonlinear optical technique, MPM has advantages
including label-free imaging, inherent optically sectioning, deep
optical penetration, and reduced specimen photobleaching and
photodamage (11–15). In this study, we used MPM to visualize
the in situ morphology of basement membrane in normal and
cancerous breast tissues based on intrinsic nonlinear optical
contrast. Our results reveal that MPM demonstrates marked
differences in the organization of basement membranes in normal
breast tissue and breast cancer tissue.
Materials and methods
Tissue specimens
A total of 30 fresh breast biopsy specimens were
obtained from 30 patients who underwent biopsy and were later
diagnosed with breast cancer by conventional histological modality.
Thirty normal breast tissues were obtained from reduction surgeries
performed at Fujian Provincial Tumor Hospital, China. Written
informed consent was required from every participant according to a
protocol approved by the Institutional Review Board of Fujian
Provincial Tumor Hospital and in accordance with the Declaration of
Helsinki. The specimens were placed in a glass-bottomed dish
(coverglass, 0.085–0.13 mm; MatTek Corporation, Ashland, MA, USA)
for multiphoton imaging. In this study, the specimen preparation
and multiphoton imaging were completed within 1 h of biopsy.
Imaging instrumentation
MPM was achieved using a nonlinear optical system
which has been described previously (16). In brief, multiphoton images were
acquired using a commercial laser scanning microscopic imaging
system (Zeiss LSM 510 META; Carl Zeiss, Jena, Germany) coupled to a
femtosecond Ti: sapphire laser (Mira 900-F, Coherent Inc., Santa
Clara, CA, USA) operating at 800 nm. The polarization direction of
the laser light was horizontal. An oil immersion objective [x63,
with numerical aperture (NA) of 1.4] was employed for focusing the
excitation beam into the tissue samples (average power, <15 mW)
and was also used to collect the backscattered intrinsic
multiphoton signals. The images were obtained at 2.56 ms per pixel.
A fine focusing stage (HRZ 200 stage; Carl Zeiss) was used to
translate the samples following an x-y scan of the samples to
obtain a large-area image, and to change the focal position for
recording various optical sections.
Histology analysis
After multiphoton imaging, histological procedures
were completed including formalin fixation, paraffin embedding and
5-µm-thick sectioning. Hematoxylin and eosin (H&E; Dako,
Carpinteria, CA, USA)-stained and antibody-stained sections were
obtained from each specimen. The collagen IV primary antibody
(rabbit polyclonal; 1:200 dilution; cat no. SAB4500369) was
obtained from Sigma-Aldrich (St. Louis, MO, USA) and the secondary
antibody (polyperoxidase rabbit anti-mouse IgG; 1:200 dilution; cat
no. zs2864967) was obtained from Beijing Zhongshan Golden Bridge
Biotechnology Co., Ltd. (Beijing, China). All specimens were
reviewed by an attendant pathologist using a light microscope
(SMZ1500; Nikon, Tokyo, Japan).
Results
Patients and tumor
characteristics
The study population comprised 60 female
participants who were divided into two groups. Group 1 consisted of
30 patients with breast cancer with a median age of 54 years (32–68
years). Group 2 comprised 30 patients who underwent breast
reduction surgeries with a median age of 56 years (39–69 years).
The average tumor size in group 1 was 2 cm (range, 1–5 cm). The
demographic and histopathological characteristics of the patients
in group 1 are summarized in Table
I.
 | Table I.Demographic and histopathological
characteristics of breast cancer patients. |
Table I.
Demographic and histopathological
characteristics of breast cancer patients.
| Characteristic | Number of
patients |
|---|
| Age, years |
|
| ﹤50 | 23 |
|
>50 | 7 |
| Tumor size |
|
| T1 | 12 |
| T2 | 18 |
| T3 | 0 |
| Grade |
|
| I | 10 |
| II | 11 |
| III | 9 |
| Tumor histology |
|
| IDC | 30 |
| Estrogen
receptor |
|
|
Positive | 21 |
|
Negative | 9 |
| Progesterone
receptor |
|
|
Positive | 19 |
|
Negative | 11 |
| HER2 |
|
|
Positive | 9 |
|
Negative | 21 |
Multiphoton images
To visualize the morphological features of basement
membranes in normal breast and breast cancer tissue, the
representative multiphoton images of the tissues are respectively
shown in Fig. 2. As expected, the MPM
technique visualizes the outline of basement membranes well. Large
morphological differences may be observed between normal breast and
breast cancer in Fig. 2. In the case
of normal tissue, the normal mammary gland is a highly organized
structure. The acini and ducts have a central lumen and are lined
by simple columnar epithelium (TPF signal, in green), basement
membrane (the interface of the epithelium and stroma), and stroma
(SHG signal, in red). The basement membrane is intact and the
surface is smooth and even. By contrast, breast carcinoma tissues
have lost this organized architecture. The basement membrane
observed in the normal case is replaced by the tumor cells and is
missing. The tumor cells (TPF signal, in green) are characterized
by irregular size and shape, enlarged nuclei, and increased
nuclear-cytoplasmic ratio.
Histological results
The basement membrane is located between the
epithelium and mesenchymal tissues. Basement membranes are
considered to form a protective barrier against the initial
infiltration of tissue by tumor cells. Here, immunohistochemical
staining of collagen IV reveals marked differences in the
organization of basement membranes in normal breast tissue and
breast cancer. Continuous basement membranes with strong staining
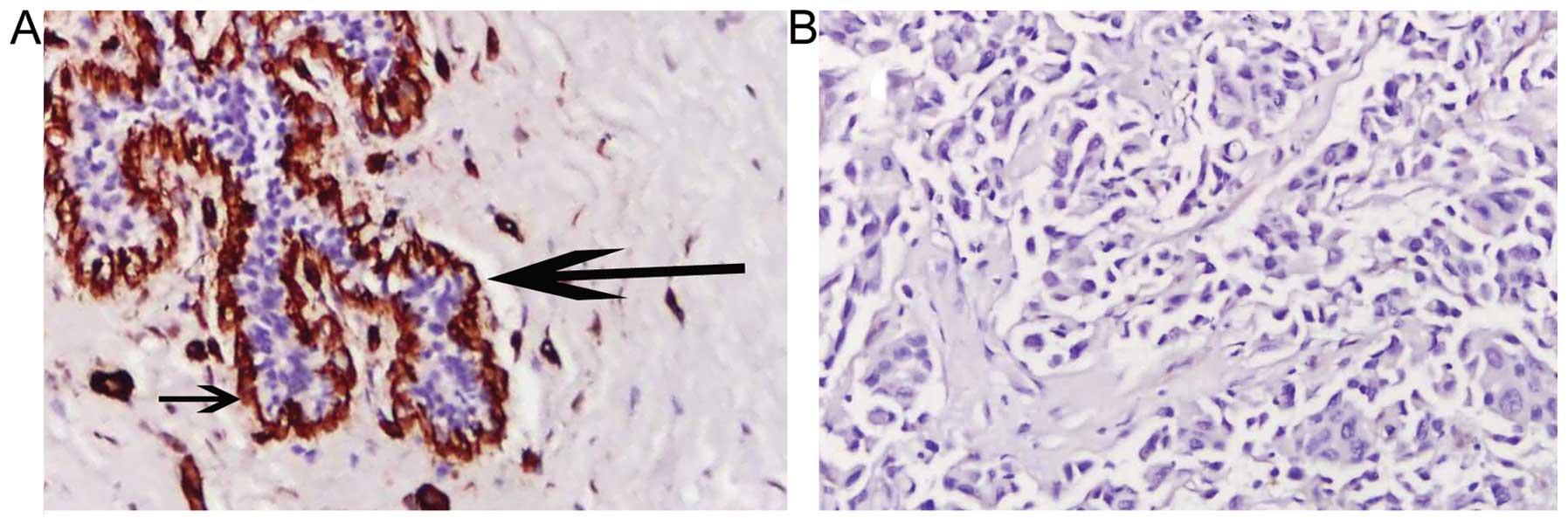
for collagen IV were observed in normal breast tissues (Fig. 3A). Conversely, breast cancer tissues
were negative for collagen IV staining (Fig. 3B), which indicated the disruption and
loss of basement membrane in invasive breast cancer. These results
were similar to those observed with MPM imaging.
Discussion
In this study, we have provided the first evidence
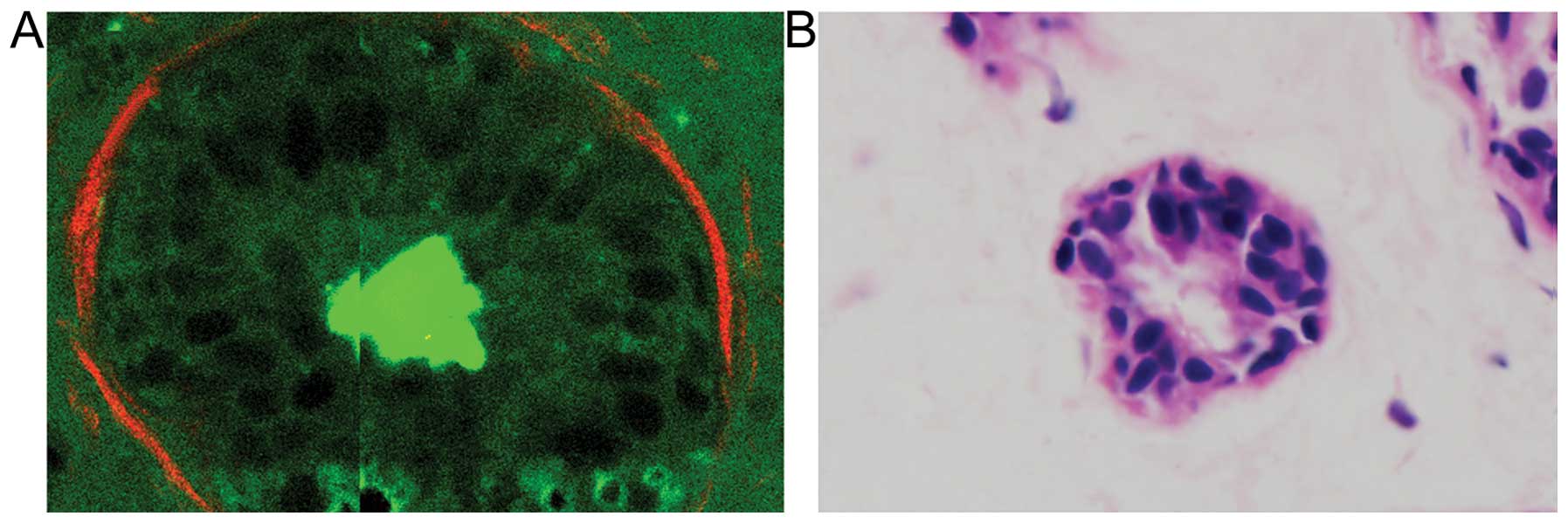
to demonstrate that MPM effectively characterizes basement
membranes in normal (Fig. 2A) and
cancerous (Fig. 2B) breast tissues.
In multiphoton images, the normal breast tissue has a highly
organized structure. The ducts are lined by simple columnar
epithelium (TPF signal, in green), basement membrane (interface of
epithelium and stroma) and stroma (SHG signal, in red). The
basement membrane is intact and the surface is smooth and even (as
indicated by the small arrow). By contrast, breast cancer tissues
have lost this organized architecture. The basement membrane
observed in the normal case is missing and replaced by tumor cells
characterized by irregular size and shape, enlarged nuclei and
increased nuclear-cytoplasmic ratio. These observations are in
accordance with traditional histological analysis (shown in
Fig. 3). The correlation between
disintegration of the basement membranes of tumors and the
increasingly anaplastic appearance supports the idea that basement
membranes may play a role in tumor invasion (4,17).
Furthermore, the absence of a basement membrane barrier may
facilitate tumor spread.
It was noted that MPM enables direct visualization
of the basement membranes in normal and cancerous breast tissues in
a manner similar to H&E staining of conventional histological
sections. Compared with H&E analysis, however, MPM presents a
couple of advantages. Firstly, basement membrane may be clearly
distinguished due to its unique structure using MPM (18), but may be difficult to identify in
H&E-stained sections (as shown in Fig. 4). Secondly, MPM introduces no
artifacts during processing due to the imaging being based on
intrinsic nonlinear optical contrast, making it possible to
diagnose biopsy specimens in situ. Finally, the multiphoton
imaging time is shorter than the turnaround time for frozen
sections involving slicing, paraffin embedding or freeze-thaw
processes. Therefore, MPM provides an in situ histological
tool with which to evaluate basement membranes without the labeling
requirement of conventional methods. The significance of this
capability results from the fact that basement membranes represent
a critical barrier against the initial infiltration of tissue by
tumor cells (6,7), and the detection of basement membrane
has so far depended heavily on traditional histological procedures
(5,6).
These results indicate that MPM holds promise for breast tissue
analysis and applications to studying the dynamics of basement
membrane changes at various stages of breast cancer in a manner
that is compatible with clinical practice.
The use of MPM does have certain limitations in its
current state. Firstly, the limited penetration depth of MPM
imaging hinders evaluation of deeper tissue change. Furthermore,
utilization of high NA objectives in MPM systems results in smaller
fields of view, which may result in missing diseased tissues. In
addition, due to sampling difficulties, this study did not include
ductal carcinoma in situ, which is a proliferation of
malignant epithelial cells within the mammary ductal system with
intact basement membrane. The dynamic alteration of basement
membrane reflects the progression of breast cancer. In future
studies, we intend to focus on evaluating the feasibility of MPM
for tracking the progression of breast cancer.
In conclusion, this study demonstrates the potential
of MPM to visualize basement membranes, the key indicators of
breast cancer progression, in a tracer-free manner. This unique
method of targeting basement membrane using MPM techniques appears
to be promising for further study, particularly as a complementary
technique with gold-standard histopathological diagnosis. Without
damaging histological procedures, this method unlocks new
possibilities for in vivo diagnosis of breast cancer. With
the capability of evaluating the basement membrane as shown in the
present study, we envisage that a MPM-based intra-fiberoptic
ductoscopy or transdermal biopsy needle (19–21) could
facilitate and benefit in vivo studies and diagnoses in the
years to come. Thus, in the future we intend to extend the
application of MPM to in vitro breast cancer studies as well
as to other types of cancer.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (81272574, 61275006 and
81271620), the Natural Science Foundation of Fujian Province
(2014J01300 and 2014J05086), the Program for Changjiang Scholars
and Innovative Research Team in University (IRT1115), and the
Program from the Education Bureau of Fujian Province (JA12057,
JA13060 and JB13127).
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Eifel P, Axelson JA, Costa J, Crowley J,
Curran WJ Jr, Deshler A, Fulton S, Hendricks CB, Kemeny M,
Kornblith AB, et al: National institutes of health consensus
development conference statement: adjuvant therapy for breast
cancer, November 1–3, 2000. J Natl Cancer Inst. 93:979–989. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Abdelkarim M, Vintonenko N, Starzec A,
Robles A, Aubert J, Martin ML, Mourah S, Podgorniak MP,
Rodrigues-Ferreira S, Nahmias C, et al: Invading basement membrane
matrix is sufficient for MDA-MB-231 breast cancer cells to develop
a stable in vivo metastatic phenotype. PLoS One. 6:e233342011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Plodinec M, Loparic M, Monnier CA,
Obermann EC, Zanetti-Dallenbach R, Oertle P, Hyotyla JT, Aebi U,
Bentires-Alj M, Lim RY and Schoenenberger CA: The nanomechanical
signature of breast cancer. Nat Nanotechnol. 7:757–765. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lipponen P, Ji H, Aaltomaa S and Syrjänen
K: Tumour vascularity and basement membrane structure in breast
cancer as related to tumour histology and prognosis. J Cancer Res
Clin Oncol. 120:645–650. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gusterson BA, Warburton MJ, Mitchell D,
Ellison M, Neville AM and Rudland PS: Distribution of myoepithelial
cells and basement membrane proteins in the normal breast and in
benign and malignant breast diseases. Cancer Res. 42:4763–4770.
1982.PubMed/NCBI
|
|
7
|
Zipfel WR, Williams RM, Christie R,
Nikitin AY, Hyman BT and Webb WW: Live tissue intrinsic emission
microscopy using multiphoton-excited native fluorescence and second
harmonic generation. Proc Natl Acad Sci USA. 100:7075–7080. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Campagnola PJ and Loew LM: Second-harmonic
imaging microscopy for visualizing biomolecular arrays in cells,
tissues and organisms. Nat Biotechnol. 21:1356–1360. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhuo S, Zheng L, Chen J, Xie S, Zhu X and
Jiang X: Depth-cumulated epithelial redox ratio and stromal
collagen quantity as quantitative intrinsic indicators for
differentiating normal, inflammatory and dysplastic epithelial
tissues. Appl Phys Lett. 97:1737012010. View Article : Google Scholar
|
|
10
|
Wu X, Zhuo S, Chen J and Liu N: Real-time
in vivo imaging collagen in lymphedematous skin using multiphoton
microscopy. Scanning. 33:463–467. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hovhannisyan VA, Su PJ and Dong CY:
Quantifying thermodynamics of collagen thermal denaturation by
second harmonic generation imaging. Appl Phys Lett. 94:2339022009.
View Article : Google Scholar
|
|
12
|
Matteini P, Ratto F, Rossi F, Cicchi R,
Stringari C, Kapsokalyvas D, Pavone FS and Pini R:
Photothermally-induced disordered patterns of corneal collagen
revealed by SHG imaging. Opt Express. 17:4868–4878. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhuo S, Chen J, Luo T and Zou D: Multimode
nonlinear optical imaging of the dermis in ex vivo human skin based
on the combination of multichannel mode and Lambda mode. Opt
Express. 14:7810–7820. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Brown E, McKee T, diTomaso E, Pluen A,
Seed B, Boucher Y and Jain RK: Dynamic imaging of collagen and its
modulation in tumors in vivo using second harmonic generation. Nat
Med. 9:796–800. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Campagnola PJ and Dong CY: Second harmonic
generation microscopy: principles and applications to disease
diagnosis. Laser Photonics Rev. 5:13–26. 2011. View Article : Google Scholar
|
|
16
|
Zhuo S, Chen J, Wu G, Xie S, Zheng L,
Jiang X and Zhu X: Quantitatively linking collagen alteration and
epithelial tumor progression by second harmonic generation
microscopy. Appl Phys Lett. 96:2137042010. View Article : Google Scholar
|
|
17
|
Place AE, Jin Huh S and Polyak K: The
microenvironment in breast cancer progression: biology and
implications for treatment. Breast Cancer Res. 13:2272011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Georgiou E, Theodossiou T and Hovhannisyan
V: Second and third optical harmonic generation in type I collagen,
by nanosecond laser irradiation, over a broad spectral region. Opt
Commun. 176:253–260. 2000. View Article : Google Scholar
|
|
19
|
Bao H, Boussioutas A, Jeremy R, Russell S
and Gu M: Second harmonic generation imaging via nonlinear
endomicroscopy. Opt Express. 18:1255–1260. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wu Y, Xi J, Cobb MJ and Li X: Scanning
fiber-optic nonlinear endomicroscopy with miniature aspherical
compound lens and multimode fiber collector. Opt Lett. 34:953–955.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rivera DR, Brown CM, Ouzounov DG, Pavlova
I, Kobat D, Webb WW and Xu C: Compact and flexible raster scanning
multiphoton endoscope capable of imaging unstained tissue. Proc
Natl Acad Sci USA. 108:17598–17603. 2011. View Article : Google Scholar : PubMed/NCBI
|