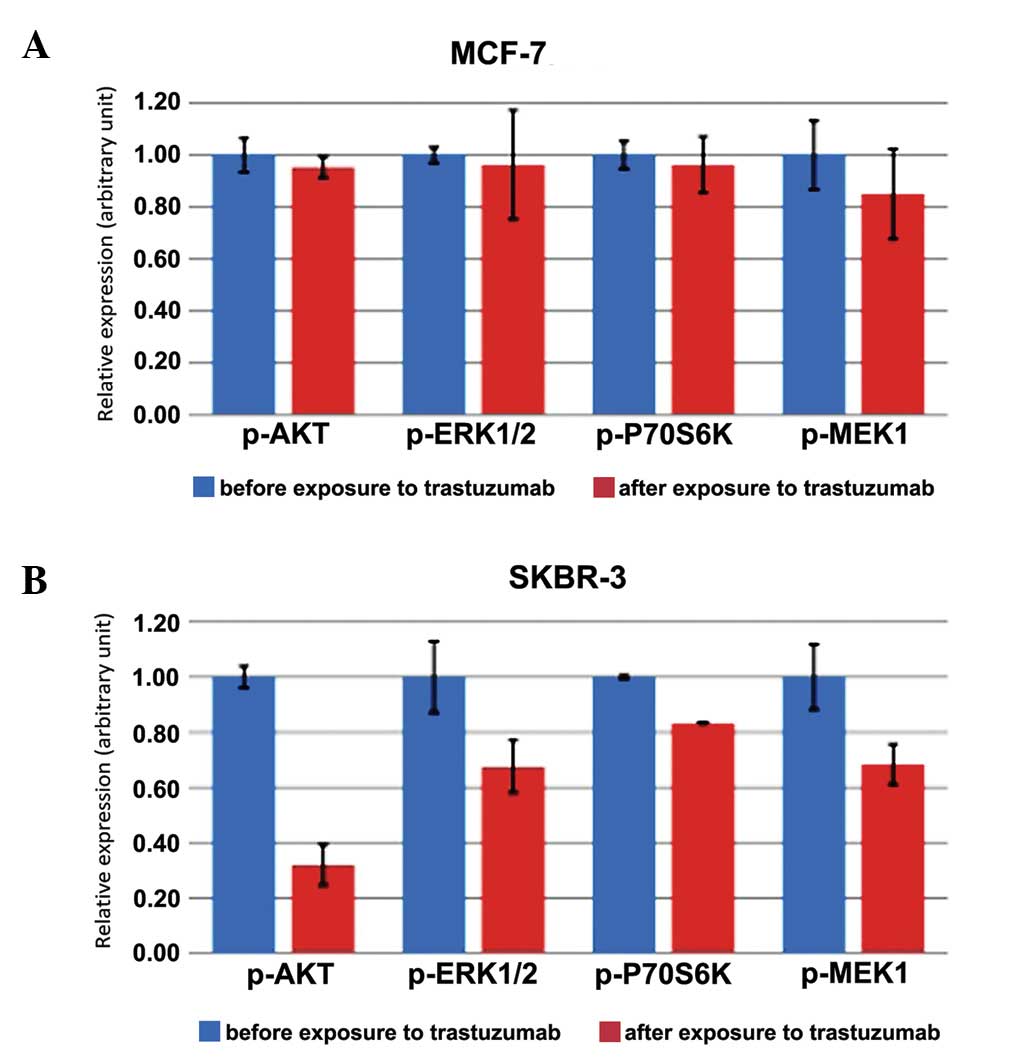
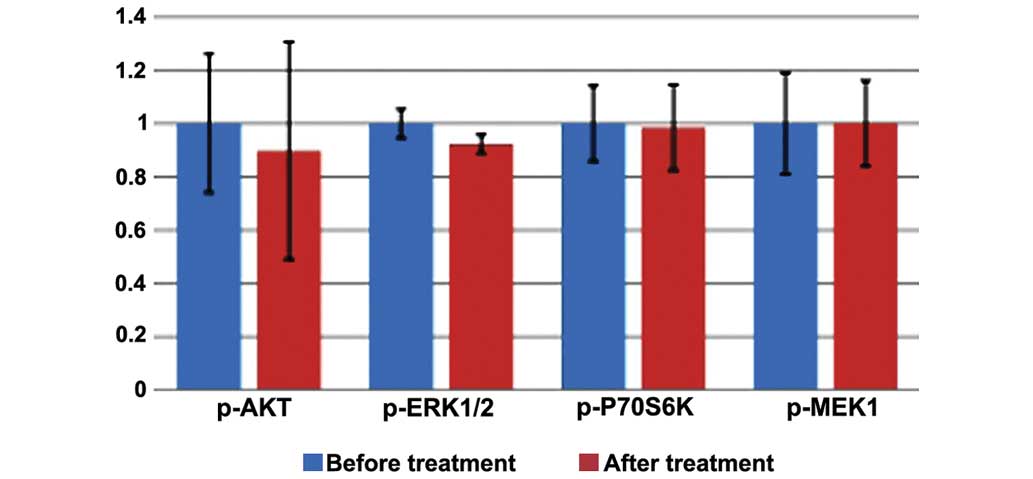
|
1
|
Hudis CA: Trastuzumab-mechanism of action
and use in clinical practice. N Engl J Med. 357:39–51. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Baselga J: Treatment of
HER2-overexpressing breast cancer. Ann Oncol. 21(Suppl 7):
vii36–vii40. 2010.PubMed/NCBI
|
|
3
|
Engelman JA, Luo J and Cantley LC: The
evolution of phosphatidylinositol 3-kinases as regulators of growth
and metabolism. Nat Rev Genet. 7:606–619. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Harlé A, Lion M, Lozano N, Husson M,
Harter V, Genin P and Merlin JL: Analysis of PIK3CA exon 9 and 20
mutations in breast cancers using PCR-HRM and PCR-ARMS: Correlation
with clinicopathological criteria. Oncol Rep. 29:1043–1052.
2013.PubMed/NCBI
|
|
5
|
Mendoza MC, Er EE and Blenis J: The
Ras-ERK and PI3K-mTOR pathways: Cross-talk and compensation. Trends
Biochem Sci. 36:320–328. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Martelli AM, Tabellini G, Bressanin D,
Ognibene A, Goto K, Cocco L and Evangelisti C: The emerging
multiple roles of nuclear Akt. Biochim Biophys Acta.
1823:2168–2178. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Park SS and Kim SW: Activated Akt
signaling pathway in invasive ductal carcinoma of the breast:
Correlation with HER2 overexpression. Oncol Rep. 18:139–143.
2007.PubMed/NCBI
|
|
8
|
Lin HJ, Hsieh FC, Song H and Lin J:
Elevated phosphorylation and activation of PDK-1/AKT pathway in
human breast cancer. Br J Cancer. 93:1372–1381. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Baker AF, Dragovich T, Ihle NT, Williams
R, Fenoglio-Preiser C and Powis G: Stability of phosphoprotein as a
biological marker of tumor signaling. Clin Cancer Res.
11:4338–4340. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
David KA and Juhl H: Immunohistochemical
detection of phosphoproteins and cancer pathways. Handbook of
Practical Immunohistochemistry. Lin F and Prichard J: Springer.
(New York, NY). 85–90. 2015.
|
|
11
|
Mandell JW: Phosphorylation state-specific
antibodies: Applications in investigative and diagnostic pathology.
Am J Pathol. 163:1687–1698. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mandell JW: Immunohistochemical assessment
of protein phosphorylation state: The dream and the reality.
Histochem Cell Biol. 130:465–471. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Menard S, Pupa SM, Campiglio M and
Tagliabue E: Biologic and therapeutic role of HER2 in cancer.
Oncogene. 22:6570–6578. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yersal O and Barutca S: Biological
subtypes of breast cancer: Prognostic and therapeutic implications.
World J Clin Oncol. 5:412–424. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Slamon DJ, Leyland-Jones B, Shak S, Fuchs
H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M,
et al: Use of chemotherapy plus a monoclonal antibody against HER2
for metastatic breast cancer that overexpresses HER2. N Engl J Med.
344:783–792. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Valabrega G, Montemurro F and Aglietta M:
Trastuzumab: Mechanism of action, resistance and future
perspectives in HER2-overexpressing breast cancer. Ann Oncol.
18:977–984. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gemmete JJ and Mukherji SK: Trastuzumab
(herceptin). AJNR Am J Neuroradiol. 32:1373–1374. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yakes FM, Chinratanalab W, Ritter CA, King
W, Seelig S and Arteaga CL: Herceptin-induced inhibition of
phosphatidylinositol-3 kinase and Akt Is required for
antibody-mediated effects on p27, cyclin D1, and antitumor action.
Cancer Res. 62:4132–4141. 2002.PubMed/NCBI
|
|
19
|
Mohsin SK, Weiss HL, Gutierrez MC,
Chamness GC, Schiff R, Digiovanna MP, Wang CX, Hilsenbeck SG,
Osborne CK, Allred DC, et al: Neoadjuvant trastuzumab induces
apoptosis in primary breast cancers. J Clin Oncol. 23:2460–2468.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nahta R, Yu D, Hung MC, Hortobagyi GN and
Esteva FJ: Mechanisms of disease: Understanding resistance to
HER2-targeted therapy in human breast cancer. Nat Clin Pract Oncol.
3:269–280. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Arnould L, Gelly M, Penault-Llorca F,
Benoit L, Bonnetain F, Migeon C, Cabaret V, Fermeaux V, Bertheau P,
Garnier J, et al: Trastuzumab-based treatment of HER2-positive
breast cancer: An antibody-dependent cellular cytotoxicity
mechanism? Br J Cancer. 94:259–267. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Clynes RA, Towers TL, Presta LG and
Ravetch JV: Inhibitory Fc receptors modulate in vivo cytotoxicity
against tumor targets. Nat Med. 6:443–446. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Spector NL and Blackwell KL: Understanding
the mechanisms behind trastuzumab therapy for human epidermal
growth factor receptor 2-positive breast cancer. J Clin Oncol.
27:5838–5847. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Molina MA, Codony-Servat J, Albanell J,
Rojo F, Arribas J and Baselga J: Trastuzumab (herceptin), a
humanized anti-Her2 receptor monoclonal antibody, inhibits basal
and activated Her2 ectodomain cleavage in breast cancer cells.
Cancer Res. 61:4744–4749. 2001.PubMed/NCBI
|
|
25
|
Andre F, Dieci MV, Dubsky P, Sotiriou C,
Curigliano G, Denkert C and Loi S: Molecular pathways: Involvement
of immune pathways in the therapeutic response and outcome in
breast cancer. Clin Cancer Res. 19:28–33. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sobin LH: TNM, sixth edition: New
developments in general concepts and rules. Semin Surg Oncol.
21:19–22. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chergui F, Chrétien AS, Bouali S, Ramacci
C, Rouyer M, Bastogne T, Genin P, Leroux A and Merlin JL:
Validation of a phosphoprotein array assay for characterization of
human tyrosine kinase receptor downstream signaling in breast
cancer. Clin Chem. 55:1327–1336. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gennari R, Menard S, Fagnoni F, Ponchio L,
Scelsi M, Tagliabue E, Castiglioni F, Villani L, Magalotti C,
Gibelli N, et al: Pilot study of the mechanism of action of
preoperative trastuzumab in patients with primary operable breast
tumors overexpressing HER2. Clin Cancer Res. 10:5650–5655. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Therasse P: Measuring the clinical
response. What does it mean? Eur J Cancer. 38:1817–1823. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Petricevic B, Laengle J, Singer J, Sachet
M, Fazekas J, Steger G, Bartsch R, Jensen-Jarolim E and Bergmann M:
Trastuzumab mediates antibody-dependent cell-mediated cytotoxicity
and phagocytosis to the same extent in both adjuvant and metastatic
HER2/neu breast cancer patients. J Transl Med. 11:3072013.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tagliabue E, Campiglio M, Pupa SM, Ménard
S and Balsari A: Activity and resistance of trastuzumab according
to different clinical settings. Cancer Treat Rev. 38:212–217. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Singh A, Nunes JJ and Ateeq B: Role and
therapeutic potential of G-protein coupled receptors in breast
cancer progression and metastases. Eur J Pharmacol. 763:178–183.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hollestelle A, Elstrodt F, Nagel JH,
Kallemeijn WW and Schutte M: Phosphatidylinositol-3-OH kinase or
RAS pathway mutations in human breast cancer cell lines. Mol Cancer
Res. 5:195–201. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Aksamitiene E, Kiyatkin A and Kholodenko
BN: Cross-talk between mitogenic Ras/MAPK and survival PI3K/Akt
pathways: A fine balance. Biochem Soc Trans. 40:139–146. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Saini KS, Loi S, de Azambuja E,
Metzger-Filho O, Saini ML, Ignatiadis M, Dancey JE and
Piccart-Gebhart MJ: Targeting the PI3K/AKT/mTOR and Raf/MEK/ERK
pathways in the treatment of breast cancer. Cancer Treat Rev.
39:935–946. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Carracedo A and Pandolfi PP: The PTEN-PI3K
pathway: Of feedbacks and cross-talks. Oncogene. 27:5527–5541.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Pinhel IF, Macneill FA, Hills MJ, Salter
J, Detre S, A'hern R, Nerurkar A, Osin P, Smith IE and Dowsett M:
Extreme loss of immunoreactive p-Akt and p-Erk1/2 during routine
fixation of primary breast cancer. Breast Cancer Res. 12:R762010.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Siddiqui S and Rimm DL: Pre-analytic
variables and phospho-specific antibodies: The Achilles heel of
immunohistochemistry. Breast Cancer Res. 12:1132010. View Article : Google Scholar : PubMed/NCBI
|