Introduction
Approximately 20,000 new cases of bone metastasis
are diagnosed each year. The most common site for bone metastasis
is the spine, with up to 70% of cancer patients developing spinal
lesions (1). The prognosis of
patients with lung cancer metastasis to the spine is poor, with a
reported 5-year survival rate of 10 to 20% (2). Treatment options include surgery,
radiation, chemotherapy and rehabilitative medicine (3,4). In
general, the life expectancy of a patient should exceed 3 months in
order that surgical intervention may be considered. Similarly, a
life expectancy of >1 month is necessary for the consideration
of radiation treatment (3,5). In cases of adenocarcinoma with bone
metastasis, epidermal growth factor receptor (EGFR) inhibitors can
improve the prognosis (6). However,
for squamous cell lung cancer patients who refuse chemotherapy and
surgery due to the side effects and risks, respectively, the
development of effective anticancer compounds and palliative
treatments with low toxicity is critical for the treatment of
unresectable spinal metastasis.
In the present study, a systemic therapy, biological
intracontrol treatment (BICT) was administered to a lung cancer
patient with multiple bone metastases. BICT consists of a
combination of early palliative care and herbal extract
combinations, including ginseng, herba agrimoniae, hairyvein
agrimonia herb, white flower patrinia herb and arginine, provided
by the Chinese State Food and Drug Administration. As herbal
extract combinations are extracted from natural compounds, it
exhibits low toxicity and is typically used for inhibiting cancer
growth and improving quality of life (QOL). In our previous
studies, this drug combination was able to inhibit EGFR and
vascular endothelial growth factor receptor (VEGFR) expression,
decrease microvessel density, inhibit expression of interleukin
(IL)-10 and transforming growth factor (TGF)-β in the tumor
microenvironment, and promote apoptosis (7,8).
Case report
A 59-year-old male who attended GuangYuan
Traditional Chinese Medicine Hospital (GuangYuan, China) was
diagnosed with pathologically confirmed squamous cell lung cancer
on 24th September, 2014, accompanied by failure of the first-line
treatment regimen of docetaxel (75 mg/m2) plus cisplatin
(40 mg/m2) after just one cycle and the development of
serious side effects, which began two days after diagnosis.
Multiple bone metastases occurred in the sixth left rib and T9
vertebra, resulting in a high risk of pathological fracture and
disability. The cancer was diagnosed as National Comprehensive
Cancer Network T2N3M1, stage IV (9).
Eastern Cooperative Oncology Group (ECOG) (10) and numerical rating scale (NRS) scores
(11) of pain were 2 and 4,
respectively. The patient described pain at his back around the T9
vertebral area. A physical examination revealed that the
respiratory volume was decreased in each lung. A large solid mass
was palpable at the back of the sixth left rib near the T6 thoracic
vertebra, and percussion of the right side of the back around the
T9 vertebra caused pain. Laboratory examination findings were as
follows: White blood cell count, 2.9×109/l [normal
range, (4.0–10.0)×109/l]; alanine aminotransferase, 45.6
U/l (normal range, 0–40 U/l); aspartate aminotransferase, 32 U/l
(normal range, 0–49 U/l); alkaline phosphatase, 44 U/l (normal
range, 34–114 U/l); serum Ca2+, 2.07 mmol/l (normal
range, 2.03–2.54 mmol/l); carcinoembryonic antigen, 1.53 U/ml
(normal range, 0–5 ng/ml); and CYFRA-211, 42.9 U/ml (normal range,
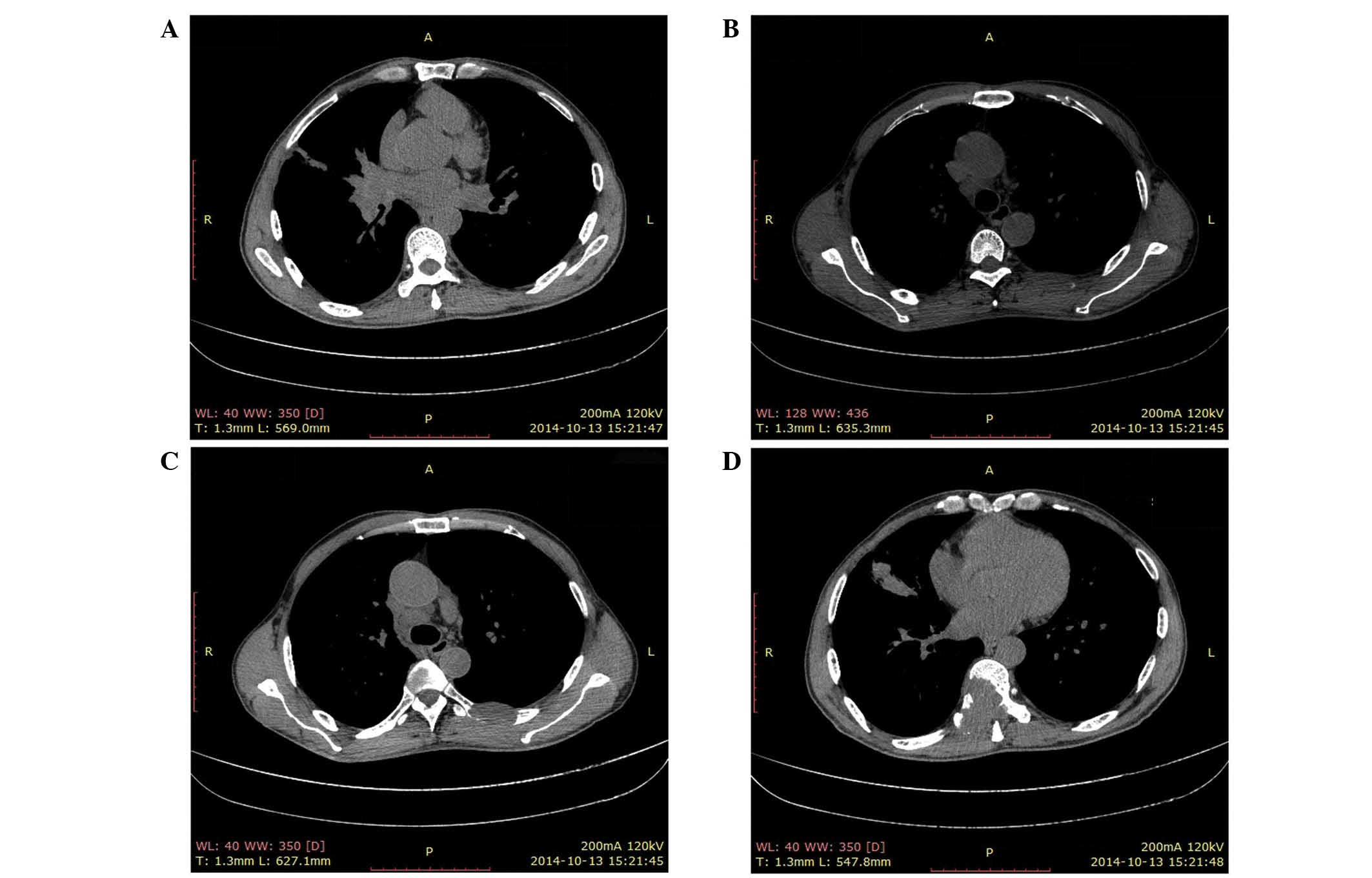
0–7 ng/ml). Computed tomography (CT) scans performed on October 13,
2014, revealed abnormal masses at the right pulmonary hilum
(Fig. 1A), the mediastinal lymph node
(Fig. 1B) and the sixth left rib near
the T6 vertebra (Fig. 1C), measuring
5.0×4.0, 2.2×1.4 and 3.5×2.0 cm, respectively. The T9 vertebra
appeared to be nearly completely replaced by a neoplasm measuring
4.7×4.9 cm. (Fig. 1D).
Although the patient received only one cycle of
chemotherapy, the tumor progressed and the patient refused to
receive any further chemotherapy or radiation therapy. The patient
was weak with an ECOG score of 3; thus, low toxicity drugs and
palliative treatment were recommended. Herbal medicine and
bisphosphonate treatments were selected as anticancer treatment and
palliative care, respectively. Herbal extract combinations were
administered orally four times daily, including Shenghuang capsule
(0.8 g), Xianhe Baijiang capsule (1.2 g) and arginine liquid (15
ml), and a single dose of bisphosphonate was administered
mid-treatment. Furthermore, hyperthermia was applied to the cancer
mass area to enhance drug absorption and kill the cancer cells
directly, and the patient was asked to discontinue heavy exercise
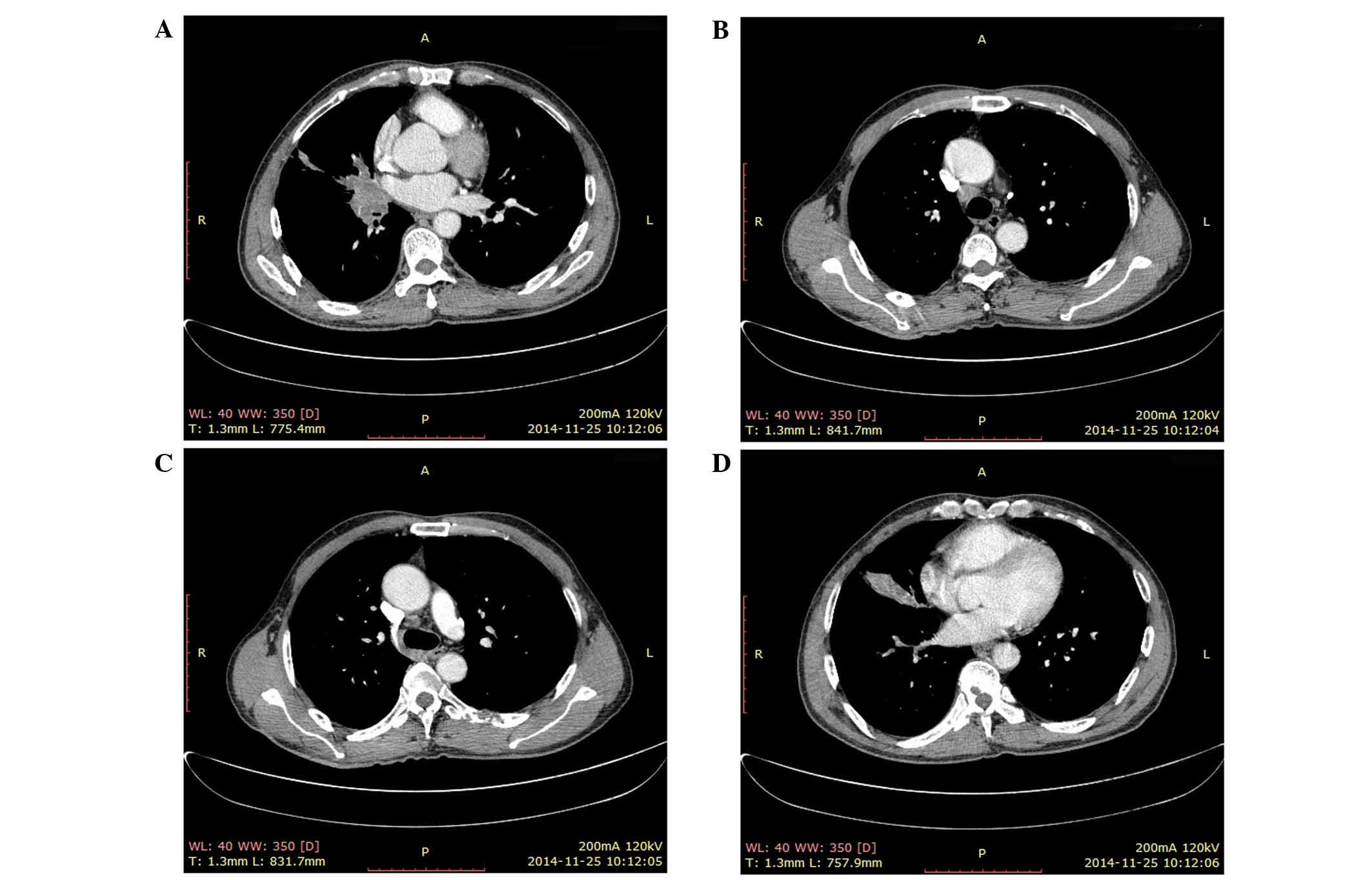
to prevent a pathological fracture of the T9 vertebra. After 40
days of treatment, a CT scan revealed a significant decrease in the
size of the masses in the T9 vertebra and the sixth left rib in the
long axis, and no new growth in the right pulmonary hilum and
mediastinal lymph node, with masses now measuring 3.8×3.1 and
1.8×1.3 cm, respectively. Additionally, no new metastatic masses
were detected (Fig. 2). Meanwhile,
the QOL of the patient had improved, and the ECOG and NRS scores of
pain had decreased to 1 and 0–1, respectively.
The patient continued to be monitored through
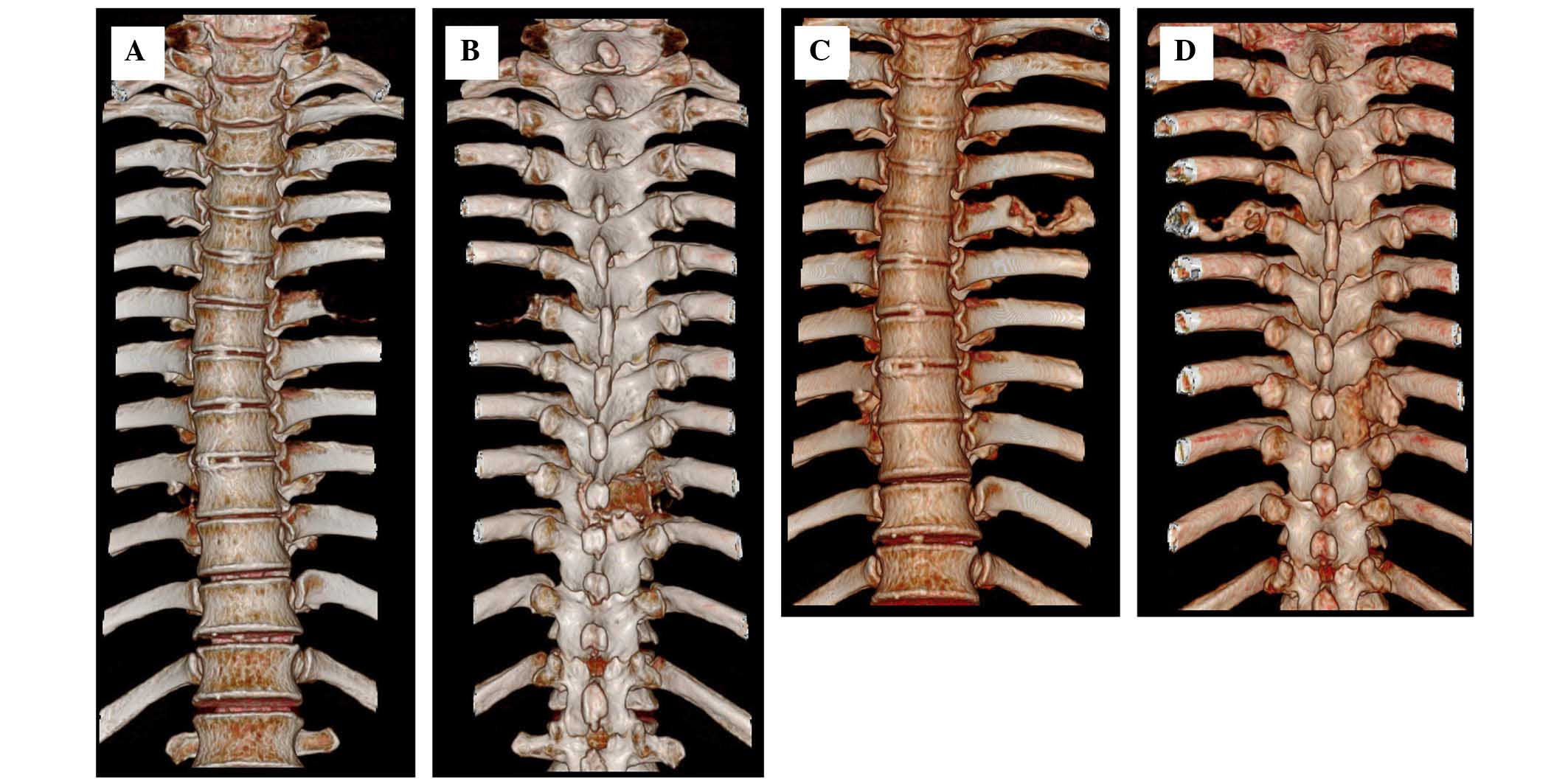
follow-up appointments. After 40 days post-treatment, on November
25, 2014, a second CT scan revealed decreases of ~90 and 95% in the
masses of the T9 vertebra and sixth left rib, respectively.
Additionally, rapid bone repair was evident (Figs. 2 and 3).
These results were accompanied by clinical and QOL improvements,
the restoration of tumor marker levels to normal values and
stabilization of the tumor masses. A partial response was achieved
according to the guidelines outlined in the Response Evaluation
Criteria in Solid Tumors (12), and
the clinical response was confirmed by CT tests. Furthermore, a
blood test confirmed that the white blood cells had recovered to
6.9×109/l, alanine aminotransferase levels to 36.9 U/l
and aspartate aminotransferase levels to 30 U/l. According to
Common Terminology Criteria for Adverse Events version 3.0, no
vomiting or hematological toxicity of more than grade 1 severity
was observed.
The patient was monitored regularly for 8 months
from the start of BICT treatment (11th October, 2014), but was
subsequently lost to follow-up after May 2015.
Discussion
The spine is one of the most common sites for bone
metastasis, and the thoracic spine is the most common site for
spinal metastasis in lung cancer. CT scans can recognize a bony
metastatic lesion up to 6 months earlier than radiography. However,
pathological compression fractures, which are common in metastatic
disease, can be readily observed, yielding a detection rate of bone
metastasis on plain radiography of ~40% (13).
Vertebral metastases cause chronic and increasing
pain, and neurological deficits due to destruction of the vertebral
body, thus increasing the risk of a pathological fracture (14). Commonly, palliative surgery and local
radiation therapy are employed. In the treatment of spinal
metastases, palliative surgery aims to reduce pain, and maintain
neurological function and spinal stability (14). Palliative radiation therapy is often
administered at 30 Gy in 10 fractions by a 6-MV X-ray (15–17).
Recently, programmed cell death protein 1 (PD-1) and its ligands,
programmed cell death 1 ligands 1 and 2, have been shown to play
important roles in treating melanoma and lung cancers (18).
Pembrolizumab and nivolumab, two humanized
monoclonal antibodies targeting PD-1, have had success in treating
non-small cell lung cancer and are currently being tested in
multiple other tumor types (19,20).
Nivolumab is currently approved by the FDA as a second-line therapy
for squamous cell lung cancer, demonstrating an objective response
rate of 14.5% and a median time to response of 3.3 months (21,22).
However, for patients classified as having an ECOG performance
status of 3–4, no standard chemotherapy regimen has been
established, owing to the associated high toxicity and low
effectiveness of these drugs. For these patients, individual care
is commonly employed. In the present case, a medicine comprised of
extracts of a combination of several herbs, plus bisphosphonates,
not only inhibited tumor growth, but also stimulated significant
and rapid bone repair. These results suggest previously unknown
properties of this combination of herbal extracts.
In our previous studies (7,8), this
combination of herbal extracts inhibited EGFR/VEGFR, IL-10 and
TGF-β expression in the tumor microenvironment, and increased serum
IL-12 expression. EGFR/VEGFR, IL-10 and TGF-β are pro-inflammatory
cytokines that promote cancer growth, whereas IL-12 functions to
inhibit cancer growth. Treatment with the herbal combination
resulted in decreased microvessel density in the tumor
microenvironment and increased tumor cell apoptosis. Although this
may explain the inhibition of cancer growth observed in the present
patient, the mechanism by which the herbal medication promotes
rapid bone repair remains under investigation.
In conclusion, the present study describes a case in
which herbal medicine promoted tumor regression and bone repair,
with an improved QOL in a short period of time. It is unclear
whether the combination of herbal medicine and bisphosphonates
synergistically enhanced bone repair. The study demonstrated a
novel individualized approach to treat lung cancer patients with an
ECOG performance status of 3 or higher. Further research is
necessary to determine the mechanisms underlying the effectiveness
of herbal combination medicine plus bisphosphonates and the
benefits associated with this treatment.
Glossary
Abbreviations
Abbreviations:
|
BICT
|
biological intracontrol treatment
|
|
CT
|
computed tomography
|
|
ECOG
|
eastern cooperative oncology group
|
|
EGFR
|
epidermal growth factor receptor
|
|
NRS
|
numerical rating scale
|
|
PD-1
|
programmed cell death protein 1
|
|
QOL
|
quality of life
|
|
TGF
|
transforming growth factor
|
|
VEGFR
|
vascular endothelial growth factor
receptor
|
References
|
1
|
American Association of Neurological
Surgeons: Patient Information, Spinal Tumors. http://www.aans.org/en/Patient%20Information/Conditions%20and%20Treatments/Spinal%20Tumors.aspxAccessed.
July. 2014
|
|
2
|
Sundaresan N, Boriani S, Rothman A and
Holtzman R: Tumors of the osseous spine. J Neurooncol. 69:273–290.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sciubba DM, Petteys RJ, Dekutoski MB,
Fisher CG, Fehlings MG, Ondra SL, Rhines LD and Gokaslan ZL:
Diagnosis and management of metastatic spine disease. A review. J
Neurosurg Spine. 13:94–108. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dodwad SN, Savage J, Scharschmidt TJ and
Patel A: Evaluation and treatment of spinal metastatic disease.
Cancer Treat Res. 162:131–150. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tokuhashi Y, Ajiro Y and Umezawa N:
Outcome of treatment for spinal metastases using scoring system for
preoperative evaluation of prognosis. Spine (Phila Pa 1976).
34:69–73. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sugiura H, Yamada K, Sugiura T, Hida T and
Mitsudomi T: Predictors of survival in patients with bone
metastasis of lung cancer. Clin Orthop Relat Res. 466:729–736.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li X, Zhang L and Li Y: Inhibition of
cancer cell proliferation by color modulation: A pilot study.
Presented at the JSMO (Japan). 2013.
|
|
8
|
Li X, Li J, Tian Y, Zhang L, Liu H, Li Z
and Yin W: Inhibition of rectal cancer growth by a new herbal
extract combinations (FUO-C4) in vivo. Presented at the JSMO
(Japan). 2014.
|
|
9
|
National Comprehensive Cancer Network
Non-small Lung Cancer Guidelines. https://www.nccn.org/store/login/login.aspx?ReturnURL=https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf2015.
|
|
10
|
Oken MM, Creech RH, Tormey DC, Horton J,
Davis TE, McFadden ET and Carbone PP: Toxicity and response
criteria of the Eastern Cooperative Oncology Group. Am J Clin
Oncol. 5:649–655. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dworkin RH, Turk DC, Farrar JT,
Haythornthwaite JA, Jensen MP, Katz NP, Kerns RD, Stucki G, Allen
RR, Bellamy N, et al: Core outcome measures for chronic pain
clinical trials: IMMPACT recommendations. Pain. 113:9–19. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Trotti A, Colevas AD, Setser A, Rusch V,
Jaques D, Budach V, Langer C, Murphy B, Cumberlin R, Coleman CN and
Rubin P: CTCAE v3.0: Development of a comprehensive grading system
for the adverse effects of cancer treatment. Semin Radiat Oncol.
13:176–181. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Salvo N, Christakis M, Rubenstein J, de Sa
E, Napolskikh J, Sinclair E, Ford M, Goh P and Chow E: The role of
plain radiographs in management of bone metastases. J Palliat Med.
12:195–198. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xu S, Yu X and Xu M: Long-term survival of
a patient with lung cancer metastasis to the spine following
surgical treatment combined with radiation and epithelial growth
factor receptor inhibitor therapy: A case report. Exp Ther Med.
9:117–119. 2015.PubMed/NCBI
|
|
15
|
Hartsell WF, Scott CB, Bruner DW,
Scarantino CW, Ivker RA, Roach M III, Suh JH, Demas WF, Movsas B,
Petersen IA, et al: Randomized trial of short-versus long-course
radiotherapy for palliation of painful bone metastases. J Natl
Cancer Inst. 97:798–804. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
No authors listed: 8 Gy single fraction
radiotherapy for the treatment of metastatic skeletal pain:
Randomised comparison with a multifraction schedule over 12 months
of patient follow-up. Bone Pain Trial Working Party. Radiother
Oncol. 52:111–121. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhao F, Ding G, Huang W, Li M, Fu Z, Yang
G, Kong L, Zhang Y and Yu J: FDG-PET predicts pain response and
local control in palliative radiotherapy with or without systemic
treatment in patients with bone metastasis from non-small-cell lung
cancer. Clin Lung Cancer. 16:e111–e119. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Azoury SC, Straughan DM and Shukla V:
Immune Checkpoint Inhibitors for Cancer Therapy: Clinical Efficacy
and Safety. Curr Cancer Drug Targets. 15:452–462. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Garon EB, Rizvi NA, Hui R, Leighl N,
Balmanoukian AS, Eder JP, Patnaik A, Aggarwal C, Gubens M, Horn L,
et al: KEYNOTE-001 Investigators: Pembrolizumab for the treatment
of non-small-cell lung cancer. N Engl J Med. 372:2018–2028. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Brahmer J, Reckamp KL, Baas P, Crinò L,
Eberhardt WE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE,
Holgado E, et al: Nivolumab versus Docetaxel in Advanced
Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med.
373:123–315. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rizvi NA, Mazières J, Planchard D,
Stinchcombe TE, Dy GK, Antonia SJ, Horn L, Lena H, Minenza E,
Mennecier B, et al: Activity and safety of nivolumab, an anti-PD-1
immune checkpoint inhibitor, for patients with advanced, refractory
squamous non-small-cell lung cancer (CheckMate 063): A phase 2,
single-arm trial. Lancet Oncol. 16:257–265. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
McDermott J and Jimeno A: Pembrolizumab:
PD-1 inhibition as a therapeutic strategy in cancer. Drugs Today
(Barc). 51:7–20. 2015. View Article : Google Scholar : PubMed/NCBI
|