Introduction
Tumor necrosis factor (TNF)-related apoptosis
inducing ligand (TRAIL) is a cytokine that belongs to the TNF
family of proteins, and is able to induce apoptosis in a variety of
cancer cells without significantly affecting normal cells (1). TRAIL promotes apoptosis by binding to
certain cognate receptors, including TRAIL-R1, or death receptor 4
(DR4), and TRAIL-R2, or death receptor 5 (DR5) (2). Fas-associated death domain (FADD) and
caspase-8 are aggregated and recruited to the DR-TRAIL complex to
form the death-inducing signaling complex (DISC) (2). This apoptotic signal is additionally
transmitted via extrinsic and intrinsic pathways (3). The extrinsic pathway is activated in a
mitochondrial-independent mechanism. Upon recruitment, caspase-8 is
activated and results in the direct cleavage of downstream effector
caspases such as caspase-3 to induce apoptosis (3). The intrinsic pathway is activated in a
mitochondrial-dependent manner. Once cleaved by caspase-8, BH3
interacting domain death agonist (Bid) translocates to the
mitochondria and activates the B-cell lymphoma (Bcl)-2 family
members Bcl-2-associated X protein (Bax) and Bcl-2 killer 1 (Bak),
which depolarize mitochondria (4).
Apoptogenic factors, including cytochrome c, are released from the
mitochondria into the cytosol to trigger the activation of
caspase-3 to induce apoptosis (4).
Studies have shown that certain cancer cells resist the apoptotic
effect of TRAIL, an occurrence that is termed ‘TRAIL resistance’
(5). However, TRAIL resistance has
been previously overcome by combining TRAIL with various
chemotherapeutic drugs (6), which
suggests that utilizing an approach that affects multiple targets
may prevent or reverse TRAIL resistance in cancer cells. The
development of effective therapies aimed at reversing TRAIL
resistance is essential to effectively kill cancer cells.
Nasopharyngeal carcinoma (NPC) is common in
Southeast Asia and in Southern parts of China (3–5/10,000), but is
rarely presented in other parts of the world (7). Until 1990, radiotherapy was the standard
treatment for all stages of NPC (8).
Despite significant progress in intensity-modulated radiation
therapy and other types of therapy such as surgery and molecular
targeted therapy, the outcomes for the patients remain
disappointing, with five-year survival rates of 34–52% (9). Consequently, the identification of more
effective treatments for NPC is required.
Death receptors have previously been detected in the
biopsy specimens of head and neck tumors, but not in the
surrounding normal tissues (10).
This finding supports the hypothesis that the presence of death
receptors in certain head and neck cancers may endow them with a
greater susceptibility to TRAIL-mediated apoptosis. One study
demonstrated the robust induction of apoptosis in the TW02 NPC cell
line by TRAIL (11); however, another
study indicated that inducing apoptosis in certain NPC cells lines
by TRAIL may be challenging (12).
The explanation for this discrepancy remains unknown. However,
previous studies indicated that the overexpression of Bcl-2 and
phosphatidylinositol-4,5-bisphosphate 3-kinase/protein kinase B
activation may contribute to TRAIL resistance in NPC cell lines
(13).
Persistent Epstein-Barr virus (EBV) infection may
cause NPC, Hodgkin's lymphoma and Burkitt's lymphoma (14). Latent membrane protein 1 (LMP1) is the
principle oncogene of EBV (15), and
LMP1 immortalizes and transforms cells by controlling the signaling
pathways that block apoptosis and stimulate cell proliferation
(16). LMP1 induces chemoresistance
and promotes cell survival in NPC (17,18).
Friboulet et al (19) showed
that EBV-positive NPC cell lines expressed increased levels of
inhibitor of apoptosis proteins (IAPs), which have anti-apoptotic
functions. Additionally, X-linked inhibitor of apoptosis protein
(XIAP) is a member of the IAP family that inhibits caspases and
induces TRAIL resistance (20).
The present study aims to test the hypothesis that
LMP1 overexpression induces TRAIL resistance in NPC cells by
enhancing XIAP, and also aims to study the associated molecular
mechanisms.
Materials and methods
Cell lines and reagents
Three human NPC cell lines, CNE-1, CNE-2 and C666-1,
were used in the present study. CNE-1 is a well-differentiated
squamous cell carcinoma NPC cell line that consistently expresses
EBV, and was established by the Cancer Research Institute, Sun
Yat-sen University (Guangdong, China). CNE-2 is poorly
differentiated cell line that was derived from the primary tumor of
a patient with poorly differentiated squamous cell carcinoma NPC
and is positive for plasma EBV DNA (established by the Chinese
Academy of Medical Sciences, Beijing, China). C666-1 is an NPC cell
line that was established by the Department of Anatomical and
Cellular Pathology, Prince of Wales Hospital, The Chinese
University of Hong Kong (Shatin, Hong Kong, China).
The non-transformed nasopharyngeal epithelium NP-69
cell line was derived from the human nasopharynx, and established
by the Department of Anatomy, Li Ka Shing Faculty of Medicine,
University of Hong Kong (Hong Kong, China). All cells were cultured
in RPMI-1640 media (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) supplemented with 10% fetal bovine serum (FBS;
Hyclone; GE Healthcare Life Sciences, Logan, UT, USA) and 100 U/ml
penicillin/streptomycin (Huabei Pharmacy Group Xiantai Medicine
Co., Ltd., Shijiazhuang, China). Cultures were maintained in a
fully-humidified atmosphere of 5% CO2 in air at 37°C.
TRAIL was obtained from Pfizer, Inc. (New York, NY, USA). Embelin
was obtained from Enzo Life Sciences, Inc. (Farmingdale, NY, USA).
Embelin was dissolved in dimethyl sulfoxide (DMSO) (Beyotime
Institute of Biotechnology, Haimen, China) at a 500 µM
concentration, and stored at −20°C until required. The following
antibodies were obtained from the indicated sources: Mouse
anti-LMP1 monoclonal antibody (Dako, Glostrup, Denmark), rabbit
anti-caspase-9 polyclonal antibody, rabbit anti-caspase-8
polyclonal antibody and rabbit anti-caspase-3 polyclonal antibody
(Cell Signaling Technology, Danvers, MA, USA). Mouse anti-Bcl-2
polyclonal antibody, mouse anti-Bcl-extra long (Bcl-XL) polyclonal
antibody, mouse anti-Bid antibody, and mouse anti-XIAP polyclonal
antibody were obtained from Santa Cruz Biotechnology, Inc.,
(Dallas, TX, USA). All antibodies were used at a dilution of
1:1,000 or 1:800.
3-(4,5-dimethylthiazolyl)-2,5-diphenyl
tetrazolium bromide (MTT) cell viability assay
Cell viability was determined using an MTT assay.
NPC cells (5,000 cells/well) were plated in 96-well plates
(Beyotime Institute of Biotechnology). Subsequent to adding the
indicated TRAIL treatment doses (0, 20, 40, 60, 80 and 100 ng/ml)
for various amounts of time (4, 8, 12, 16, 20 and 24 h), cells were
incubated for 2 h with 0.5 mg/ml of MTT (Sigma-Aldrich, St. Louis,
MO, USA), and DMSO was used to solubilize the formazan product.
Control wells were treated with DMSO only. The optical density (OD)
of each well was measured at 570 nm with a microplate reader
(MA68II; X-Rite, Inc., Grand Rapids, MI, USA) (survival rate =
ODtreat / ODcontrol).
Determination of apoptosis by annexin
V/propidium iodide (PI) staining
Levels of TRAIL-mediated apoptosis were determined
using the Annexin V/PI staining kit (catalogue no. P0018A; Beyotime
Institute of Biotechnology). Subsequent to incubation with the
indicated TRAIL treatment doses (20, 40, 60, 80 and 100 ng/ml) for
various lengths of time (4, 8, 12, 18 and 24 h), cells were
harvested, and the cell pellets were suspended in 500 µl binding
buffer [10 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid
(pH 7.4), 140 mM NaCl, 1 mM MgCl2, 5 mM KCl and 2.5 mM
CaCl2] at a density of 1×106 cells⁄ml.
Samples were incubated with 1 µl annexin V-fluorescein
isothiocyanate (FITC) and 5 µl PI for 5 min at room temperature in
the dark and measured on a FACSort flow cytometer (BD Biosciences,
Franklin Lakes, NJ, USA). Annexin V-FITC and PI fluorescence were
detected in the FL-1 (green) and FL-2 (red) channels, respectively.
Data were analyzed using CellQuest Pro software version 2.7 (BD
Biosciences).
LMP1 complementary DNA (cDNA)
transfection
The LMP1-expression vector (pGL6-LMP1) and pGL6
vector were obtained from Beyotime Institute of Biotechnology.
Transfection was performed using the Lipofectamine 2000
(Invitrogen; Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol. In brief, cells were seeded in a 6-well
plate (Beyotime Institute of Biotechnology) at a density of
105 cells/well and cultured for 24 h until cells
attained a confluence of 60–70%. The vector pGL6-LMP1 or the
negative pGL6 vector (10 µg) was diluted in 100 µl of Opti-MEM
(Thermo Fisher Scientific, Inc.) for 5 min at room temperature.
During the incubation period, 5 µl of Lipofectamine 2000 was
diluted in 100 µl Opti-MEM. These two mixtures were combined, mixed
gently, incubated for 20 min at room temperature, and then
incubated with the cells for 20 min at room temperature. Stably
transfected clones were selected in medium containing 0.5 mg/ml
geneticin sulfate (G418; Amresco Inc., Framingham, MA, USA).
Established clones were grown in RPMI-1640 medium supplemented with
10% FBS containing 0.5 mg/ml G418 sulfate (Beyotime Institute of
Biotechnology).
Flow cytometric analysis of TRAIL
receptors
NPC cells at a density of 1×106 cells
were suspended in 500 µl of the medium and incubated with 5 µl of
anti-DR4 (catalogue no. SAB4700541) or anti-DR5 (catalogue no.
SAB1303637) polyclonal rabbit antibodies (dilution, 1:100;
Sigma-Aldrich) for 1 h. Subsequent to washing in phosphate-buffered
saline (PBS) (Beyotime Institute of Biotechnology) three times (5
min each), FITC-conjugated goat anti-rabbit polyclonal antibody
(dilution, 1:200; catalogue no. A0528; Beyotime Institute of
Biotechnology) was added to the cell suspensions and incubated for
1 h on ice. Subsequent to rinsing in PBS, samples were analyzed
with a FACSort flow cytometer. Data were analyzed using CellQuest
Pro software version 2.7.
XIAP-small interfering RNA (siRNA)
transfection
The siRNA-targeting XIAP messenger RNA (mRNA) was
predesigned and validated according to the following sequences:
Sense 5′-GGAGAUACCGUGCGGUGCUdTdT-3′ and antisense
5′-AGCACCGCACGGUAUCUCCdTdT-3′. The negative control siRNA was
designed by the Invitrogen brand (Thermo Fisher Scientific, Inc.)
and had the following sequences: Sense
5′-UUCUCCGAACGUGUCACGUdTdT-3′ and antisense
5′-ACGUGACACGUUCGGAGAAdTdT-3′. Cells were seeded into a 6-well
plate at a density of 105 cells/well and cultured to a
confluence of 60–70%. Cells were then transfected with 100 nM
specific or nonspecific siRNA. The transfection was accomplished
with 30 ml INTERFERin (Polyplus transfection; Ozyme, St.
Quentin-en-Yvelines, France). The siRNA transfection reagent was
added to the siRNA duplex in 500 µl of Opti-MEM. Subsequent to 48 h
incubation, the transfected cells were treated with TRAIL.
Western blot analysis
Cells were washed three times for 5 min in PBS, and
then dissolved in lysis buffer (20 mM Na2PO4,
150 mM NaCl, 1% Triton X-100, 1% aprotinin, 1 mM
phenylmethylsulfonyl fluoride, 100 mM NaF and 2 mM
Na3VO4). Proteins were quantified with a
Bradford kit (catalaogue no. P0006; Beyotime Institute of
Biotechnology). Next, proteins (20 µg/lane) were separated by
sodium dodecyl sulfate-polyacrylamide gel electrophoresis and
transferred to a polyvinylidene difluoride membrane. Membranes were
incubated with the primary antibody [mouse anti-LMP1 monoclonal
antibody (catalogue nos. M0897, IR753 and IS753; Dako), rabbit
anti-caspase-9 polyclonal antibody (catalogue no. 9506; Cell
Signaling Technology), rabbit anti-caspase-8 polyclonal antibody
(catalogue no. 9496s; Cell Signaling Technology), rabbit
anti-caspase-3 polyclonal antibody (catalogue no. 9665s; Cell
Signaling Technology), mouse anti-Bcl-2 polyclonal antibody
(catalogue no. sc-7382; Santa Cruz Biotechnology, Inc.), mouse
anti-Bcl-XL polyclonal antibody (catalogue no. sc-8392; Santa Cruz
Biotechnology, Inc.), mouse anti-Bid antibody (catalogue no. 5C9;
Santa Cruz Biotechnology, Inc.) and mouse anti-XIAP polyclonal
antibody (catalogue no. sc-55552; Santa Cruz Biotechnology, Inc.)]
overnight at 4°C. The membranes were then washed in PBS three times
for 5 min, and incubated with horseradish peroxidase-conjugated
goat anti-rabbit secondary antibodies (1:500; catalogue no. A0192;
Beyotime Institute of Biotechnology). Proteins were detected using
an enhanced chemiluminescence kit (catalogue no. P0018A; Beyotime
Institute of Biotechnology).
Semi-quantitative reverse
transcription-polymerase chain reaction (RT-PCR)
Total RNA was extracted and purified with TRIzol
Total RNA Extraction Kit (catalogue no. R0016; Beyotime Institute
of Biotechnology), using DNase (catalogue no. R0021; Beyotime
Institute of Biotechnology). Total RNA (1 µg) was reverse
transcribed by High-Capacity cDNA Reverse Transcription Kits
(Applied Biosystems; Thermo Fisher Scientific, Inc.), according to
the manufacturer's protocol. The primers for LMP1 and β-actin were
synthesized by Sangon Biological Engineering Technology and
Services Co. Ltd. (Shanghai, China) as follows: LMP1, sense
5′-TGGAGGGAGAGTCAGTCAGGC-3′ and antisense
5′-ATTGACGGAAGAGGTTGAAAAC-3′ to generate a 254-bp fragment;
glyceraldehyde 3-phosphate dehydrogenase (GAPDH), sense
5′-ACCCACTCCTCCACCTTTG-3′ and antisense 5′-ACCCACTCCTCCACCTTTG-3′
to generate a 188-bp fragment. The RT products (1 µl) were
amplified by PCR using the following conditions: 95°C for 5 min;
then 32 cycles at 95°C for 30 sec, 58°C for 30 sec and 72°C for 30
sec; and a final extension at 72°C for 5 min. PCR products were
electrophoresed on 1.5% agarose gels at 100 V for 40 min, using DNA
ladder (catalogue no. D0107; Beyotime Institute of Biotechnology).
Bands were visualized with ethidium bromide (Shanghai Rong Bo
Biotechnology Co., Ltd., Shanghai, China). LMP1 mRNA expression
levels were quantified by Quantity One software version 4.31
(Bio-Rad Laboratories, Inc., Hercules, CA, USA) and represented as
the densitometric ratio of the targeted gene relative to GAPDH. PCR
experiments were repeated three times.
Assessment of mitochondrial
transmembrane potential (Δψm)
Assessment of the Δψm was performed using cationic
lipophilic fluorochrome 3,3′-dihexyloxacarbocyanine iodide
[DiOC6(3); 460 ng/ml;
Molecular Probes; Thermo Fisher Scientific, Inc.). The cells were
treated for 12 h with 100 ng/ml TRAIL and incubated for 30 min at
37.8°C in complete medium consisting of DMSO with
DiOC6(3). The cells were
then washed twice in PBS three times for 5 min, and analyzed using
a FACSort flow cytometer and analyzed with Cell Quest Pro
software.
Statistical analysis
All tests were performed in triplicate and
statistical analyses were performed using SPSS software (version
15.0; SPSS, Inc., Chicago, IL, USA). Descriptive statistics were
expressed as the mean ± standard deviation, and Student's t-tests
were used to analyze the significant differences between paired
datasets. An α value of P<0.05 was considered to indicate a
statistically significant difference.
Results
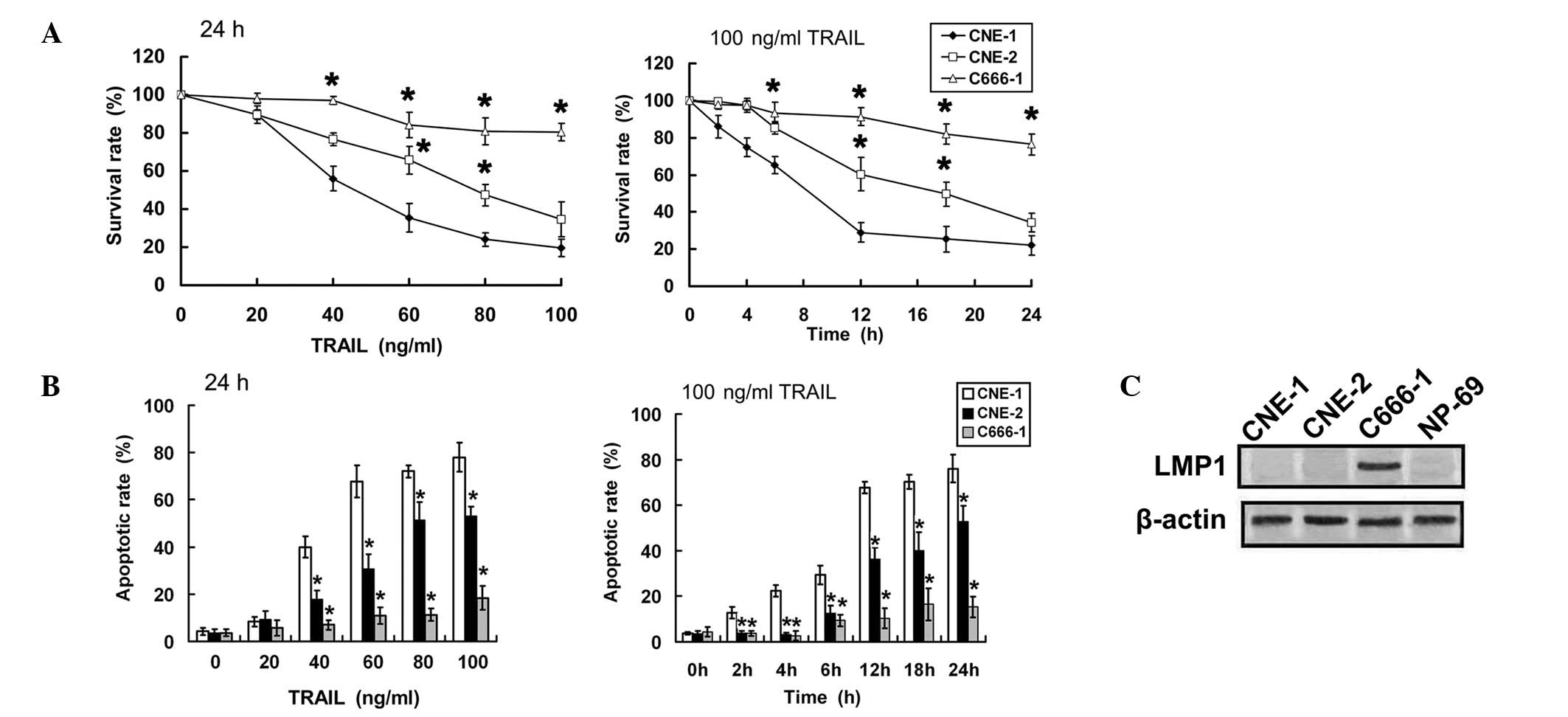
Sensitivity to TRAIL varies among NPC
cells
First, the growth inhibition effects of TRAIL
against NPC cell lines were assessed by MTT assay. TRAIL induced
cell death in a dose- and time-dependent manner. C666-1 cells were
highly resistant to TRAIL, whereas CNE-1 cells were highly
sensitive (Fig. 1A). The apoptosis
rate was additionally analyzed by annexin V/PI staining and flow
cytometry to confirm whether the difference in cell death was due
to various apoptotic responses. The apoptotic effect of TRAIL was
also time- and dose-dependent. The percentage of apoptotic cells
was highest in CNE-1 cells and lowest in C666-1 cells in response
to TRAIL treatment (Fig. 1B). LMP1
was detected in C666-1, but not in either CNE-1 or CNE-2 cells. The
level of LMP1 expression was also tested in the non-transformed
nasopharyngeal epithelial cell line NP-69, which did not show
expression of LMP1 (Fig. 1C). From
the results, LMP1-positive NPC cells were concluded to be more
sensitive to TRAIL compared with LMP1-negative NPC cells.
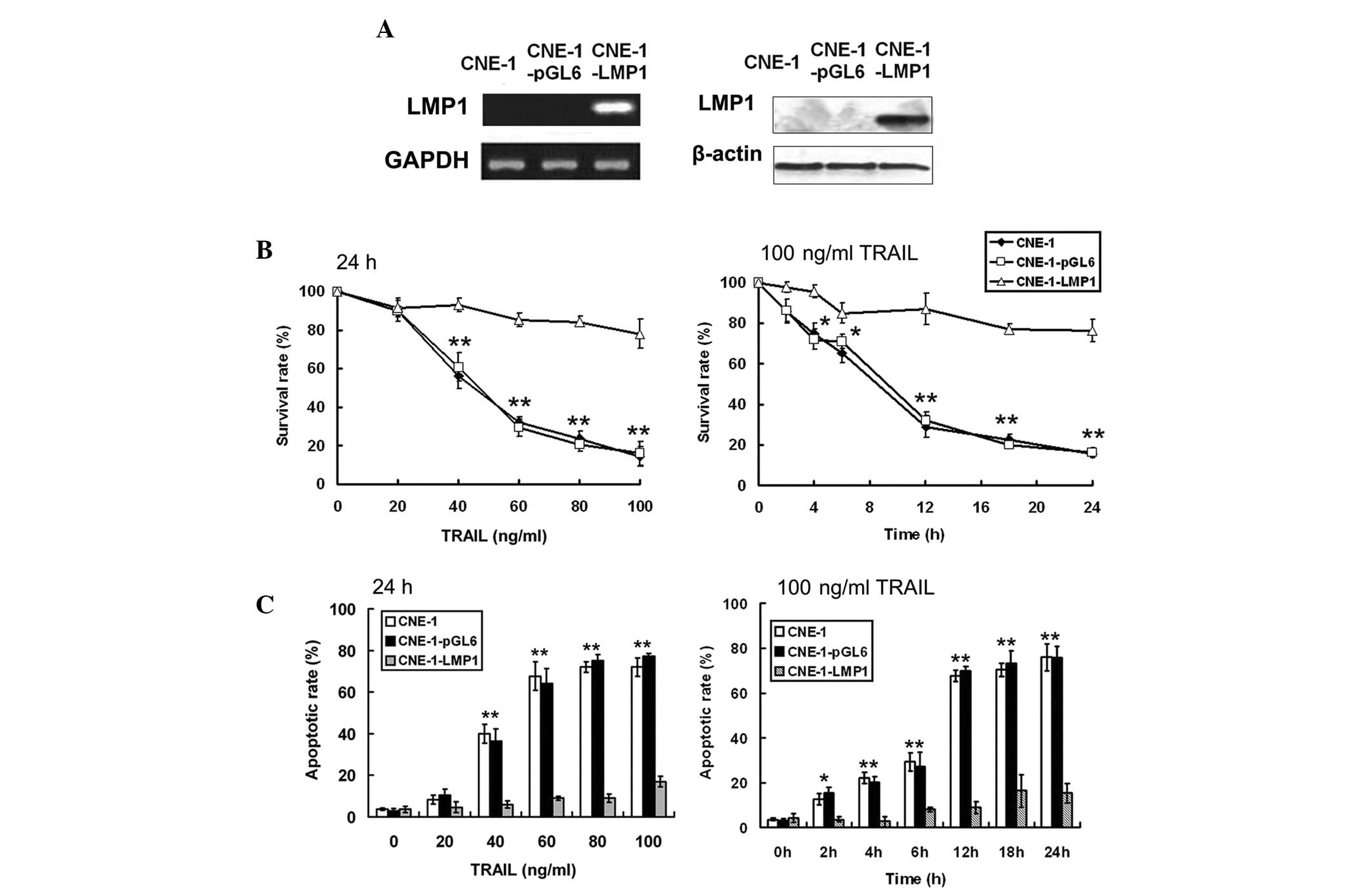
Overexpression of LMP1 inhibits the
apoptotic effect of TRAIL
LMP1-positive NPC cells were indicated to be more
sensitive to TRAIL compared with LMP1-negative NPC cells.
Therefore, a genetic approach was used to upregulate LMP1
expression in CNE-1 cell lines. The pGL6-LMP1 vector was
transfected into CNE-1 cells. Stable pGL6-LMP1 transfected cells
and empty vector transfected control cells were designated as
CNE-1-LMP1 and CNE-1-pGL6, respectively. Semi-quantitative RT-PCR
and western blot analyses revealed that LMP1 expression was
enhanced in CNE-1-LMP1 cells (Fig.
2A). The effect of TRAIL on cell survival was evaluated by MTT
cell viability assays (Fig. 2B).
Treatment with TRAIL resulted in cell death in CNE-1 and CNE-1-pGL6
cells in a dose- and time-dependent manner but had a dampened
effect on CNE-1-LMP1 cells. Similarly, TRAIL induced significant
apoptosis in CNE-1 and CNE-1-pGL6 cells in a time- and
dose-dependent manner, but the apoptosis level was not as high in
CNE-1-LMP1 cells (Fig. 2C). The
results suggested that the overexpression of LMP1 may inhibit
TRAIL-induced apoptosis.
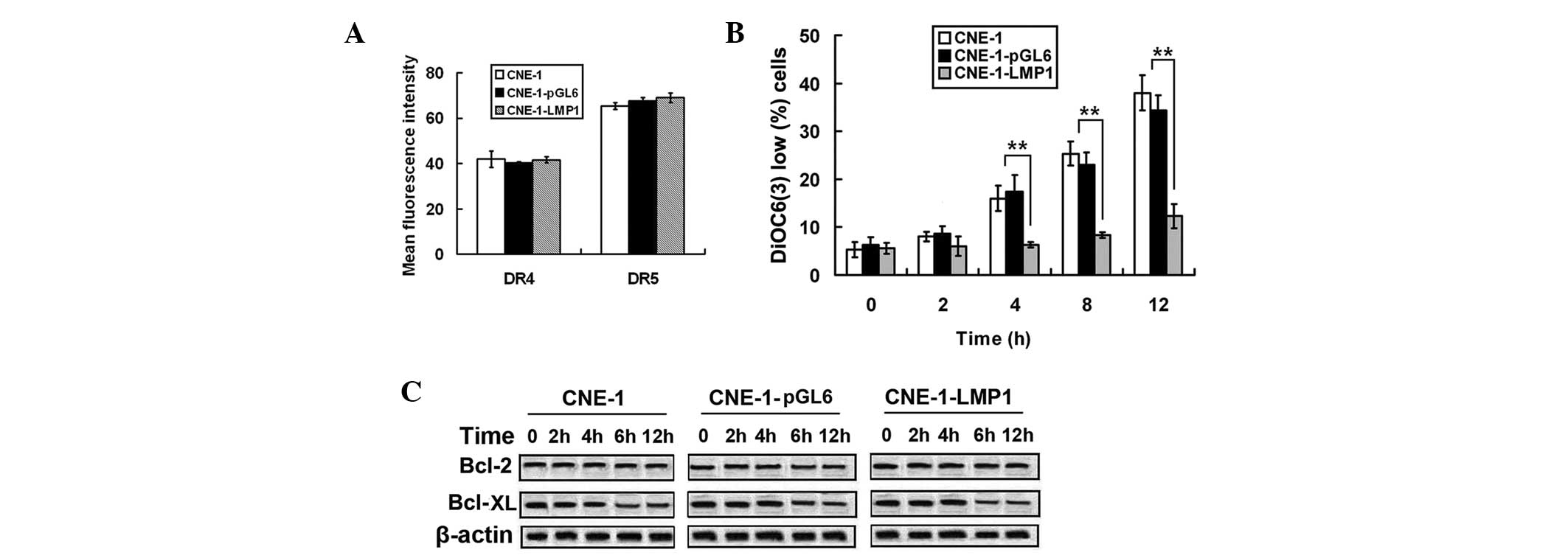
Overexpression of LMP1 inhibits
mitochondrial-dependent apoptosis in NPC cells
To elucidate the molecular mechanism responsible for
LMP1-indued TRAIL resistance, the effect of LMP1 on the
TRAIL-induced apoptotic pathway was examined. Since TRAIL is known
to trigger apoptosis via binding to DR4 and DR5, the expression of
DR4 and DR5 was examined (Fig. 3A).
LMP1 overexpression did not change the cell surface expression
levels of either DR4 or DR5 (P=0.087). Furthermore, the
DiOC6(3) negative cell
rate was increased in CNE-1 and CNE-1-pGL6 cells compared with the
CNE-1-LMP1 cells during TRAIL treatment. This result suggested that
mitochondrial depolarization was inhibited by LMP1 overexpression
(Fig. 3B). The expression levels of
the pro-survival proteins Bcl-2 and Bcl-XL, were unaffected by LMP1
overexpression (Fig. 3C). The results
showed that LMP1 inhibited the mitochondrial-dependent apoptotic
pathway without having any impact on Bcl-2 and Bcl-XL.
LMP1 inhibits TRAIL-induced caspase
and Bid cleavage in NPC cells
Analysis of caspase activation showed that the
cleaved, mature forms of caspase-8 (p43/41), caspase-9 (p35) and
caspase-3 (p17, p10) were generated in CNE-1 and CNE-1-pGL6 cells
during TRAIL treatment, in a time-dependent manner (Fig. 4). However, CNE-1-LMP1 showed minimal
caspase activation during TRAIL treatment. This observation
indicated that LMP1 inhibited TRAL-induced caspase activation.
Previously, the present study demonstrated that LMP1 inhibited
TRAIL-induced mitochondrial-dependent apoptotic pathways.
Therefore, the effects of LMP1 on TRAIL-induced Bid cleavage, which
is an important event in mitochondrial-dependent apoptotic
pathways, were additionally examined. Since the anti-Bid antibody
recognized only the uncleaved Bid protein and not the cleaved
product, the cleavage of Bid was assessed by determining the extent
of the decreased levels of uncleaved Bid protein. TRAIL treatment
resulted in a reduction in uncleaved Bid (indicating Bid cleavage)
in CNE-1 and CNE-1-pGL6 cells. However, the reduction in Bid was
inhibited in CNE-1-LMP1 cells, suggesting that LMP1 affected the
upstream cleavage of Bid.
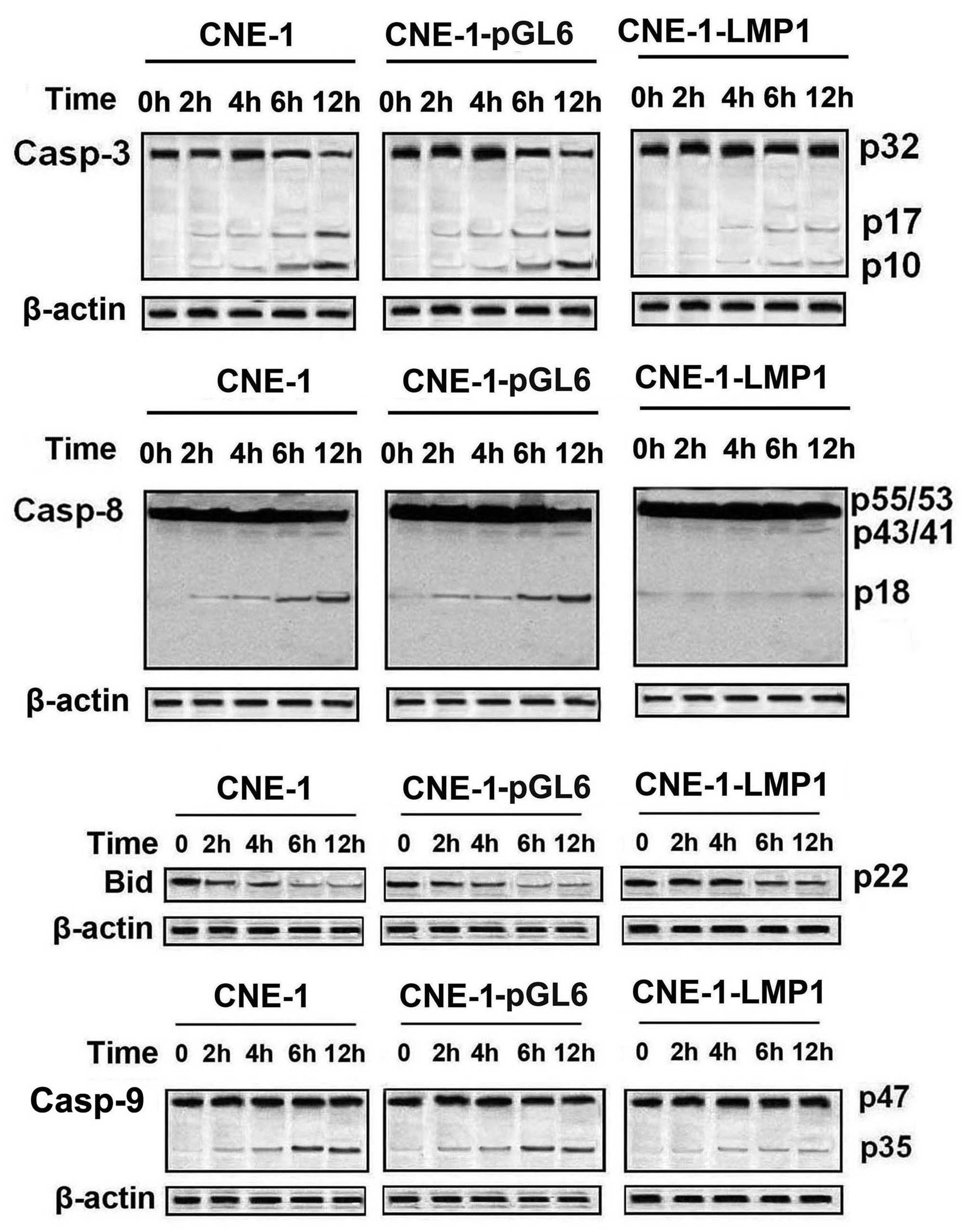 | Figure 4.LMP1 induced TRAIL resistance via
inhibition of caspase and Bid cleavage. Cleavage of caspases-3, −8
and −9 and Bid was assessed by western blot analysis during TRAIL
treatment (100 ng/ml) for various periods of time. Caspase-3,
p32-proform, p17, p10-cleavage fragments; caspase-8,
p55/53-proform, p43/41, p18-cleavage fragments; caspase-9:
p47-proform, p35-cleavage fragments. LMP1, latent membrane protein
1 TRAIL, tumor necrosis factor-related apoptosis-inducing ligand;
Bid, BH3 interacting domain death agonist. |
XIAP is involved in LMP1-induced TRAIL
resistance in NPC cells
Based on the initial results that showed that
caspase activation was inhibited by LMP1 overexpression, the
expression of XIAP proteins, which are known to antagonize caspase
activity, were analyzed. Western blot analysis revealed that XIAP
was upregulated in CNE-1-LMP1 cells (Fig.
5A). To determine the role of XIAP in LMP1 function, the
expression of XIAP in CNE-1-LMP1 cells was knocked-down by siRNA
(Fig. 5B). XIAP was indicated to be
efficiently inhibited by the specific siRNA, and the apoptotic rate
of CNE-1-LMP1 cells was increased during TRAIL treatment (P=0.021).
Upon treatment with TRAIL, the extent of XIAP downregulation
increased the cleavage of caspase-3, −8 and −9 and Bid in
CNE-1-LMP1 cells (Fig. 5C).
Furthermore, as shown in Fig. 5D, the
decreased expression of XIAP increased the
DiOC6(3) negative cell
rate. These observations suggest that XIAP was involved in
LMP1-induced TRAIL resistance in NPC cells.
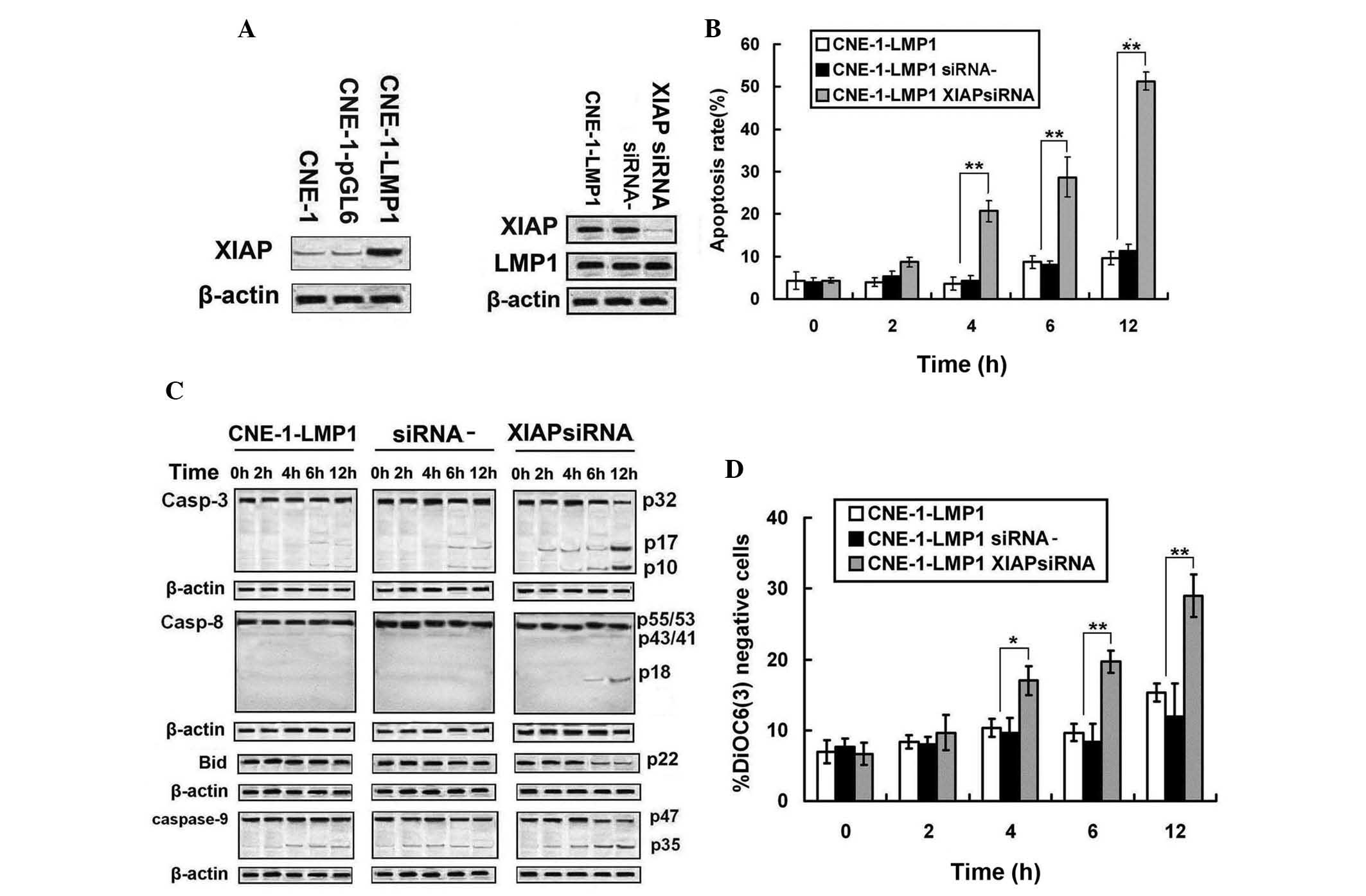 | Figure 5.Upregulation of XIAP was involved in
LMP1-induced TRAIL resistance. (A) Expressions of XIAP were
assessed by western blot analysis. (B) Upon treatment with TRAIL
(100 ng/ml) for various periods of time, apoptosis in CNE-1-LMP1
transfected with XIAP-siRNA was determined by flow cytometric
analysis. (C) Upon treatment with TRAIL (100 ng/ml) for various
periods of time, caspase-3, −8 and −9 and Bid cleavage was assessed
by western blot analysis in CNE-1-LMP1 cells transfected with
XIAP-siRNA. (D) Upon treatment with TRAIL (100 ng/ml) for various
periods of time, mitochondrial depolarization was measured by
DiOC6(3) fluorescence in
CNE-1-LMP1 cells transfected with XIAP-siRNA. siRNA-, CNE-1-LMP1
transfected with negative control siRNA; XIAP-siRNA, CNE-1-LMP1
transfected with XIAP-siRNA. *P<0.05 compared to CNE-1-LMP1
transfected with XIAP-siRNA; **P<0.01 compared to CNE-1-LMP1
cells transfected with XIAP-siRNA. XIAP, X-linked inhibitor of
apoptosis protein; LMP1, latent membrane protein 1 TRAIL, tumor
necrosis factor-related apoptosis-inducing ligand; siRNA, small
interfering RNA; Bid, BH3 interacting domain death agonist;
DiOC6(3),
3,3′-dihexyloxacarbocyanine iodide. |
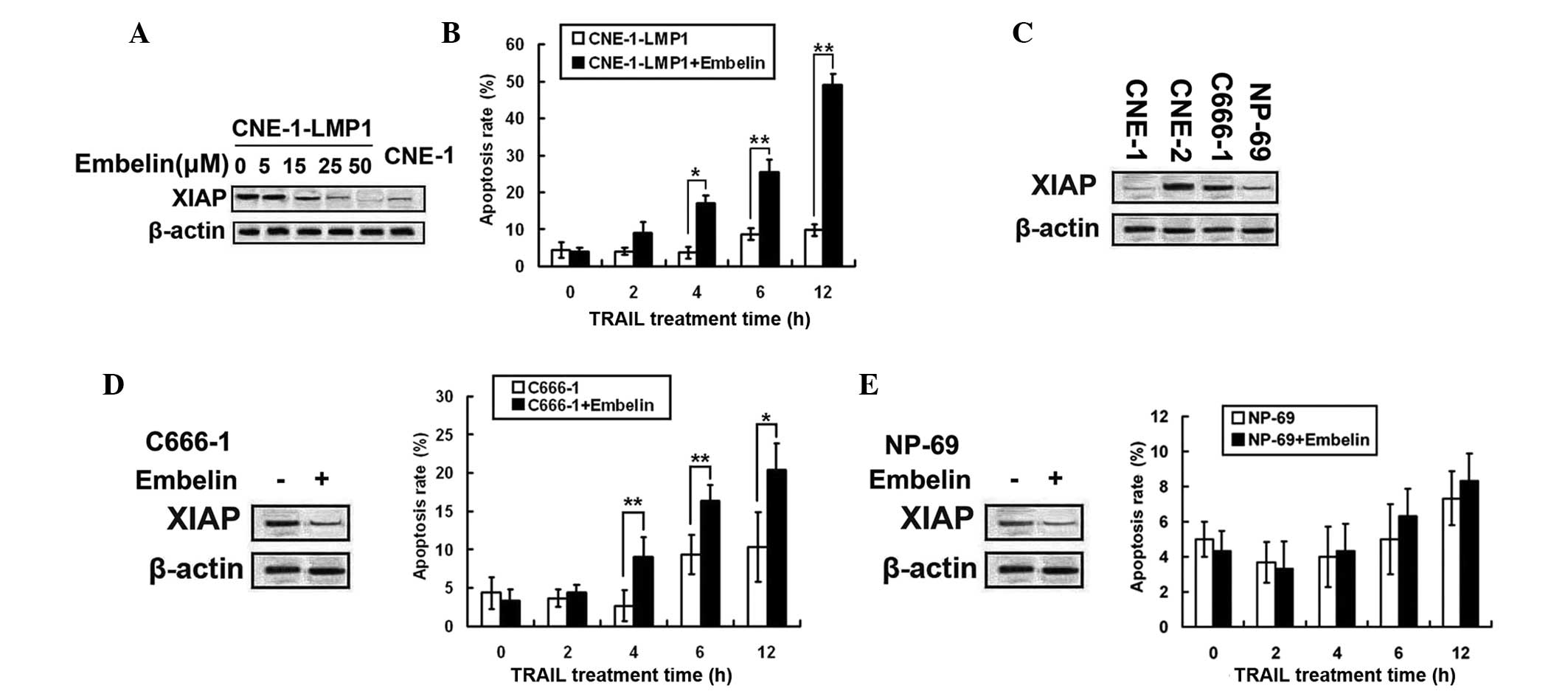
The XIAP special inhibitor, embelin,
prevented LMP1-indued TRAIL resistance
As previously mentioned, XIAP was involved in
LMP1-induced TRAIL resistance in NPC cells. Therefore, the present
study sought to determine whether embelin (XIAP inhibitor) prevents
LMP1-induced TRAIL resistance. CNE-1-LMP1 cells were treated with
various concentrations of embelin (5, 15, 25 and 50 µM) for 24 h
(Fig. 6A). The level of protein
expression of XIAP in CNE-1-LMP1 cells was indicated to be
decreased to the level observed in CNE-1 cells, following treatment
with 25 µM embelin. Additionally, embelin pretreated CNE-1-LMP1
cells, co-treated with TRAIL, revealed that embelin pretreatment
increased TRAIL-induced apoptosis in CNE-1-LMP1 cells (Fig. 6B).
Embelin prevented the LMP1-indued
TRAIL resistance
As embelin prevented LMP1-induced TRAIL resistance,
the effect of embelin on C666-1 cells, the EBV-positive NPC cell
line, was examined. The expression of XIAP was determined in three
NPC cell lines and in NP-69 cells. Notably, XIAP expression levels
were not increased in C666-1 cells. However, CNE-2 demonstrated the
greatest expression levels of the cell lines (Fig. 6C). C666-1 cells were treated with 25
µM of embelin for 24 h to dampen XIAP expression, following TRAIL
treatment. The apoptotic effect of the combined treatment was
stronger compared with treatment with TRAIL alone (Fig. 6D). The results show that embelin
enhanced TRAIL sensitivity of EBV-positive NPC cells.
The interest in TRAIL is due in large part to its
selective tumoricidal effects and relatively low toxicity profile
towards normal cells. The present study selected NP-69, which was
used as a model of the benign nasopharyngeal epithelium cell line
to test the toxicity of combined treatment against benign cells
(Fig. 6E). Treatment with embelin was
indicated to effectively decrease XIAP protein levels and not to
enhance TRAIL-induced apoptosis in NP-69 cells.
Discussion
The present study indicated that LMP1-positive NPC
cells are more sensitive to TRAIL compared with LMP1-negative NPC
cells. Therefore LMP1 was hypothesized to induce TRAIL resistance
in NPC cells. CNE-1-LMP1 cell lines that express LMP1 were
established, and the overexpression of LMP1 was indicated to induce
TRAIL resistance. The knockdown of XIAP by siRNA showed that the
upregulation of XIAP was involved in mediating the role of LMP1. In
addition, the XIAP inhibitor, embelin, suppressed the expression of
the XIAP protein and prevented LMP1-induced TRAIL resistance in
CNE-1-LMP1 and EBV-positive C666-1 NPC cells. The present study
showed that LMP1 induces TRAIL resistance in NPC cells via XIAP
upregulation. Furthermore, embelin may be additionally evaluated as
an agent for combination therapy with TRAIL in EBV-positive NPC
patients.
At present, numerous factors have been identified
that may affect TRAIL-induced apoptosis. However, the underlying
molecular mechanisms remain to be defined. Snow et al
(21) reported that resistance to
TRAIL-induced apoptosis was observed in EBV-positive spontaneous
lymphoblastoid cell lines that were derived from patients with
post-transplant lymphoproliferative disease. This observation
suggested a role for EBV in TRAIL resistance. The expression of EBV
genes is indicated in the majority of NPC cell lines (22). These genes encode viral proteins that
may contribute to malignant phenotypes (15). Among these genes, LMP1 is considered a
primary viral oncogene (23). The
presence of LMP1 in NPC tissues suggests the contribution of LMP1
to EBV-mediated tumorigenesis (24).
Silencing of the LMP1 gene by siRNA enhances the chemosensitivity
of EBV-positive NPC cells to bleomycin and cisplatin, suggesting a
possible role for LMP1 in chemoresistance in NPC (17).
The present study tested the effect of LMP1 on
TRAIL-induced apoptosis in NPC cells. C666-1 cells were indicated
to be less sensitive to TRAIL compared with CNE-1 and CNE-2. A
genetic approach was used to upregulate LMP1 in CNE-1, and the
overexpression of LMP1 decreased sensitivity to TRAIL via the
inhibition of mitochondrial depolarization. By contrast, Zhang
et al (25) indicated that
LMP1 increased cisplatin-induced mitochondrial depolarization and
apoptosis in cervical carcinoma-derived HeLa cells. This
inconsistency between findings may be due to the varying apoptotic
mechanisms induced by cisplatin and TRAIL. Bcl-2 or Bcl-XL
overexpression during Fas-induced apoptosis defines the two types
of cells as having a differential dependence on the mitochondrial
pathway. In type II cells, where apoptosis is associated with a
mitochondrial-dependent pathway, the overexpression of Bcl-2 or
Bcl-XL prevents TRAIL-induced apoptosis (26). The present study indicated that TRAIL
induced mitochondrial depolarization in CNE-1 cells, such that
Bcl-2 and Bcl-XL may potentially regulate the sensitivity of CNE-1
cells to TRAIL. However, Bcl-2 and Bcl-XL were unaffected by the
increased expression of LMP1, suggesting that LMP1 regulates
TRAIL-induced apoptosis without the effect on Bcl-2 and Bcl-XL. It
has been previously reported that LMP1 augments Bcl-2 expression in
certain cells, including B cells and certain types of NPC cell
lines (27,28). LMP1 may therefore differentially
modulate Bcl-2 expression in various cell types and cell
differentiated types.
The present study also indicated that the
TRAIL-induced cleavage of caspase-8,-3 and −9 was inhibited by the
enhanced expression of LMP1. ‘Initiator’ caspases with long
prodomains, such as caspase-8, either directly or indirectly
activate ‘effector’ caspases, such as caspase-3 (29). Vier et al (30) showed that the enhancement of death
receptor expression induces caspase-8 cleavage. However, the
present study demonstrated that the death receptors were expressed
at similar levels in CNE-1, CNE-1-pGL6 and CNE-1-LMP1 cells,
suggesting that LMP1 inhibited caspase-8 cleavage via an additional
mechanism. For example, a previous study indicated that LMP1
inhibited caspase-8 cleavage through the upregulation of the FLIP
protein, which possesses homology to caspase-8 (31). Following Bid cleavage, caspase-9 is
activated and mitochondria are disrupted to activate caspase-3. In
the present study, these events were inhibited by LMP1
upregulation. Consistent with the results of the present study,
Kijima et al (14)
knocked-down the LMP1 gene in NPC cells and identified that the
cleavage of caspase-3 and −8 and poly(ADP-ribose) polymerase 1 was
increased. However, in another study, LMP1 activated caspase-8, −9,
−3 and −7 via the induction of Fas overexpression in lymphoblastoid
cell lines (32). The inconsistencies
observed between these studies may be attributed to the dual
effects of LMP1 to induce apoptosis, which was also observed in the
study conducted by Chen and Chen (33).
In the present study, the enhanced expression of
XIAP was indicated to be involved in LMP1-induced TRAIL resistance.
IAPs are a group of anti-apoptotic factors that render cancer cells
insensitive to apoptotic stimulation (34). A previous study considered XIAP to be
the most potent IAP member for apoptosis inhibition and to often be
overexpressed in various cancers (35). Furthermore, the overexpression of XIAP
in cancer is often associated with a poor prognosis and resistance
to chemotherapy (36,37). Certain studies have clearly
demonstrated that XIAP may inhibit TRAIL-induced apoptosis through
the inactivation of caspase-3 and −9 (38). Consistent with previous reports, the
present study indicated that the downregulation of XIAP reversed
LMP1-induced TRAIL resistance by a mechanism that was dependent on
increased mitochondrial depolarization and the cleavage of
caspase-3 and −9. XIAP inhibits apoptosis by binding to, and
thereby inactivating, certain caspases, including the initiator
caspase-9 and the effector caspases-3 and −7 (39,40).
Previously, the binding affinity of XIAP to caspase-3 and −7 was
indicated to be increased compared with the binding affinity to the
initiator caspase-9; whereas the binding affinity of XIAP to the
initiator caspase-8 was undetectable (35). This affinity profile places XIAP as an
inhibitor of the common effector phase of apoptosis; therefore,
XIAP acts downstream of the Fas death-receptor stimulation and the
receptor proximal signaling events within the DISC, including
FADD-mediated recruitment and activation of caspase-8. By contrast,
and concordant with previous reports, the present study indicated
that the downregulation of XIAP increased the cleavage of caspase-8
(35). Ferreira et al
(41) indicated that in the
hepatocytic type I Fas signaling pathway, active p17 (a claved
fragment of caspase-3) feeds back to caspase-8, which cleaves the
partially processed p43 fragment into the fully processed p18
species. XIAP siRNA-induced caspase-8 activation may, therefore, be
attributed to caspase-3 activation feedback. In addition, the
downregulation of XIAP in the present study resulted in decreased
Bid expression levels in response to TRAIL treatment. This
observation suggested that enhanced LMP1-induced XIAP expression
reduced sensitivity to TRAIL via the inhibition of Bid
cleavage.
Embelin has previously been shown to have antitumor,
anti-inflammatory and analgesic properties (42). Embelin has also been identified as a
small molecular inhibitor of XIAP (43). Certain studies have shown that embelin
suppresses the expression of XIAP and sensitizes tumor cells to
TRAIL-induced apoptosis (44). The
present study tested whether embelin could inhibit the
LMP1-mediated upregulation of XIAP and TRAIL resistance. XIAP
expression levels were decreased in CNE-1-LMP1 cells by treatment
with embelin, and LMP1-induced resistance to TRAIL was prevented.
This finding indicated that embelin may replace XIAP-siRNA in
preventing LMP1-induced TRAIL resistance in NPC cells.
In order to understand the association between LMP1
and XIAP in various NPC cell lines, the expression levels of XIAP
in three NPC cell lines and NP-69 was tested. Notably, the
expression levels of XIAP were not high in C666-1 cells, but CNE-2
cells demonstrated the highest expression levels, suggesting that
LMP1 was not the only regulatory factor of XIAP expression. Embelin
was indicated to enhance TRAIL-induced apoptosis in C666-1 cells,
an EBV-positive NPC cell line, and additionally suggested that
embelin may sensitize EBV-positive NPC patients to TRAIL treatment.
However, a significantly decreased apoptotic response was
demonstrated in C666-1 cells that were treated with embelin and
TRAIL, compared with CNE-1-LMP1 cells. The difference in these
responses may be attributable to the various cell sources and other
EBV genes. In Fig. 6E, embelin did
not affect TRAIL-induced apoptosis in NP-69 cells, suggesting that
embelin failed to enhance TRAIL-induced apoptosis in benign cells.
The results of the present study are considered to indicate that
combined treatment with embelin fails to augment the toxicity of
TRAIL.
In conclusion, the present study has shown that,
following the overexpression of LMP1, NPC cells acquired resistance
to TRAIL-mediated apoptosis through the upregulation of XIAP.
Embelin may exhibit efficacy in combinatorial therapy with TRAIL in
an attempt to manage EBV-positive NPC patients.
References
|
1
|
Walczak H and Krammer PH: The CD95
(APO-1/Fas) and the TRAIL (APO-2L) apoptosis systems. Exp Cell Res.
256:58–66. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pan G, Ni J, Wei YF, Yu G, Gentz R and
Dixit VM: An antagonist decoy receptor and a death
domain-containing receptor for TRAIL. Science. 277:815–818. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kaufmann SH and Earnshaw WC: Induction of
apoptosis by cancer chemotherapy. Exp Cell Res. 256:42–49. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Luo X, Budihardjo I, Zou H, Slaughter C
and Wang X: Bid, a Bcl-2 interacting protein, mediates cytochrome c
release from mitochondria in response to activation of cell surface
death receptors. Cell. 94:481–490. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Siddiqui IA, Malik A, Adhami VM, Asim M,
Hafeez BB, Sarfaraz S and Mukhtar H: Green tea polyphenol EGCG
sensitizes human prostate carcinoma LNCaP cells to TRAIL-mediated
apoptosis and synergistically inhibits biomarkers associated with
angiogenesis and metastasis. Oncogene. 27:2055–2063. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yamaguchi K, Uzzo RG, Pimkina J, Makhov P,
Golovine K, Crispen P and Kolenko VM: Methylseleninic acid
sensitizes prostate cancer cells to TRAIL-mediated apoptosis.
Oncogene. 24:5868–5877. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tang L, Mao Y, Liu L, Liang S, Chen Y, Sun
Y, Liao X, Lin A, Liu M, Li L and Ma J: The volume to be irradiated
during selective neck irradiation in nasopharyngeal carcinoma:
Analysis of the spread patterns in lymph nodes by magnetic
resonance imaging. Cancer. 115:680–688. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Isobe K, Ito H, Shigematsu N, Kawada T,
Yasuda S, Hara R, Machida N, Takano H, Uchida Y, Uno T, et al:
Advanced nasopharyngeal carcinoma treated with chemotherapy and
radiotherapy: Distant metastasis and local recurrence. Int J Oncol.
12:1183–1187. 1998.PubMed/NCBI
|
|
9
|
Shao JY, Wang HY, Huang XM, Feng QS, Huang
P, Feng BJ, Huang LX, Yu XJ, Li JT, Hu LF, et al: Genome-wide
allelotype analysis of sporadic primary nasopharyngeal carcinoma
from southern China. Int J Oncol. 17:1267–1275. 2000.PubMed/NCBI
|
|
10
|
Teng MS, Brandwein Gensler MS, Teixeira
MS, Martignetti JA and Duffey DC: A Study of TRAIL receptors in
squamous cell carcinoma of the head and neck. Arch Otolaryngol Head
Neck Surg. 131:407–412. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen LC, Chung IC, Hsueh C, Tsang NM, Chi
LM, Liang Y, Chen CC, Wang LJ and Chang YS: The antiapoptotic
protein, FLIP, is regulated by heterogeneous nuclear
ribonucleoprotein K and correlates with poor overall survival of
nasopharyngeal carcinoma patients. Cell Death Differ. 17:1463–1473.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ozören N, Fisher MJ, Kim K, Liu CX, Genin
A, Shifman Y, Dicker DT, Spinner NB, Lisitsyn NA and El-Deiry WS:
Homozygous deletion of the death receptor DR4 gene in a
nasopharyngeal cancer cell line is associated with TRAIL
resistance. Int J Oncol. 16:917–925. 2000.PubMed/NCBI
|
|
13
|
Li SS, Tang QL, Wang SH, Wang S and Yang
XM: Simultaneously targeting bcl-2 and Akt pathways sensitizes
nasopharyngeal carcinoma to tumor necrosis factor-related
apoptosis-inducing ligand. Cancer Biother Radiopharm. 27:88–95.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kijima T, Kinukawa N, Gooding WE and Uno
M: Association of Epstein-Barr virus with tumor cell proliferation:
Clinical implication in nasopharyngeal carcinoma. Int J Oncol.
18:479–485. 2001.PubMed/NCBI
|
|
15
|
Tang YL, Lu JH, Cao L, Wu MH, Peng SP,
Zhou HD, Huang C, Yang YX, Zhou YH, Chen Q, et al: Genetic
variations of EBV-LMP1 from nasopharyngeal carcinoma biopsies:
Potential loss of T cell epitopes. Braz J Med Biol Res. 41:110–116.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Du CW, Wen BG, Li DR, Peng X, Hong CQ,
Chen JY, Lin ZZ, Hong X, Lin YC, Xie LX, et al: Arsenic trioxide
reduces the invasive and metastatic properties of nasopharyngeal
carcinoma cells in vitro. Braz J Med Biol Res. 39:677–685. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mei YP, Zhou JM, Wang Y, Huang H, Deng R,
Feng GK, Zeng YX and Zhu XF: Silencing of LMP1 induces cell cycle
arrest and enhances chemosensitivity through inhibition of AKT
signaling pathway in EBV-positive nasopharyngeal carcinoma cells.
Cell Cycle. 6:1379–1385. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dawson CW, Tramountanis G, Eliopoulos AG
and Young LS: Epstein-Barr virus latent membrane protein 1 (LMP1)
activates the phosphatidylinositol 3-kinase/Akt pathway to promote
cell survival and induce actin filament remodeling. J Biol Chem.
278:3694–7304. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Friboulet L, Pioche-Durieu C, Rodriguez S,
Valent A, Souquère S, Ripoche H, Khabir A, Tsao SW, Bosq J, Lo KW
and Busson P: Recurrent overexpression of c-IAP2 in EBV-associated
nasopharyngeal carcinomas: Critical role in resistance to Toll-like
receptor 3-mediated apoptosis. Neoplasia. 10:1183–1194. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hornle M, Peters N, Thayaparasingham B,
Vörsmann H, Kashkar H and Kulms D: Caspase-3 cleaves XIAP in a
positive feedback loop to sensitize melanoma cells to TRAIL-induced
apoptosis. Oncogene. 30:575–587. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Snow AL, Vaysberg M, Krams SM and Martinez
OM: EBV B lymphoma cell lines from patients with post-transplant
lymphoproliferative disease are resistant to TRAIL-induced
apoptosis. Am J Transplant. 6:976–985. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hitt MM, Allday MJ, Hara T, Karran L,
Jones MD, Busson P, Tursz T, Ernberg I and Griffin BE: EBV gene
expression in an nasopharyngeal carcinoma-related tumor. EMBO J.
8:2639–2651. 1989.PubMed/NCBI
|
|
23
|
Wang D, Liebowitz D and Kieff E: An EBV
membrane protein expressed in immortalized lymphocytes transforms
established rodent cells. Cell. 43:831–840. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lin SY, Tsang NM, Kao SC, Hsieh YL, Chen
YP, Tsai CS, Kuo TT, Hao SP, Chen IH and Hong JH: Presence of
Epstein-Barr virus latent membrane protein 1 gene in the
nasopharyngeal swabs from patients with nasopharyngeal carcinoma.
Head Neck. 23:194–200. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang X, Sanmun D, Hu L, Fadeel B and
Ernberg I: Epstein-Barr virus-encoded LMP1 promotes
cisplatin-induced caspase activation through JNK and NF-kappaB
signaling pathways. Biochem Biophys Res Commun. 360:263–268. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wei MC, Zong WX, Cheng EH, Lindsten T,
Panoutsakopoulou V, Ross AJ, Roth KA, MacGregor GR, Thompson CB and
Korsmeyer SJ: Proapoptotic BAX and BAK: A requisite gateway to
mitochondrial dysfunction and death. Science. 292:727–730. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pratt ZL, Zhang J and Sugden B: The latent
membrane protein 1 (LMP1) oncogene of Epstein-Barr virus can
simultaneously induce and inhibit apoptosis in B cells. J Virol.
86:4380–4393. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lee H, Seo SY, Tiwari I and Jang KL:
Epstein-Barr Virus latent membrane protein 1 overcomes all-trans
retinoic acid-induced apoptosis by inhibiting retinoic acid
receptor-β2 expression. Biochem Biophys Res Commun.
423:313–318. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Thornberry NA and Lazebnik Y: Caspases:
Enemies within. Science. 281:1312–1316. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Vier J, Gerhard M, Wagner H and Häcker G:
Enhancement of death-receptor induced caspase-8-activation in the
death-inducing signalling complex by uncoupling of oxidative
phosphorylation. Mol Immunol. 40:661–670. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li SS, Yang S, Wang S, Yang XM, Tang QL
and Wang SH: Latent membrane protein 1 mediates the resistance of
nasopharyngeal carcinoma cells to TRAIL-induced apoptosis by
activation of the PI3K/Akt signaling pathway. Oncol Rep.
26:1573–1579. 2011.PubMed/NCBI
|
|
32
|
Le Clorennec C, Ouk TS, Youlyouz-Marfak I,
Panteix S, Martin CC, Rastelli J, Adriaenssens E, Zimber-Strobl U,
Coll J, Feuillard J and Jayat-Vignoles C: Molecular basis of
cytotoxicity of Epstein-Barr virus (EBV) latent membrane protein 1
(LMP1) in EBV latency III B cells: LMP1 induces type II
ligand-independent autoactivation of CD95/Fas with caspase
8-mediated apoptosis. J Virol. 82:6721–6733. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chen Y and Chen XY: Effect of Epstein-Barr
virus latent membrane protein 1 (LMP1) on apoptosis of
nasopharyngeal carcinoma cell line CNE-1. Ai Zheng. 21:498–503.
2002.(In Chinese). PubMed/NCBI
|
|
34
|
Wei Y, Fan T and Yu M: Inhibitor of
apoptosis proteins and apoptosis. Acta Biochim Biophys Sin
(Shanghai). 40:278–288. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Eckelman BP, Salvesen GS and Scott FL:
Human inhibitor of apoptosis proteins: Why XIAP is the black sheep
of the family. EMBO Rep. 7:988–994. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mizutani Y, Nakanishi H, Li YN, Matsubara
H, Yamamoto K, Sato N, Shiraishi T, Nakamura T, Mikami K, Okihara
K, et al: Overexpression of XIAP expression in renal cell carcinoma
predicts a worse prognosis. Int J Oncol. 30:919–925.
2007.PubMed/NCBI
|
|
37
|
Checinska A, Hoogeland BS, Rodriguez JA,
Giaccone G and Kruyt FA: Role of XIAP in inhibiting
cisplatin-induced caspase activation in non-small cell lung cancer
cells: A small molecule Smac mimic sensitizes for
chemotherapy-induced apoptosis by enhancing caspase-3 activation.
Exp Cell Res. 313:1215–1224. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ndozangue-Touriguine O, Sebbagh M, Mérino
D, Micheau O, Bertoglio J and Bréard J: A mitochondrial block and
expression of XIAP lead to resistance to TRAIL-induced apoptosis
during progression to metastasis of a colon carcinoma. Oncogene.
27:6012–6022. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Deveraux QL, Leo E, Stennicke HR, Welsh K,
Salvesen GS and Reed JC: Cleavage of human inhibitor of apoptosis
protein XIAP results in fragments with distinct specificities for
caspases. EMBO J. 18:5242–5251. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Riedl SJ, Renatus M, Schwarzenbacher R,
Zhou Q, Sun C, Fesik SW, Liddington RC and Salvesen GS: Structural
basis for the inhibition of caspase-3 by XIAP. Cell. 104:791–800.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ferreira KS, Kreutz C, Macnelly S, Neubert
K, Haber A, Bogyo M, Timmer J and Borner C: Caspase-3 feeds back on
caspase-8, Bid and XIAP in type I Fas signaling in primary mouse
hepatocytes. Apoptosis. 17:503–515. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Feresin GE, Tapia A, Sortino M, Zacchino
S, de Arias AR, Inchausti A, Yaluff G, Rodriguez J, Theoduloz C and
Schmeda-Hirschmann G: Bioactive alkyl phenols and embelin from
Oxalis erythrorhiza. J Ethnopharmacol. 88:241–247. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Nikolovska-Coleska Z, Xu L, Hu Z, Tomita
Y, Li P, Roller PP, Wang R, Fang X, Guo R, Zhang M, et al:
Discovery of embelin as a cell-permeable, small-molecular weight
inhibitor of XIAP through structure-based computational screening
of a traditional herbal medicine three-dimensional structure
database. J Med Chem. 47:2430–2440. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Mori T, Doi R, Kida A, Nagai K, Kami K,
Ito D, Toyoda E, Kawaguchi Y and Uemoto S: Effect of the XIAP
inhibitor Embelin on TRAIL-induced apoptosis of pancreatic cancer
cells. J Surg Res. 142:281–286. 2007. View Article : Google Scholar : PubMed/NCBI
|