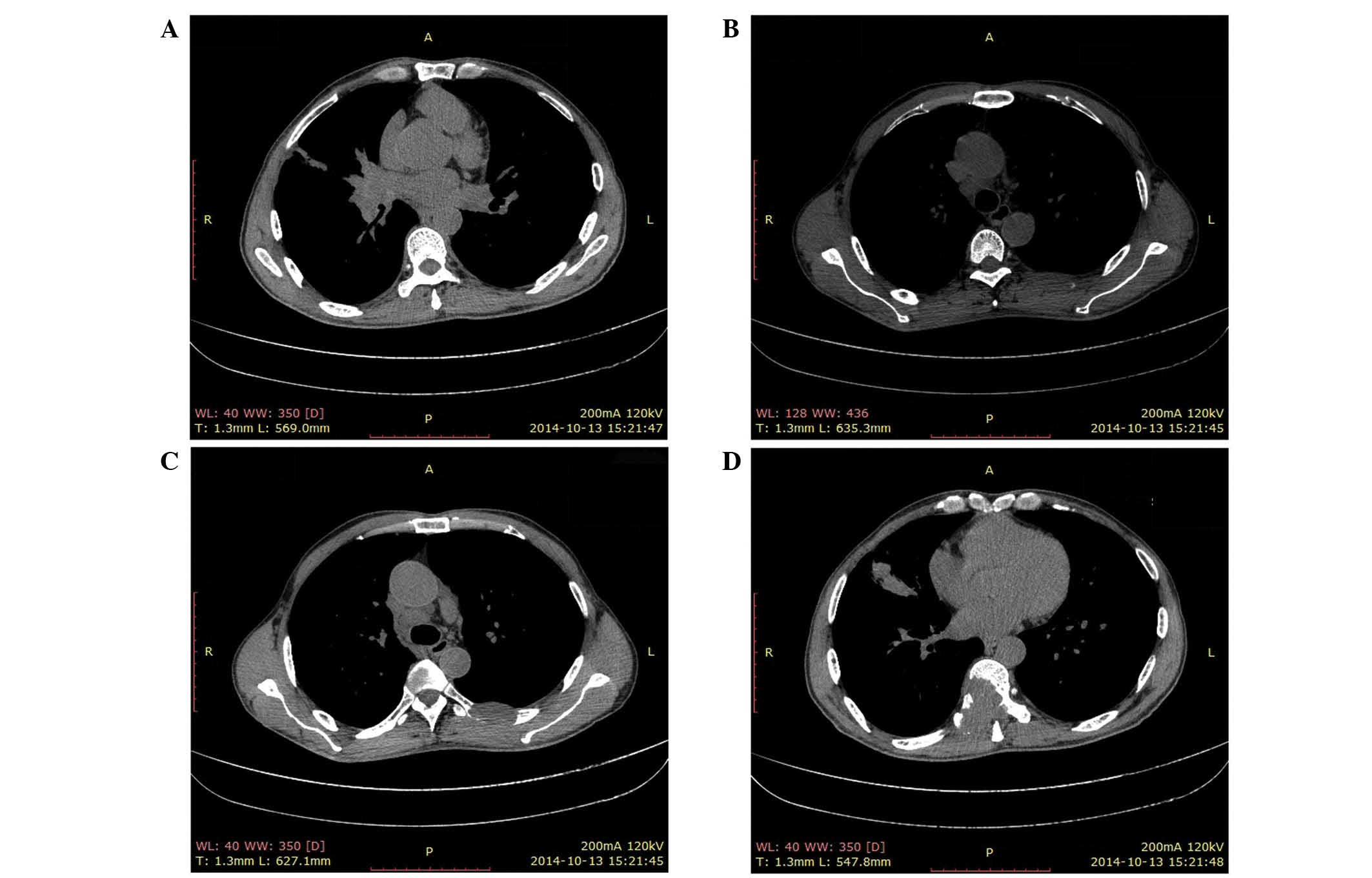
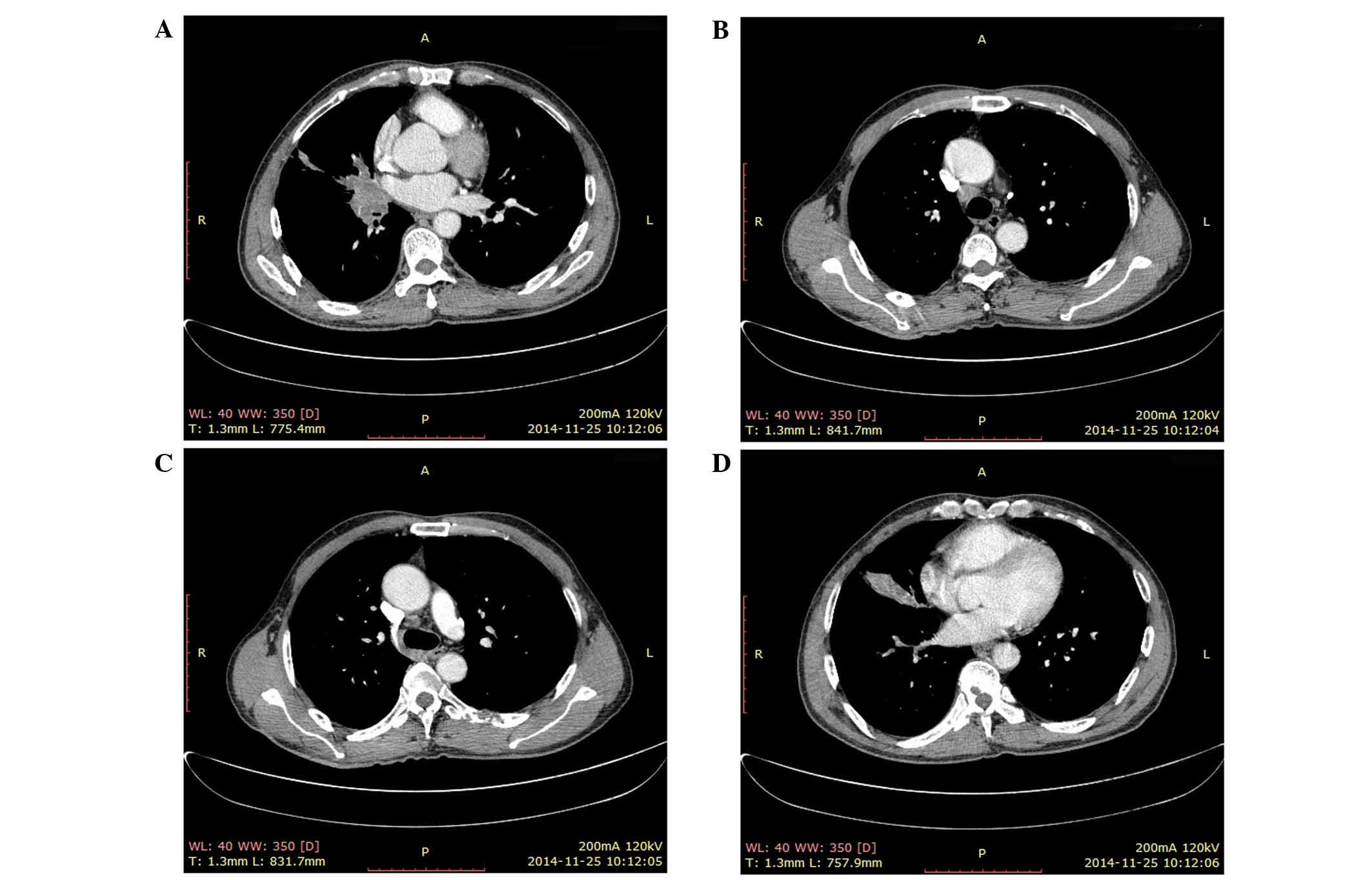
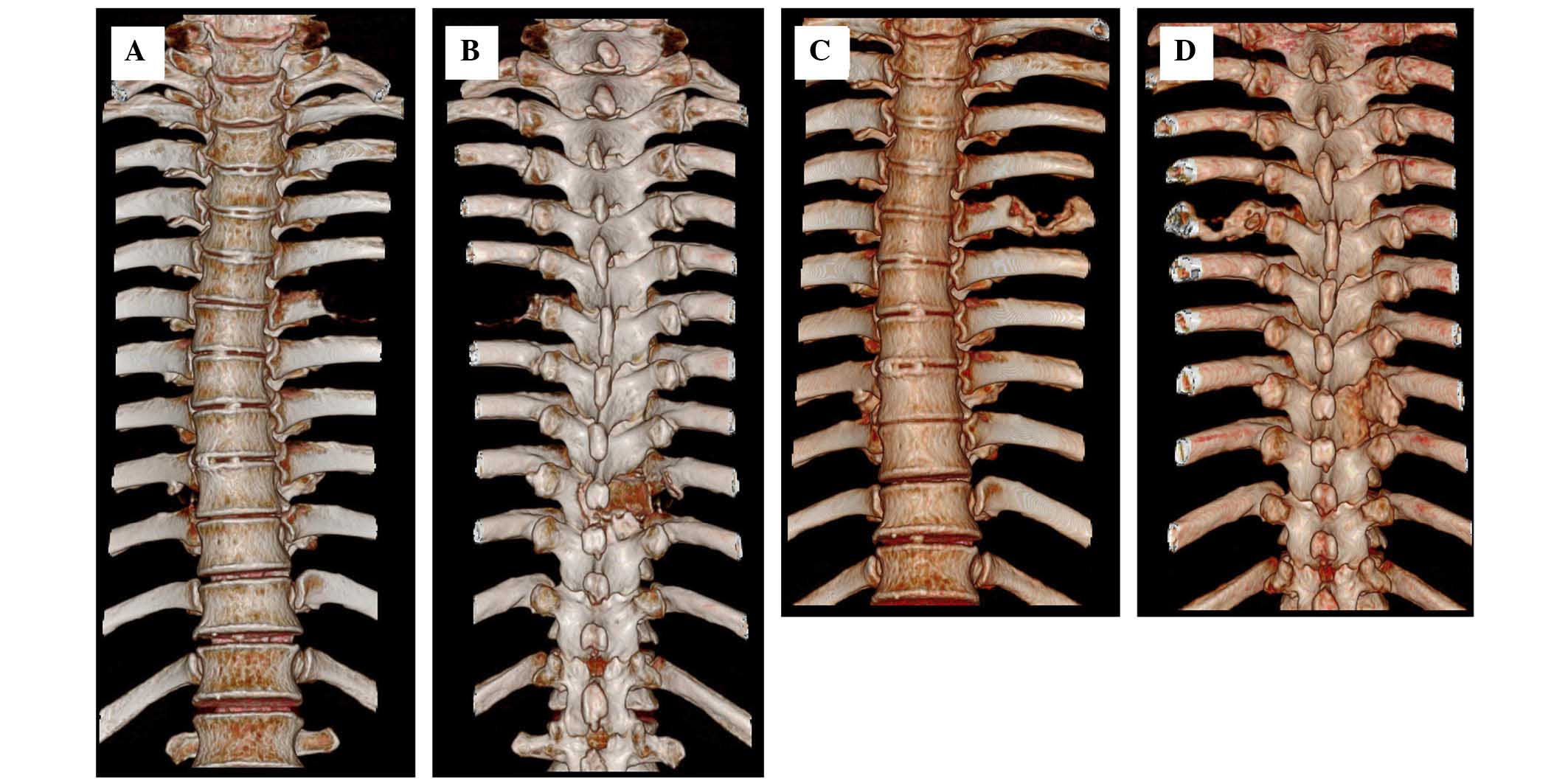
|
1
|
American Association of Neurological
Surgeons: Patient Information, Spinal Tumors. http://www.aans.org/en/Patient%20Information/Conditions%20and%20Treatments/Spinal%20Tumors.aspxAccessed.
July. 2014
|
|
2
|
Sundaresan N, Boriani S, Rothman A and
Holtzman R: Tumors of the osseous spine. J Neurooncol. 69:273–290.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sciubba DM, Petteys RJ, Dekutoski MB,
Fisher CG, Fehlings MG, Ondra SL, Rhines LD and Gokaslan ZL:
Diagnosis and management of metastatic spine disease. A review. J
Neurosurg Spine. 13:94–108. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dodwad SN, Savage J, Scharschmidt TJ and
Patel A: Evaluation and treatment of spinal metastatic disease.
Cancer Treat Res. 162:131–150. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tokuhashi Y, Ajiro Y and Umezawa N:
Outcome of treatment for spinal metastases using scoring system for
preoperative evaluation of prognosis. Spine (Phila Pa 1976).
34:69–73. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sugiura H, Yamada K, Sugiura T, Hida T and
Mitsudomi T: Predictors of survival in patients with bone
metastasis of lung cancer. Clin Orthop Relat Res. 466:729–736.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li X, Zhang L and Li Y: Inhibition of
cancer cell proliferation by color modulation: A pilot study.
Presented at the JSMO (Japan). 2013.
|
|
8
|
Li X, Li J, Tian Y, Zhang L, Liu H, Li Z
and Yin W: Inhibition of rectal cancer growth by a new herbal
extract combinations (FUO-C4) in vivo. Presented at the JSMO
(Japan). 2014.
|
|
9
|
National Comprehensive Cancer Network
Non-small Lung Cancer Guidelines. https://www.nccn.org/store/login/login.aspx?ReturnURL=https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf2015.
|
|
10
|
Oken MM, Creech RH, Tormey DC, Horton J,
Davis TE, McFadden ET and Carbone PP: Toxicity and response
criteria of the Eastern Cooperative Oncology Group. Am J Clin
Oncol. 5:649–655. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dworkin RH, Turk DC, Farrar JT,
Haythornthwaite JA, Jensen MP, Katz NP, Kerns RD, Stucki G, Allen
RR, Bellamy N, et al: Core outcome measures for chronic pain
clinical trials: IMMPACT recommendations. Pain. 113:9–19. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Trotti A, Colevas AD, Setser A, Rusch V,
Jaques D, Budach V, Langer C, Murphy B, Cumberlin R, Coleman CN and
Rubin P: CTCAE v3.0: Development of a comprehensive grading system
for the adverse effects of cancer treatment. Semin Radiat Oncol.
13:176–181. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Salvo N, Christakis M, Rubenstein J, de Sa
E, Napolskikh J, Sinclair E, Ford M, Goh P and Chow E: The role of
plain radiographs in management of bone metastases. J Palliat Med.
12:195–198. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xu S, Yu X and Xu M: Long-term survival of
a patient with lung cancer metastasis to the spine following
surgical treatment combined with radiation and epithelial growth
factor receptor inhibitor therapy: A case report. Exp Ther Med.
9:117–119. 2015.PubMed/NCBI
|
|
15
|
Hartsell WF, Scott CB, Bruner DW,
Scarantino CW, Ivker RA, Roach M III, Suh JH, Demas WF, Movsas B,
Petersen IA, et al: Randomized trial of short-versus long-course
radiotherapy for palliation of painful bone metastases. J Natl
Cancer Inst. 97:798–804. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
No authors listed: 8 Gy single fraction
radiotherapy for the treatment of metastatic skeletal pain:
Randomised comparison with a multifraction schedule over 12 months
of patient follow-up. Bone Pain Trial Working Party. Radiother
Oncol. 52:111–121. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhao F, Ding G, Huang W, Li M, Fu Z, Yang
G, Kong L, Zhang Y and Yu J: FDG-PET predicts pain response and
local control in palliative radiotherapy with or without systemic
treatment in patients with bone metastasis from non-small-cell lung
cancer. Clin Lung Cancer. 16:e111–e119. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Azoury SC, Straughan DM and Shukla V:
Immune Checkpoint Inhibitors for Cancer Therapy: Clinical Efficacy
and Safety. Curr Cancer Drug Targets. 15:452–462. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Garon EB, Rizvi NA, Hui R, Leighl N,
Balmanoukian AS, Eder JP, Patnaik A, Aggarwal C, Gubens M, Horn L,
et al: KEYNOTE-001 Investigators: Pembrolizumab for the treatment
of non-small-cell lung cancer. N Engl J Med. 372:2018–2028. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Brahmer J, Reckamp KL, Baas P, Crinò L,
Eberhardt WE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE,
Holgado E, et al: Nivolumab versus Docetaxel in Advanced
Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med.
373:123–315. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rizvi NA, Mazières J, Planchard D,
Stinchcombe TE, Dy GK, Antonia SJ, Horn L, Lena H, Minenza E,
Mennecier B, et al: Activity and safety of nivolumab, an anti-PD-1
immune checkpoint inhibitor, for patients with advanced, refractory
squamous non-small-cell lung cancer (CheckMate 063): A phase 2,
single-arm trial. Lancet Oncol. 16:257–265. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
McDermott J and Jimeno A: Pembrolizumab:
PD-1 inhibition as a therapeutic strategy in cancer. Drugs Today
(Barc). 51:7–20. 2015. View Article : Google Scholar : PubMed/NCBI
|