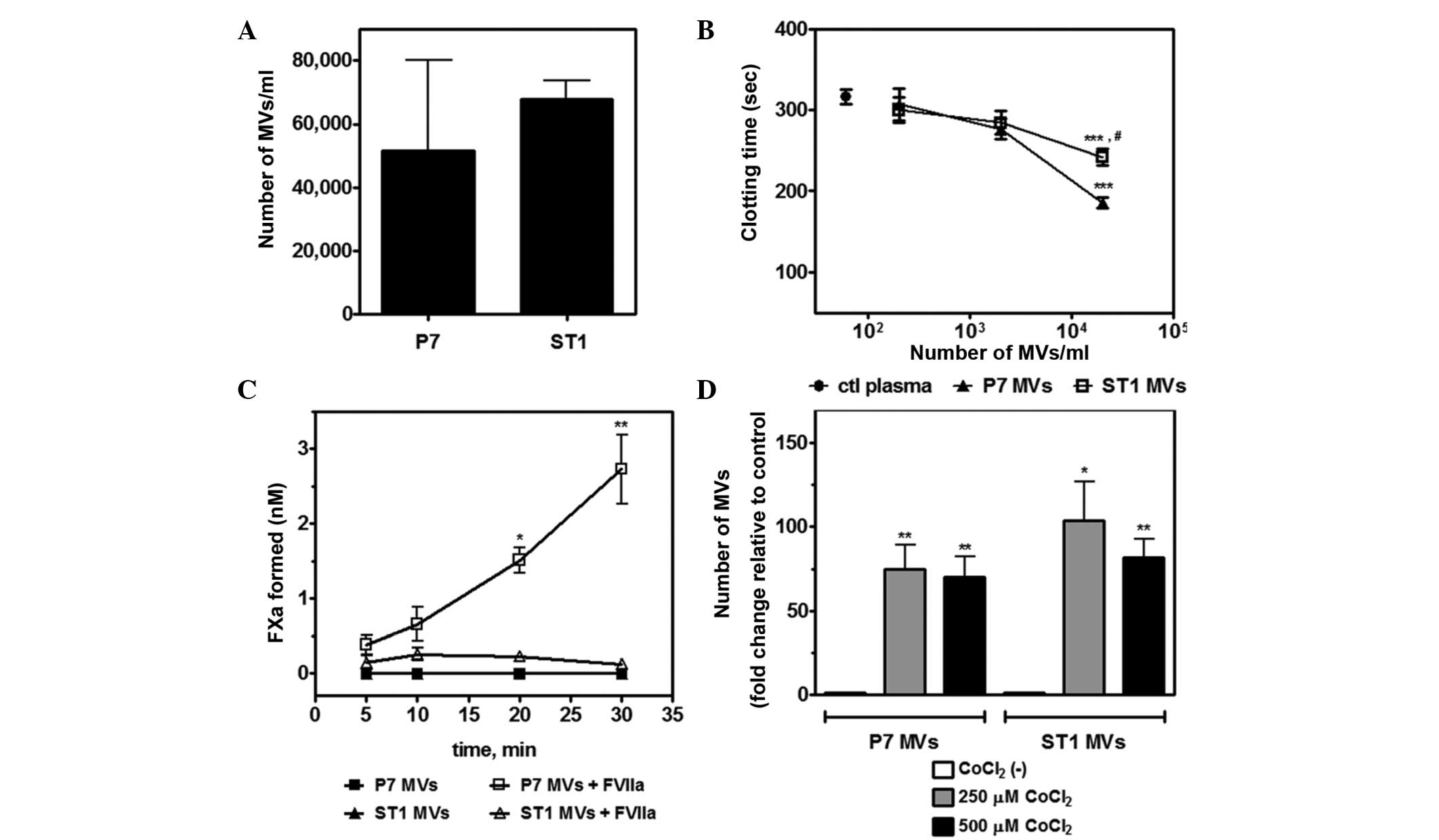
|
1
|
Williams JC and Mackman N: Tissue factor
in health and disease. Front Biosci. 1:358–372. 2012. View Article : Google Scholar
|
|
2
|
Østerud B and Bjørklid E: Sources of
tissue factor. Semin Thromb Hemost. 32:11–23. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Francischetti IM, Seydel KB and Monteiro
RQ: Blood coagulation, inflammation, and malaria. Microcirculation.
15:81–107. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Brat DJ and Van Meir EG: Vaso-occlusive
and prothrombotic mechanisms associated with tumor hypoxia,
necrosis, and accelerated growth in glioblastoma. Lab Invest.
84:397–405. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rak J, Milsom C, Magnus N and Yu J: Tissue
factor in tumour progression. Best Pract Res Clin Haematol.
22:71–83. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kasthuri RS, Taubman MB and Mackman N:
Role of tissue factor in cancer. J Clin Oncol. 27:4834–4838. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ruf W, Disse J, Carneiro-Lobo TC, Yokota N
and Schaffner F: Tissue factor and cell signalling in cancer
progression and thrombosis. J Thromb Haemost. 9(Suppl 1):
S306–S315. 2011. View Article : Google Scholar
|
|
8
|
van den Berg YW, Osanto S, Reitsma PH and
Versteeg HH: The relationship between tissue factor and cancer
progression: Insights from bench and bedside. Blood. 119:924–932.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lima LG and Monteiro RQ: Activation of
blood coagulation in cancer: Implications for tumour progression.
Biosci Rep. 33:e000642013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lima LG, Oliveira AS, Campos LC, Bonamino
M, Chammas R, Werneck C, Vicente CP, Barcinski MA, Petersen LC and
Monteiro RQ: Malignant transformation in melanocytes is associated
with increased production of procoagulant microvesicles. Thromb
Haemost. 106:712–723. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhou L, Qi XL, Xu MX, Mao Y, Liu ML and
Song HM: Microparticles: New light shed on the understanding of
venous thromboembolism. Acta Pharmacol Sin. 35:1103–1110. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Boire A, Covic L, Agarwal A, Jacques S,
Sherifi S and Kuliopulos A: PAR1 is a matrix metalloprotease-1
receptor that promotes invasion and tumorigenesis of breast cancer
cells. Cell. 120:303–313. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Albrektsen T, Sørensen BB, Hjortø GM,
Fleckner J, Rao LV and Petersen LC: Transcriptional program induced
by factor VIIa-tissue factor, PAR1 and PAR2 in MDA-MB-231 cells. J
Thromb Haemost. 5:1588–1597. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wen PY and Kerasi S: Malignant gliomas in
adults. N Engl J Med. 359:492–507. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Evans SM, Judy KD, Dunphy I, Jenkins WT,
Nelson PT, Lustig RA, Jenkins K, Magarelli DP, Hahn SM, Collins RA,
et al: Hypoxia is important in the biology and aggression of human
glial brain tumors. Clin Cancer Res. 10:8177–8184. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Brown JM and Wilson WR: Exploiting tumour
hypoxia in cancer treatment. Nat Rev Cancer. 4:437–447. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Anand M and Brat DJ: Oncogenic regulation
of tissue factor and thrombosis in cancer. Thromb Res. 129(Suppl
1): S46–S49. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Magnus N, D'Asti E, Meehan B, Garnier D
and Rak J: Oncogenes and the coagulation system-forces that
modulate dormant and aggressive states in cancer. Thromb Res.
133(Suppl 2): S1–S9. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rong Y, Post DE, Pieper RO, Durden DL, Van
Meir EG and Brat DJ: PTEN and hypoxia regulate tissue factor
expression and plasma coagulation by glioblastoma. Cancer Res.
65:1406–1413. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Magnus N, Garnier D and Rak J: Oncogenic
epidermal growth factor receptor up-regulates multiple elements of
the tissue factor signaling pathway in human glioma cells. Blood.
116:815–818. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
D'Asti E, Fang Y and Rak J: Brain
neoplasms and coagulation-lessons from heterogeneity. Rambam
Maimonides Med J. 5:e00302014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Carneiro-Lobo TC, Lima MT,
Mariano-Oliveira A, Dutra-Oliveira A, Oba-Shinjo SM, Marie SK,
Sogayar MC and Monteiro RQ: Expression of tissue factor signaling
pathway elements correlates with the production of vascular
endothelial growyh factor and interleukin-8 in human astrocytoma
patients. Oncol Rep. 31:679–686. 2014.PubMed/NCBI
|
|
23
|
Armelin MC and Armelin HÀ: Glucocorticoid
hormone modulation of both cell surface and cytoskeleton related to
growth control of rat glioma cells. J Cell Biol. 97:459–465. 1983.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Armelin MC, Stocco RC and Armelin HA:
Control of rat C6 glioma cell proliferation: Uncoupling of the
inhibitory effects of hydrocortisone hormone in suspension and
monolayer cultures. J Cell Biol. 97:455–458. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2-ΔΔCt. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hamada K, Kuratsu J, Saitoh Y, Takeshima
H, Nishi T and Ushio Y: Expression of tissue factor correlates with
grade of malignancy in human glioma. Cancer. 77:1877–1883. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Guan M, Jin J, Su B, Liu WW and Lu Y:
Tissue factor expression and angiogenesis in human glioma. Clin
Biochem. 35:321–325. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fernandes RS, Kirszberg C, Rumjanek VM and
Monteiro RQ: On the molecular mechanisms for the highly
procoagulant pattern of C6 glioma cells. J Thromb Haemost.
4:1546–1552. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kirszberg C, Lima LG, Da Silva de Oliveira
A, Pickering W, Gray E, Barrowcliffe TW, Rumjanek VM and Monteiro
RQ: Simultaneous tissue factor expression and phosphatidylserine
exposure account for the highly procoagulant pattern of melanoma
cell lines. Melanoma Res. 19:301–308. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Brat DJ, Castellano-Sanchez AA, Hunter SB,
Pecot M, Cohen C, Hammond EH, Devi SN, Kaur B and Van Meir EG:
Pseudopalisades in glioblastoma are hypoxic, express extracellular
matrix proteases and are formed by an actively migrating cell
population. Cancer Res. 64:920–927. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Rautou PE and Mackman N: Microvesicles as
risk markers for venous thrombosis. Expert Rev Hematol. 6:91–101.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Svensson KJ, Kucharzewska P, Christianson
HC, Sköld S, Löfstedt T, Johansson MC, Mörgelin M, Bengzon J, Ruf W
and Belting M: Hypoxia triggers a proangiogenic pathway involving
cancer cell microvesicles and PAR-2-mediated heparin-binding EGF
signaling in endothelial cells. Proc Natl Acad Sci USA.
108:13147–13152. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Naldini A, Filippi I, Ardinghi C, Silini
A, Giavazzi R and Carraro F: Identification of a functional role
for the protease-activated receptor-1 in hypoxic breast cancer
cells. Eur J Cancer. 45:454–460. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hammoud MA, Sawaya R, Shi W, Thall PF and
Leeds NE: Prognostic significance of preoperative MRI scans in
glioblastoma multiforme. J Neurooncol. 27:65–73. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tehrani M, Friedman TM, Olson JJ and Brat
DJ: Intravascular thrombosis in central nervous system
malignancies: A potential role in astrocytoma progression to
glioblastoma. Brain Pathol. 18:164–171. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Blanco VM, Chu Z, Vallabhapurapu SD,
Sulaiman MK, Kendler A, Rixe O, Warnick RE, Franco RS and Qi X:
Phosphatidylserine-selective targeting and anticancer effects of
SapC-DOPS nanovesicles on brain tumors. Oncotarget. 5:7105–7118.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang T, Gilkes DM, Takano N, Xiang L, Luo
W, Bishop CJ, Chaturvedi P, Green JJ and Semenza GL:
Hypoxia-inducible factors and RAB22A mediate formation of
microvesicles that stimulate breast cancer invasion and metastasis.
Proc Natl Acad Sci USA. 111:E3234–E3242. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Marras LC, Geerts WH and Perry JR: The
risk of venous thromboembolism is increased throughout the course
of malignant glioma: An evidence-based review. Cancer. 89:640–664.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Thaler J, Ay C, Mackman N, Bertina RM,
Kaider A, Marosi C, Key NS, Barcel DA, Scheithauer W, Kornek G, et
al: Microparticle-associated tissue factor activity, venous
thromboembolism and mortality in pancreatic, gastric, colorectal
and brain cancer patients. J Thromb Haemost. 10:1363–1370. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Thaler J, Preusser M, Ay C, Kaider A,
Marosi C, Zielinski C, Pabinger I and Hainfellner JA: Intratumoral
tissue factor expression and risk of venous thromboembolism in
brain tumor patients. Thromb Res. 131:162–165. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lima LG, Leal AC, Vargas G, Porto-Carreiro
I and Monteiro RQ: Intercellular transfer of tissue factor via the
uptake of tumor-derived microvesicles. Thromb Res. 132:450–456.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhang Y, Zhan H, Xu W, Yuan Z, Lu P, Zhan
L and Li Q: Upregulation of matrix metalloproteinase-1 and
proteinase-activated receptor-1 promotes the progression of human
gliomas. Pathol Res Pract. 207:24–29. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Itsekson-Hayosh Z, Shavit-Stein E, Last D,
Goez D, Daniels D, Bushi D, Gera O, Zibly Z, Mardor Y, Chapman J
and Harnof S: Thrombin activity and thrombin receptor in rat
glioblastoma model: Possible markers and targets for intervention?
J Mol Neurosci. 56:644–651. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Dutra-Oliveira A, Monteiro RQ and
Mariano-Oliveira A: Protease-activated receptor-2 (PAR2) mediates
VEGF production through the ERK1/2 pathway in human glioblastoma
cell lines. Biochem Biophys Res Commun. 421:221–227. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Harter PN, Dützmann S, Drott U, Zachskorn
C, Hattingen E, Capper D, Gessler F, Senft C, Seifert V, Plate KH,
et al: Anti-tissue factor (TF9-10H10) treatment reduces tumor cell
invasiveness in a novel migratory glioma model. Neuropathology.
33:515–525. 2013.PubMed/NCBI
|
|
46
|
Luo R, Wang X, Dong Y, Wang L and Tian C:
Activation of protease-activated receptor 2 reduces glioblastoma
cell apoptosis. J Biomed Sci. 21:252014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Carneiro-Lobo TC, Konig S, Machado DE,
Nasciutti LE, Forni MF, Francischetti IM, Sogayar MC and Monteiro
RQ: Ixolaris, a tissue factor inhibitor, blocks primary tumor
growth and angiogenesis in a glioblastoma model. J Thromb Haemost.
7:1855–1864. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Carneiro-Lobo TC, Schaffner F, Disse J,
Ostergaard H, Francischetti IM, Monteiro RQ and Ruf W: The
tick-derived inhibitor Ixolaris prevents tissue factor signaling on
tumor cells. J Thromb Haemost. 10:1849–1858. 2012. View Article : Google Scholar : PubMed/NCBI
|