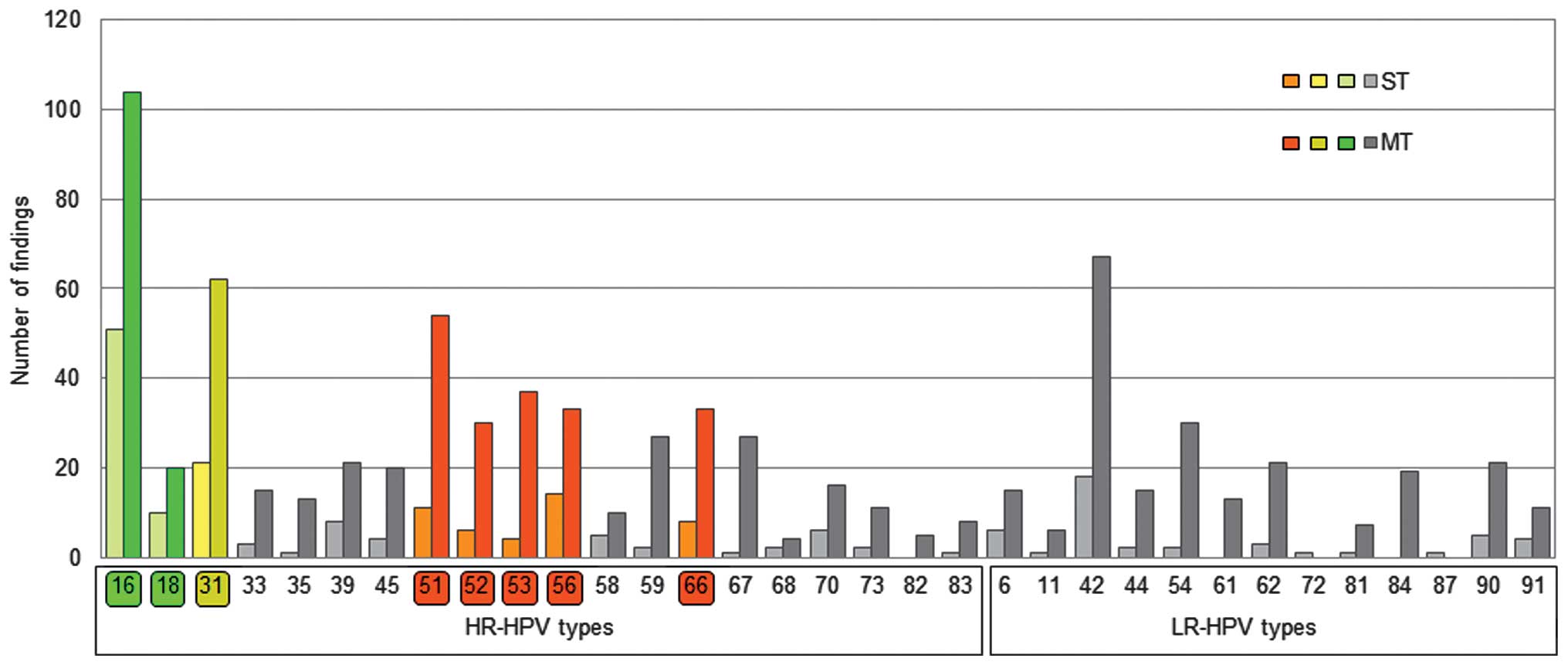
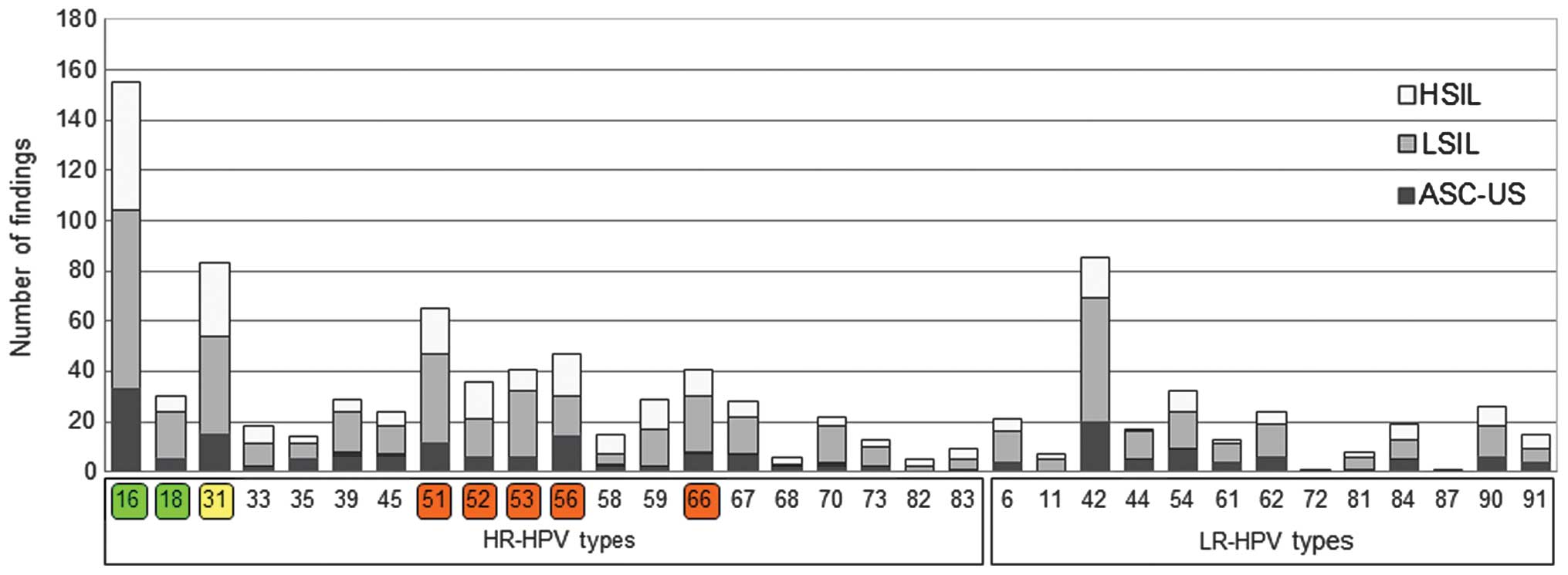
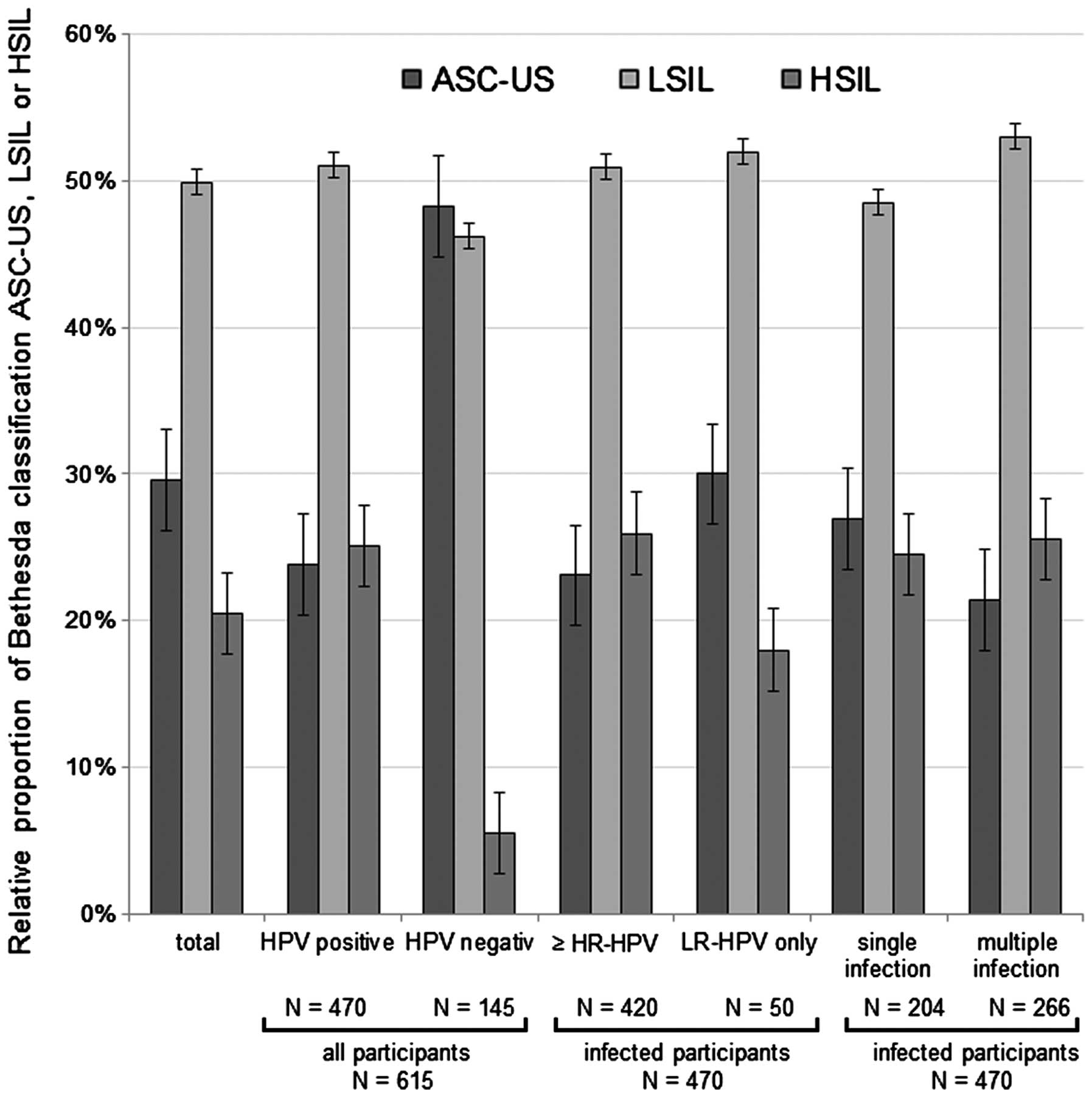
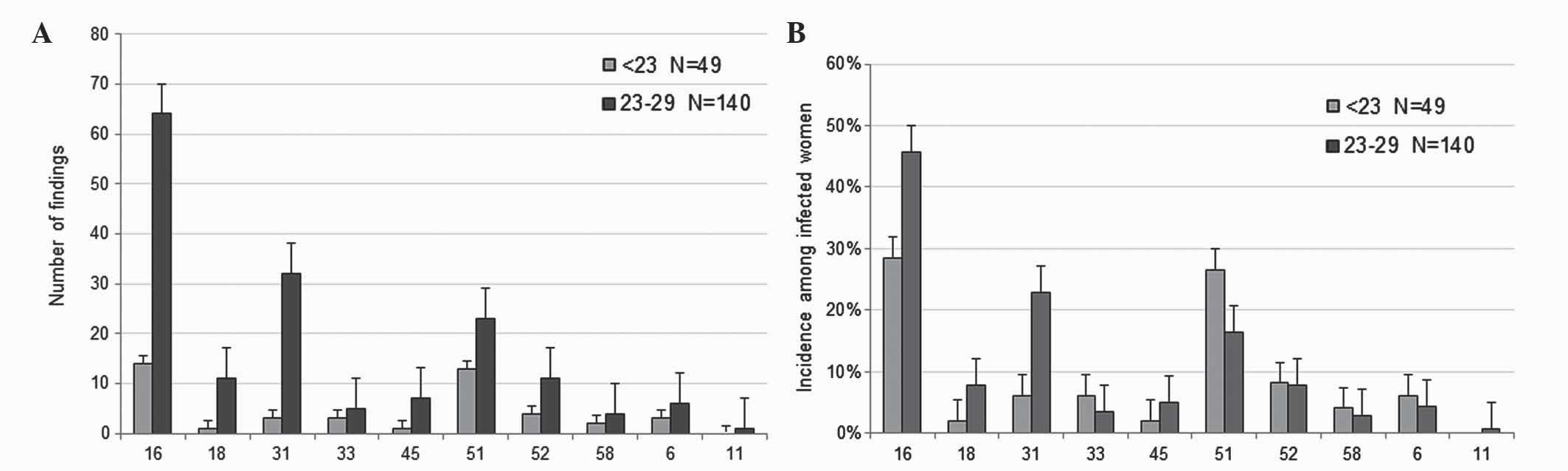
|
1
|
de Villiers EM, Fauquet C, Broker TR,
Bernard HU and zur Hausen H: Classification of papillomaviruses.
Virology. 324:17–27. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
de Villiers EM: Cross-roads in the
classification of papillomaviruses. Virology. 445:2–10. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Schiffman MH, Bauer HM, Hoover RN, Glass
AG, Cadell DM, Rush BB, Scott DR, Sherman ME, Kurman RJ, Wacholder
S, et al: Epidemiologic evidence showing that human papillomavirus
infection causes most cervical intraepithelial neoplasia. J Natl
Cancer Inst. 85:958–964. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Walboomers JM, Jacobs MV, Manos MM, Bosch
FX, Kummer JA, Shah KV, Snijders PJ, Peto J, Meijer CJ and Muñoz N:
Human papillomavirus is a necessary cause of invasive cervical
cancer worldwide. J Pathol. 189:12–19. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kjaer SK, van den Brule AJ, Bock JE, Poll
PA, Engholm G, Sherman ME, Walboomers JM and Meijer CJ: Human
papillomavirus - the most significant risk determinant of cervical
intraepithelial neoplasia. Int J Cancer. 65:601–606. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bosch FX, Lorincz A, Muñoz N, Meijer CJ
and Shah KV: The causal relation between human papillomavirus and
cervical cancer. J Clin Pathol. 55:244–265. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
IARC: Latest World Cancer Statistics.
Global cancer burden rises to 14.1 million new cases in 2012:
Marked increase in breast cancers must be addressed. IARC/WHO Press
Release Dec 223. 2013.
|
|
8
|
Robert Koch-Institute: Cancer in Germany
2009–2010: Incidences and Trends. (9th). Robert Koch Institute and
the Association of Population-based Cancer Registries in Germany
(eds) (RKI-Hausdruckerei, Berlin). 2014.
|
|
9
|
Burd EM: Human papillomavirus and cervical
cancer. Clin Microbiol Rev. 16:1–17. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Halec G, Alemany L, Lloveras B, Schmitt M,
Alejo M, Bosch FX, Tous S, Klaustermeier JE, Guimerà N, Grabe N, et
al: Retrospective International Survey and HPV Time Trends Study
Group; Retrospective International Survey and HPV Time Trends Study
Group: Pathogenic role of the eight probably/possibly carcinogenic
HPV types 26, 53, 66, 67, 68, 70, 73 and 82 in cervical cancer. J
Pathol. 234:441–451. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Muñoz N, Bosch FX, de Sanjosé S, Herrero
R, Castellsagué X, Shah KV, Snijders PJ and Meijer CJ:
International Agency for Research on Cancer Multicenter Cervical
Cancer Study Group: Epidemiologic classification of human
papillomavirus types associated with cervical cancer. N Engl J Med.
348:518–527. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
de Sanjose S, Quint WG, Alemany L, et al:
Retrospective International Survey and HPV Time Trends Study Group:
Human papillomavirus genotype attribution in invasive cervical
cancer: A retrospective cross-sectional worldwide study. Lancet
Oncol. 11:1048–1056. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Schiffman M, Clifford G and Buonaguro FM:
Classification of weakly carcinogenic human papillomavirus types:
Addressing the limits of epidemiology at the borderline. Infect
Agent Cancer. 4:82009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Clifford GM, Smith JS, Plummer M, Muñoz N
and Franceschi S: Human papillomavirus types in invasive cervical
cancer worldwide: A meta-analysis. Br J Cancer. 88:63–73. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Clifford GM, Gallus S, Herrero R, et al:
IARC HPV Prevalence Surveys Study Group: Worldwide distribution of
human papillomavirus types in cytologically normal women in the
International Agency for Research on Cancer HPV prevalence surveys:
A pooled analysis. Lancet. 366:991–998. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Smith JS, Lindsay L, Hoots B, Keys J,
Franceschi S, Winer R and Clifford GM: Human papillomavirus type
distribution in invasive cervical cancer and high-grade cervical
lesions: A meta-analysis update. Int J Cancer. 121:621–632. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lowy DR and Schiller JT: Prophylactic
human papillomavirus vaccines. J Clin Invest. 116:1167–1173. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
ECDC: Introduction of HPV vaccines in
European Union countries - an update. ECDC, Stockholm: 2012.
|
|
19
|
Robert Koch-Institute: Recommendations of
the Standing Committee on Vaccination (STIKO) at the Robert Koch
Institute/Effective: August 2014. Epid Bull. 34:305–339. 2014.
|
|
20
|
Paavonen J, Jenkins D, Bosch FX, et al:
HPV PATRICIA study group: Efficacy of a prophylactic adjuvanted
bivalent L1 virus-like-particle vaccine against infection with
human papillomavirus types 16 and 18 in young women: An interim
analysis of a phase III double-blind, randomised controlled trial.
Lancet. 369:2161–2170. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Paavonen J, Naud P, Salmerón J, Wheeler
CM, Chow SN, Apter D, Kitchener H, Castellsague X, Teixeira JC,
Skinner SR, et al: HPV PATRICIA Study Group: Efficacy of human
papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical
infection and precancer caused by oncogenic HPV types (PATRICIA):
Final analysis of a double-blind, randomised study in young women.
Lancet. 374:301–314. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
FUTURE II Study Group: Quadrivalent
vaccine against human papillomavirus to prevent high-grade cervical
lesions. N Engl J Med. 356:1915–1927. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lehtinen M, Paavonen J, Wheeler CM, et al:
Overall efficacy of HPV-16/18 AS04-adjuvanted vaccine against grade
3 or greater cervical intraepithelial neoplasia: 4-year
end-of-study analysis of the randomised, double-blind PATRICIA
trial. Lancet Oncol. 13:89–99. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tjalma WA: There are two prophylactic
human papillomavirus vaccines against cancer, and they are
different. J Clin Oncol. 33:964–965. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Malagón T, Drolet M, Boily MC, Franco EL,
Jit M, Brisson J and Brisson M: Cross-protective efficacy of two
human papillomavirus vaccines: A systematic review and
meta-analysis. Lancet Infect Dis. 12:781–789. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wheeler CM, Castellsagué X, Garland SM, et
al: HPV PATRICIA Study Group: Cross-protective efficacy of
HPV-16/18 AS04-adjuvanted vaccine against cervical infection and
precancer caused by non-vaccine oncogenic HPV types: 4-year
end-of-study analysis of the randomised, double-blind PATRICIA
trial. Lancet Oncol. 13:100–110. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Drolet M, Bénard É, Boily MC, Ali H,
Baandrup L, Bauer H, Beddows S, Brisson J, Brotherton JM, Cummings
T, Donovan B, et al: Population-level impact and herd effects
following human papillomavirus vaccination programmes: a systematic
review and meta-analysis. Lancet Infect Dis. 15:565–580. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Plummer M, Vaccarella S and Franceschi S:
Multiple human papillomavirus infections: the exception or the
rule? J Infect Dis. 203:891–893. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wagner D: Munich nomenclature II for
gynaecologic cytodiagnosis. Acta Cytol. 34:900–901. 1990.
|
|
30
|
Solomon D, Davey D, Kurman R, Moriarty A,
O'Connor D, Prey M, Raab S, Sherman M, Wilbur D, Wright T Jr, et
al: The 2001 Bethesda System: Terminology for reporting results of
cervical cytology. JAMA. 287:2114–2119. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Apgar BS, Zoschnick L and Wright TC Jr:
The 2001 Bethesda System terminology. Am Fam Physician.
68:1992–1998. 2003.PubMed/NCBI
|
|
32
|
WHO: WHO Guidelines: Guidelines for
screening and treatment of precancerous lesions for cervical cancer
prevention. World Health Organization. Geneva: 2013.
|
|
33
|
Cannavo I, Benchetrit M, Loubatier C,
Michel G, Lemichez E and Giordanengo V: Characterization of a
cluster of oncogenic mutations in E6 of a human papillomavirus 83
variant isolated from a high-grade squamous intraepithelial lesion.
J Gen Virol. 92:2428–2436. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Robert Koch Institute: Vaccination against
human papillomavirus among girls aged 12–17 years - recommendation
and explanatory statement. Epidemiol Bull. 12:97–103. 2007.(In
German).
|
|
35
|
Bruni L, Diaz M, Castellsagué X, Ferrer E,
Bosch FX and de Sanjosé S: Cervical human papillomavirus prevalence
in 5 continents: Meta-analysis of 1 million women with normal
cytological findings. J Infect Dis. 202:1789–1799. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Meloni A, Pilia R, Campagna M, Usai A,
Masia G, Caredda V and Coppola RC: Prevalence and molecular
epidemiology of human papillomavirus infection in italian women
with cervical cytological abnormalities. J Public Health Res.
3:1572014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Klug SJ, Hukelmann M, Hollwitz B, Düzenli
N, Schopp B, Petry KU and Iftner T: Prevalence of human
papillomavirus types in women screened by cytology in Germany. J
Med Virol. 79:616–625. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Iftner T, Eberle S, Iftner A, Holz B,
Banik N, Quint W and Straube AN: Prevalence of low-risk and
high-risk types of human papillomavirus and other risk factors for
HPV infection in Germany within different age groups in women up to
30 years of age: An epidemiological observational study. J Med
Virol. 82:1928–1939. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Argyri E, Papaspyridakos S, Tsimplaki E,
Michala L, Myriokefalitaki E, Papassideri I, Daskalopoulou D,
Tsiaoussi I, Magiakos G and Panotopoulou E: A cross sectional study
of HPV type prevalence according to age and cytology. BMC Infect
Dis. 13:532013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Plummer M, Schiffman M, Castle PE,
Maucort-Boulch D and Wheeler CM: ALTS Group: A 2-year prospective
study of human papillomavirus persistence among women with a
cytological diagnosis of atypical squamous cells of undetermined
significance or low-grade squamous intraepithelial lesion. J Infect
Dis. 195:1582–1589. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
41
|
Heard I, Tondeur L, Arowas L, Falguières
M, Demazoin MC and Favre M: Human papillomavirus types distribution
in organised cervical cancer screening in France. PLoS One.
8:e793722013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Resende LS, Rabelo-Santos SH, Sarian LO,
Alves Figueiredo RR, Ribeiro AA, Zeferino LC and Derchain S: A
portrait of single and multiple HPV type infections in Brazilian
women of different age strata with squamous or glandular cervical
lesions. BMC Infect Dis. 14:2142014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Cobo F, Concha A and Ortiz M: Human
papillomavirus (HPV) type distribution in females with abnormal
cervical cytology. A correlation with histological study. Open
Virol J. 3:60–66. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
García-Espinosa B, Moro-Rodríguez E and
Alvarez-Fernández E: Genotype distribution of human papillomavirus
(HPV) in histological sections of cervical intraepithelial
neoplasia and invasive cervical carcinoma in Madrid, Spain. BMC
Cancer. 12:5332012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
van der Marel J, Berkhof J, Ordi J, Torné
A, Del Pino M, van Baars R, Schiffman M, Wentzensen N, Jenkins D
and Quint WG: Attributing oncogenic human papillomavirus genotypes
to high-grade cervical neoplasia: Which type causes the lesion? Am
J Surg Pathol. 39:496–504. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kuznetsov AV, Müller RA, Ruzicka T,
Herzinger T and Kuznetsov L: Knowledge of sexually transmitted HPV
infection, genitoanal warts, cancer and their prevention among
young females after vaccine introduction in Germany. J Eur Acad
Dermatol Venereol. 27:1527–1534. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Rieck T, Feig M, Deleré Y and Wichmann O:
Utilization of administrative data to assess the association of an
adolescent health check-up with human papillomavirus vaccine uptake
in Germany. Vaccine. 32:5564–5569. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Deleré Y, Remschmidt C, Leuschner J,
Schuster M, Fesenfeld M, Schneider A, Wichmann O and Kaufmann AM:
Human Papillomavirus prevalence and probable first effects of
vaccination in 20 to 25 year-old women in Germany: A
population-based cross-sectional study via home-based
self-sampling. BMC Infect Dis. 14:872014. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Paul P and Fabio A: Literature review of
HPV vaccine delivery strategies: Considerations for school- and
non-school based immunization program. Vaccine. 32:320–326. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Yang Z, Cuzick J, Hunt WC and Wheeler CM:
Concurrence of multiple human papillomavirus infections in a large
US population-based cohort. Am J Epidemiol. 180:1066–1075. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Tota JE, Ramanakumar AV, Jiang M, Dillner
J, Walter SD, Kaufman JS, Coutlée F, Villa LL and Franco EL:
Epidemiologic approaches to evaluating the potential for human
papillomavirus type replacement postvaccination. Am J Epidemiol.
178:625–634. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Safaeian M and Rodriguez AC: Invited
commentary: Multiple human papillomavirus infections and type
replacement-anticipating the future after human papillomavirus
vaccination. Am J Epidemiol. 180:1076–1081. 2014. View Article : Google Scholar : PubMed/NCBI
|