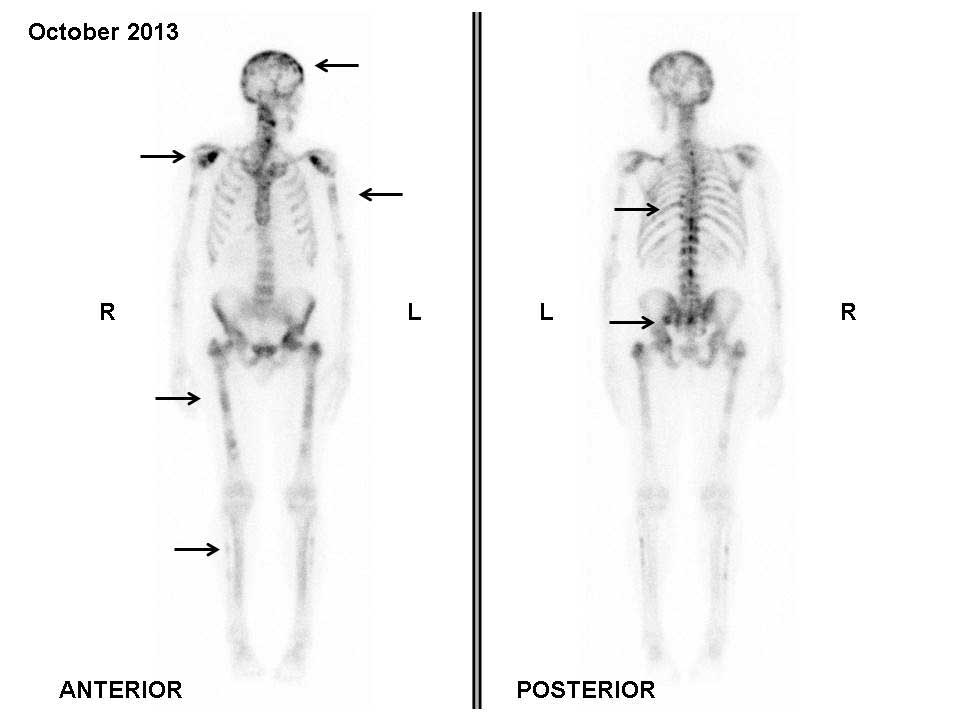
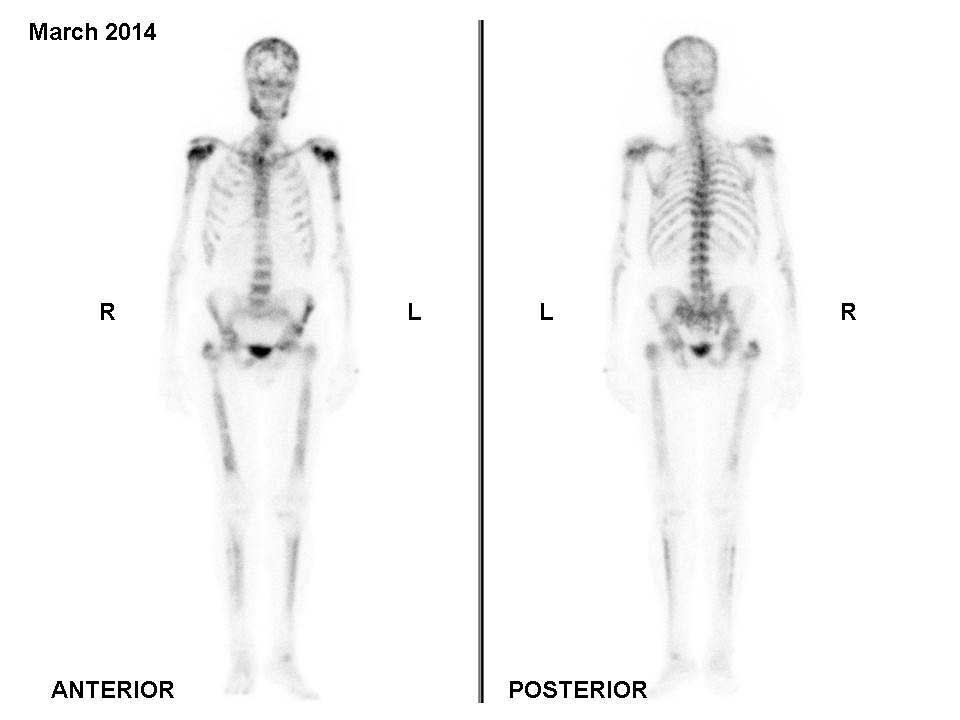
|
1
|
World Health Organization: Cancer Pain
Relief: With a Guide to Opioid Availability (2nd). WHO. Geneva,
Switzerland: 36–37. 1996.
|
|
2
|
National Comprehensive Cancer Network
(NCCN): NCCN Guidelines for Supportive Care: Adult cancer pain.
Version 2. 2013.http://www.nccn.org/professionals/physician_gls/pdf/pain.pdfAccessed.
January. 2016
|
|
3
|
Hamdy NA and Papapoulos SE: The palliative
management of skeletal metastases in prostate cancer: Use of
bone-seeking radionuclides and bisphosphonates. Semin Nucl Med.
31:62–68. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Herranz Anido U, Fernández Calvo O, Afonso
Afonso FJ, de Rodríguez Martínez Llano S, Lázaro Quintela M, León
Mateos L, Vázquez Estévez S and Antón Aparicio LM: Radium-223
dichloride: A new paradigm in the treatment of prostate cancer.
Expert Rev Anticancer Ther. 15:339–348. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Parker C, Nilsson S, Heinrich D, Helle SI,
O'Sullivan JM, Fosså SD, Chodacki A, Wiechno P, Logue J, Seke M, et
al: Alpha emitter radium-223 and survival in metastatic prostate
cancer. N Engl J Med. 369:213–223. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sartor O, Coleman R, Nilsson S, Heinrich
D, Helle SI, O'Sullivan JM, Fosså SD, Chodacki A, Wiechno P, Logue
J, et al: Effect of radium-223 dichloride on symptomatic skeletal
events in patients with castration-resistant prostate cancer and
bone metastases: Results from a phase 3, double-blind, randomised
trial. Lancet Oncol. 15:738–746. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nilsson S, Franzén L, Parker C, Tyrrell C,
Blom R, Tennvall J, Lennernäs B, Petersson U, Johannessen DC, Sokal
M, et al: Two-year survival follow-up of the randomized,
double-blind, placebo-controlled phase II study of radium-223
chloride in patients with castration-resistant prostate cancer and
bone metastases. Clin Genitourin Cancer. 11:20–26. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nilsson S, Strang P, Aksnes AK, Franzèn L,
Olivier P, Pecking A, Staffurth J, Vasanthan S, Andersson C and
Bruland ØS: A randomized, dose-response, multicenter phase II study
of radium-223 chloride for the palliation of painful bone
metastases in patients with castration-resistant prostate cancer.
Eur J Cancer. 48:678–686. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nilsson S, Larsen RH, Fosså SD, Balteskard
L, Borch KW, Westlin JE, Salberg G and Bruland OS: First clinical
experience with alpha-emitting radium-223 in the treatment of
skeletal metastases. Clin Cancer Res. 11:4451–4459. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nilsson S, Franzén L, Parker C, Tyrrell C,
Blom R, Tennvall J, Lennernäs B, Petersson U, Johannessen DC, Sokal
M, et al: Bone-targeted radium-223 in symptomatic,
hormone-refractory prostate cancer: A randomised, multicentre,
placebo-controlled phase II study. Lancet Oncol. 8:587–594. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Humphrey PA: Gleason grading and
prognostic factors in carcinoma of the prostate. Mod Pathol.
17:292–306. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
National Collaborating Centre for Cancer
(UK): TNM staging for prostate cancer. Prostate Cancer: Diagnosis
and Treatment. National Collaborating Centre for Cancer (UK).
(Cardiff). Appendix 2. 2008.
|
|
13
|
Sonpavde G, Pond GR, Armstrong AJ, Galsky
MD, Leopold L, Wood BA, Wang SL, Paolini J, Chen I, Chow-Maneval E,
et al: Radiographic progression by Prostate Cancer Working Group
(PCWG)-2 criteria as an intermediate endpoint for drug development
in metastatic castration-resistant prostate cancer. BJU Int.
114:E25–E31. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sgouros G, Roeske JC, McDevitt MR, Palm S,
Allen BJ, Fisher DR, Brill AB, Song H, Howell RW, Akabani G, et al:
MIRD pamphlet no. 22 (abridged): Radiobiology and dosimetry of
alpha-particle emitters for targeted radionuclide therapy. J Nucl
Med. 51:311–328. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lassmann M and Nosske D: Dosimetry of
Ra-223-chloride: Dose to normal organs and tissues. Eur J Nucl Med
Mol Imaging. 40:207–212. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pandit-Taskar N, Larson SM and
Carrasquillo JA: Bone-seeking radiopharmaceuticals for treatment of
osseous metastases, part 1: α therapy with 223Ra-dichloride. J Nucl
Med. 55:268–274. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Parker CC, Pascoe S, Chodacki A,
O'Sullivan JM, Germá JR, O'Bryan-Tear CG, Haider T and Hoskin P: A
randomized, double-blind, dose finding, multicenter, phase 2 study
of radium chloride (Ra 223) in patients with bone metastases and
castration-resistant prostate cancer. Eur Urol. 63:189–197. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Carrasquillo JA, O'Donoghue JA,
Pandit-Taskar N, Humm JL, Rathkopf DE, Slovin SF, Williamson MJ,
Lacuna K, Aksnes AK, Larson SM, et al: Phase I pharmacokinetic and
biodistribution study with escalating doses of
223Ra-dichloride in men with castration-resistant
metastatic prostate cancer. Eur J Nucl Med Mol Imaging.
40:1384–1393. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Scher HI, Halabi S, Tannock I, Morris M,
Sternberg CN, Carducci MA, Eisenberger MA, Higano C, Bubley GJ,
Dreicer R, et al: Design and end points of clinical trials for
patients with progressive prostate cancer and castrate levels of
testosterone: Recommendations of the Prostate Cancer Clinical
Trials Working Group. J Clin Oncol. 26:1148–1159. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Carducci MA, Padley RJ, Breul J, Vogelzang
NJ, Zonnenberg BA, Daliani DD, Schulman CC, Nabulsi AA,
Humerickhouse RA, Weinberg MA, et al: Effect of endothelin-A
receptor blockade with atrasentan on tumor progression in men with
hormone-refractory prostate cancer: A randomized, phase II,
placebo-controlled trial. J Clin Oncol. 21:679–689. 2003.
View Article : Google Scholar : PubMed/NCBI
|