Introduction
Hepatocellular carcinoma (HCC) is one of the most
common malignant tumors and its incidence is increasing.
Furthermore, HCC is the third leading cause of cancer-associated
mortality worldwide, partly due to its high recurrence rate and
early metastasis (1,2). In 2000, there were 564,000 new cases and
549,000 mortalities from HCC worldwide, indicating the devastating
prognosis of this tumor (3). In 2008,
746,300 new cases of HCC were diagnosed worldwide, and 695,900
HCC-related mortalities were reported. In total, >700,000 new
cases are diagnosed each year throughout the world and >600,000
mortalities are attributed to HCC each year (4). At present, the majority of patients with
HCC are diagnosed at the advanced stage due to lack of specific
clinical manifestations, meaning that patients often miss out on
the chance of receiving curative treatments, such as liver
resection (5). In addition, patients
with HCC often have a poor prognosis due to the aggressive nature
of the malignancy, including a high recurrence rate and metastasis.
Therefore, an improved understanding of the mechanisms underlying
the recurrence and metastasis of HCC is required in order to
identify effective prognostic and therapeutic biomarkers of
HCC.
Branched-chain amino acid transaminase 1 (BCAT1),
which is also known as cytosolic branched-chain aminotransferase
and ECA39, is located at chromosome 12p12.1. It encodes the
cytosolic form of the branched-chain amino acid transaminase
enzyme, which catalyzes the reversible transamination of
branched-chain α-keto acids to branched-chain L-amino acids
essential for cell growth (6–10). It has previously been suggested that
the aberrant expression of BCAT1, and the concomitant defect in
branched-chain amino acid transamination, leads to hypervalinemia
and hyperleucine-isoleucinemia, and may have an important role in
the cell growth, proliferation and apoptosis of numerous tumor
types (8,11–14).
Furthermore, BCAT1 overexpression has been reported in
non-neoplastic diseases of the liver, including chronic hepatitis C
and non-alcoholic fatty liver disease (15–17).
However, the expression and role of BCAT1 in HCC remains
unclear.
Previous studies have reported that BCAT1 serves as
an oncogenic protein that is upregulated by several signaling
molecules, including c-Myc (18–20). c-Myc
is an oncogene and transcription factor involved in the
tumorigenesis of multiple cancers, including Burkitt's lymphoma and
breast cancer, by targeting genes harboring the c-Myc-binding
element (CACGTG) downstream of their transcription start site
(11). Therefore, c-Myc may have an
important role in the development and progression of HCC (20).
BCAT1 has previously been associated with numerous
malignancies due to its role in cell proliferation, cell cycle
progression, differentiation and apoptosis (8,10–14). However, little is known regarding the
role of BCAT1 in HCC. To the best of our knowledge, the present
study is the first to assess the association between BCAT1 and HCC.
The study aimed to determine whether BCAT1 may serve as a potential
prognostic and therapeutic biomarker for HCC.
Materials and methods
Cell lines
The L-02, SMMC-7721, BEL-7402, Huh-7, HepG2 and
MHCC-97H cell lines were obtained from the Type Culture Collection
of the Chinese Academy of Sciences (Shanghai, China). All cells
were maintained in Gibco Dulbecco's modified Eagle's medium (DMEM;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with
10% fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific,
Inc.), 100 U/ml penicillin and 100 µg/ml streptomycin
(Sigma-Aldrich; Merck Millipore, Darmstadt, Germany), and cultured
in a humidified 5% CO2 incubator at 37°C.
Patients and specimens
A total of 74 HCC and matched normal adjacent
samples (>2 cm distance from the margin of the resection) were
obtained from pathologically confirmed HCC patients who had
undergone surgical resection at the First Affiliated Hospital of
Xi'an Jiaotong University (Xi'an, China) between October 2005 and
September 2008. None of the patients had received any pre-operative
chemotherapy or radiotherapy, and patients with evidence of
concomitant extrahepatic disease were excluded from the analysis.
HCC stage was classified according to the seventh edition of the
tumor-node-metastasis (TNM) classification criteria of the
International Union Against Cancer (21). The present study included 56 males and
18 females with a median age of 52 years (range, 33–75 years). All
HCC tissues and matched pericarcinous liver tissues were
immediately snap-frozen in liquid nitrogen following surgery and
stored at −80°C until use. Hepatitis B surface antigen (HBsAg) and
α-fetoprotein (AFP) levels were obtained from the results of
laboratory tests, capsule formation was observed during surgery and
Edmonson-Steiner grade (22) was
evaluated by an experienced pathologist. All information was
recorded for each case. All patients provided informed consent
prior to surgery, and all protocols were performed in accordance
with the 1975 Declaration of Helsinki. The present study was
approved by the Ethics Committee of The First Affiliated Hospital
of Xi'an Jiaotong University.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from HCC cell lines and
tissues using TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol. In
order to avoid DNA contamination, the extracted RNA was treated
with RNase-free DNase I (Invitrogen; Thermo Fisher Scientific,
Inc.) and quantified by spectrophotometry. Subsequently, cDNA was
synthesized using the RevertAid Premium First Strand cDNA Synthesis
kit (Fermentas; Thermo Fisher Scientific, Inc.). qPCR was performed
using the Applied Biosystems 7500 Real-Time PCR system (Thermo
Fisher Scientific, Inc.) and SYBR® Premix Ex Taq™ II
(Tli RNaseH Plus; Takara Bio, Inc., Otsu, Japan). The primer
sequences were as follows: BCAT1 forward,
5′-CCAAAGCCCTGCTCTTTGTA-3′ and reverse, 5′-TGGAGGAGTTGCCAGTTCTT-3′;
and β-actin (internal control) forward, 5′-GGGAAATCGTGCGTGACAT-3′
and reverse, 5′-CTGGAAGGTGGACAGCGAG-3′. The reaction conditions for
the PCR program were as following: Initial denaturation at 95°C for
30 sec, followed by 40 cycles at 95°C for 5 sec and 60°C for 64
sec. Melting curve analyses were performed to confirm the
specificity of the PCR product. Relative mRNA expression levels
were determined using the 2−ΔΔCq method (23). Reactions were performed in
triplicate.
Immunoblotting
Total protein was extracted from HCC cell lines and
tissues using radioimmunoprecipitation buffer (catalog no. WB009;
HEART Biological Technology Co. Ltd., Xi'an, China). Protein
concentration was determined using the bicinchoninic acid kit
(Pierce; Thermo Fisher Scientific, Inc.). Denatured protein samples
(25 µg) were separated by 10% polyacrylamide gel electrophoresis,
and then electrophoretically transferred onto polyvinylidene
fluoride membranes (Merck Millipore). The membranes were blocked
with 5% skimmed milk in Tris-buffered saline with Tween (TBST) at
37°C for 2 h, and subsequently incubated with the primary
antibodies overnight at 4°C; the membranes were incubated with
rabbit anti-BCAT1 polyclonal antibody (cat. no. ab197941; 1:1,000
dilution; Abcam, Cambridge, UK), mouse anti-c-Myc monoclonal
antibody (cat. no. ab32; 1:1,000 dilution; Abcam) and mouse
anti-β-actin monoclonal antibody (cat. no. ab6276; 1:10,000
dilution; Abcam). Subsequently, the membranes were incubated with
horseradish peroxidase-conjugated goat anti-rabbit (cat. no.
ab6721; 1:3,000 dilution; Abcam) and rabbit anti-mouse (cat. no.
ab6728; 1:3,000 dilution; Abcam) secondary antibodies at 37°C for 2
h, followed by washing three times with TBST. Protein bands were
detected using the Western Blotting Luminol Reagent (cat. no.
sc-2048, Santa Cruz Biotechnology, Inc., Dallas, TX, USA). The
reactions were detected using the HyGLO™ Chemiluminescent HRP
Detection kit (Denville Scientific, Inc., Holliston, MA, USA). The
band density was measured using Image Lab 4.0 (Bio-Rad Laboratories
Inc., Hercules, CA, USA) imaging software. Expression levels of the
protein are assessed by a densitometric ratio of the targeted
protein to the β-actin housekeeping protein.
Immunohistochemical staining
Immunohistochemical staining was performed using
paraformaldehyde-fixed, paraffin-embedded tissue sections, which
were prepared according to a method described previously (14). The tissue sections were incubated with
rabbit anti-BCAT1 polyclonal antibody (cat. no. ab197941; 1:50
dilution; Abcam) and mouse anti-C-Myc monoclonal antibody (cat. no.
ab32; 1:200 dilution; Abcam) overnight at 4°C, followed by
incubation with biotinylated goat anti-rabbit (cat. no. SV0002;
Wuhan Boster Biological Technology, Ltd., Wuhan, China) and rabbit
anti-mouse (cat. no. SV0001; Wuhan Boster Biological Technology,
Ltd.) secondary antibodies at 37°C for 1 h. Each slide was colored
with DAB (Sigma-Aldrich, St. Louis, MO, USA) in a dark room, then
all the sections were rinsed with running water and counterstained
with hematoxylin (cat. no. ST047; HEART Biological Technology Co.
Ltd.). Subsequently, the tissue sections were assessed by light
microscopy and evaluated blindly and independently by two
experienced pathologists. To evaluate the association between the
expression of BCAT1 and c-Myc, a semi-quantitative scoring system
based on the staining intensity and the percentage of positive
liver cells was applied. Immunostaining intensity was evaluated as
one of the following four grades: 0, negative; 1 weak; 2, moderate;
and 3, strong. The percentage of positive liver cells was
categorized into one of the following groups: 0, 0%; 1, 1–10%; 2,
11–50%; 3, 51–80%; and 4, >80%. The immunostaining intensity and
average percentage of positive cells were evaluated for 10
independent high magnification fields. The final weighted
expression score (0–12) was obtained by multiplying the staining
intensity with the percentage of positive cells. The total
expression scores for BCAT1 and c-Myc were listed as continuous
variables for the correlation analyses. In order to evaluate the
effect of BCAT1 protein expression on overall survival, the
weighted expression scores of BCAT1 protein were divided into high
and low scores using the median expression score as the cutoff
point.
Small interfering RNA (siRNA)
transfection
siRNAs targeting c-Myc (cat. no. sc-29226) and BCAT1
(cat. no. sc-77222), as well as control siRNA (cat. no. sc-37007),
were purchased from Santa Cruz Biotechnology, Inc. MHCC-97H tumor
cells were seeded at a density of 2×105 cells per well
into six-well plates and cultured overnight in a humidified 5%
CO2 incubator at 37°C. Subsequently, the cells were
transfected with 100 nM of the BCAT1, c-Myc or control siRNA using
Lipofectamine RNAi MAX Reagent (Invitrogen; Thermo Fisher
Scientific, Inc.). Further experiments were performed after 48 h of
transfection.
Transwell invasion assay
Matrigel was diluted in serum-free DMEM (1:3) and
added to the upper chamber of a 24-well Transwell plate. HCC cells
were trypsinized and counted manually under a light microscope. A
cell suspension of 5×104 cells/ml in serum-free medium
was prepared and 100 µl of the suspension was loaded into the upper
chamber. The lower chambers were filled with 10% FBS in DMEM.
Invasion was halted in a 37°C incubator (5% CO2) after
~24 h by removing the non-migrated cells from the upper chamber
using a cotton swab. The HCC cells that had migrated through the
membrane were stained with 0.05% crystal violet after fixing with
4% paraformaldehyde, and were counted under a microscope. At least
five fields were randomly selected for counting the mean number of
invaded cells in each membrane using ImageJ v1.48 software (NIH,
Bethesda, MD, USA). At least three experimental replicates were
performed.
Wound healing assay
MHCC97H cells transfected with BCAT1 or control
siRNA were seeded at a concentration of 5×105 per well
onto 6-well plates and cultured to full confluency. Scratch wounds
were made across the surface of the plates using a 10-µl pipette
tip and the suspension cells were removed using phosphate-buffered
saline. Cells were cultured in serum-free DMEM medium in a
humidified 5% CO2 incubator at 37°C for 48 h, after
which images of the plates were captured using a phase-contrast
microscope. At least five replicate experiments were performed.
Follow-up
Follow-up of the patients in the present study was
performed on December 31, 2013. The duration was defined as the
interval between the date of surgery and the date of mortality or
last follow-up. The follow-up time ranged from 6–78 months and the
median time was 58.5 months. All patients received follow-up visits
once every 1–3 months in the first year and every 3–6 months
thereafter. The follow-up protocol included a physical examination,
measurement of serum AFP levels, a chest X-ray and abdominal
ultrasonography. Computed tomography, magnetic resonance imaging or
positron emission tomography was performed to assess the occurrence
of tumor recurrence. During the follow-up period, 59 patients
(79.7%) were shown to have intrahepatic tumor recurrence and 11
patients (14.9%) had developed distant tumor metastases.
Statistical analysis
Statistical analyses were performed using SPSS 16.0
software (SPSS Inc., Chicago, IL, USA). The Spearman's rank
correlation coefficient was applied to evaluate the association
between ordinal data, and the χ2 test or Fisher's exact
test was performed for comparisons of categorical data. The
expression levels between groups were compared using the
Mann-Whitney U test. Overall survival and disease-free survival
rates, and mortalities associated with tumor recurrence or
metastasis, were analyzed using the Kaplan-Meier method, and
differences between curves were assessed using the log-rank test.
Independent prognostic factors were assessed by the Cox
proportional hazards stepwise regression model. Data are presented
as the mean ± standard error of the mean. P-values were two-sided.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Expression of BCAT1 in HCC tissues and
cells
The expression levels of BCAT1 in cell lines and
tissues were determined using RT-qPCR and western blotting. The
expression levels of BCAT1 were significantly lower in the L-02
cells compared with the HCC cell lines (all P<0.001; Fig. 1A and B). Similarly, BCAT1 expression
levels were significantly higher in HCC tissues compared with
adjacent non-cancerous liver tissues (P<0.001; Fig. 1C and D).
Association between BCAT1 expression
and clinicopathological parameters
To investigate the clinical significance of BCAT1 in
patients with HCC, the associations between the BCAT1 expression
levels (high or low) and clinicopathological parameters, including
patient gender, age, detection of HBsAg, AFP level, tumor size,
tumor number, vascular invasion, cirrhosis, capsule formation,
Edmondson-Steiner grade and TNM stage, were investigated. The
median expression score of BCAT1 protein was used as a cutoff point
to divide patients into high and low expression groups. Notably,
the expression levels of BCAT1 were significantly associated with
the Edmondson-Steiner grade, tumor number, vascular invasion and
TNM stage (all P<0.05). However, no significant association was
observed between the expression levels of BCAT1 and the patient
gender, age, HBsAg, AFP level, cirrhosis and capsule formation (all
P>0.05). The results are shown in Table I.
 | Table I.Associations between BCAT1 expression
and clinicopathologic features of patients with hepatocellular
carcinoma. |
Table I.
Associations between BCAT1 expression
and clinicopathologic features of patients with hepatocellular
carcinoma.
|
|
| BCAT1 protein, n |
|
|---|
|
|
|
|
|
|---|
| Characteristics | n | High | Low | P-value |
|---|
| Gender |
|
|
| 0.787 |
|
Female | 18 | 8 | 10 |
|
| Male | 56 | 29 | 27 |
|
| Age, years |
|
|
| 1.000 |
| ≤45 | 17 | 9 | 8 |
|
|
>45 | 57 | 28 | 29 |
|
| HBsAg status |
|
|
| 0.674 |
|
Negative | 6 | 4 | 2 |
|
|
Positive | 68 | 33 | 35 |
|
| Cirrhosis |
|
|
| 0.754 |
| No | 12 | 7 | 5 |
|
|
Yes | 62 | 30 | 32 |
|
| AFP, µg/l |
|
|
| 0.634 |
|
≤400 | 29 | 13 | 16 |
|
|
>400 | 45 | 24 | 21 |
|
| Tumor size, cm |
|
|
| 0.087 |
| ≤5 | 26 | 17 | 9 |
|
|
>5 | 48 | 20 | 28 |
|
| Tumor number |
|
|
| 0.003a |
|
Single | 47 | 17 | 30 |
|
|
Multiple | 27 | 20 | 7 |
|
| Tumor capsule |
|
|
| 0.074 |
|
Complete | 52 | 30 | 22 |
|
|
Incomplete | 22 | 7 | 15 |
|
| Vascular
invasion |
|
|
| 0.017a |
| No | 54 | 22 | 32 |
|
|
Yes | 20 | 15 | 5 |
|
| Edmondson
grade |
|
|
| 0.027a |
|
I/II | 48 | 19 | 29 |
|
|
III/IV | 26 | 18 | 8 |
|
| TNM stage |
|
|
| 0.017a |
|
I+II | 45 | 17 | 28 |
|
|
III+IV | 29 | 20 | 9 |
|
High expression levels of BCAT1 are
associated with a poor HCC prognosis
The median expression score of BCAT1 protein was
used as a cutoff point to divide patients into high and low
expression groups for a clinical association analysis. Univariate
prognostic analyses and multivariate Cox regression models were
applied to assess the association between the expression levels of
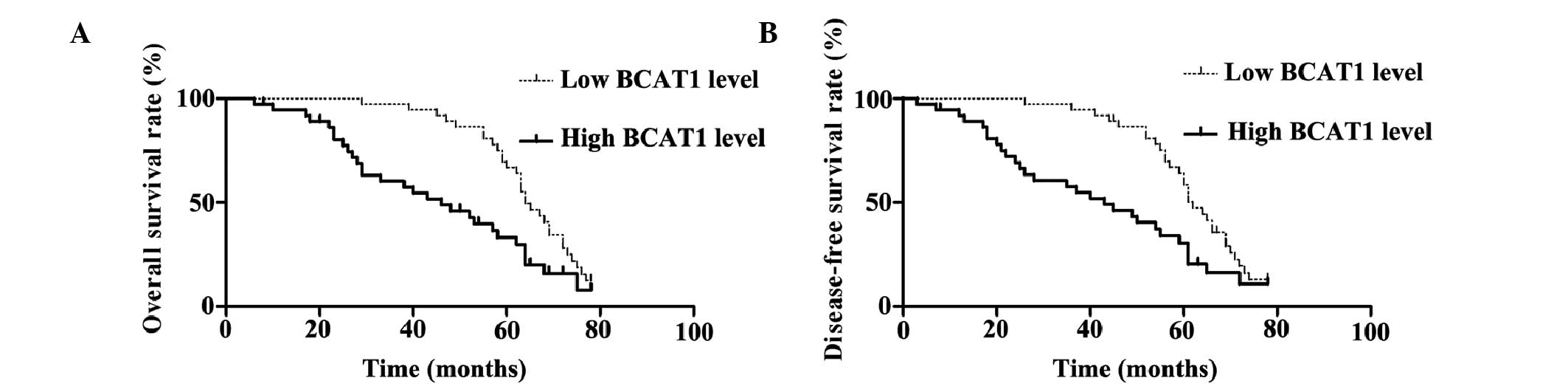
BCAT1 and the overall and disease-free survival rates (Fig. 2). The patients with high BCAT1
expression levels showed significantly reduced overall and
disease-free survival rates (P=0.002). The 5-year overall survival
rate of the low BCAT1 expression group was 66.8%, which was
significantly higher than that of the high BCAT1 expression group
(33.2%) (P=0.002). In addition, the 5-year disease-free survival
rate of the low BCAT1 expression group was 58.5%, which was also
significantly higher compared with that of the high BCAT1
expression group (30.5%) (Fig. 2).
The associations between the overall and disease-free survival
rates and the clinicopathological parameters in the HCC patients
were determined by a univariate analysis. The univariate analysis
demonstrated that vascular infiltration, tumor number,
Edmonson-Steiner classification, TNM stage and the expression level
of BCAT1 were all significant prognostic factors for HCC
(P<0.05). The results are shown in Table II.
 | Table II.Univariate prognostic analysis of
overall and disease-free survival rates in patients with
hepatocellular carcinoma. |
Table II.
Univariate prognostic analysis of
overall and disease-free survival rates in patients with
hepatocellular carcinoma.
|
|
| Overall survival
rate | Disease-free
survival rate |
|---|
|
|
|
|
|
|---|
| Variable | n | 3-year, % | 5-year, % | P-value | 3-year, % | 5-year, % | P-value |
|---|
| Gender |
|
|
| 0.891 |
|
| 0.895 |
|
Female | 18 | 82.4 | 51.0 |
| 82.4 | 44.9 |
|
|
Male | 56 | 78.2 | 50.1 |
| 74.6 | 44.5 |
|
| Age, years |
|
|
| 0.644 |
|
| 0.732 |
|
≤45 | 17 | 81.3 | 68.8 |
| 75.6 | 56.7 |
|
|
>45 | 57 | 78.6 | 44.7 |
| 76.8 | 41.1 |
|
| HBsAg status |
|
|
| 0.079 |
|
| 0.073 |
|
Negative | 6 | 50.0 | 33.3 |
| 50.0 | 33.3 |
|
|
Positive | 68 | 81.9 | 52.0 |
| 78.9 | 45.7 |
|
| Cirrhosis |
|
|
| 0.235 |
|
| 0.318 |
| No | 12 | 66.7 | 50.0 |
| 66.7 | 50.0 |
|
|
Yes | 62 | 81.8 | 50.4 |
| 78.5 | 43.5 |
|
| AFP, µg/l |
|
|
| 0.122 |
|
| 0.224 |
|
≤400 | 29 | 68.1 | 41.0 |
| 64.7 | 33.2 |
|
|
>400 | 45 | 86.4 | 56.3 |
| 84.1 | 51.8 |
|
| Tumor size, cm |
|
|
| 0.764 |
|
| 0.778 |
| ≤5 | 26 | 60.1 | 47.5 |
| 60.4 | 47.7 |
|
|
>5 | 48 | 89.4 | 52.0 |
| 85.2 | 47.8 |
|
| Tumor number |
|
|
|
<0.001a |
|
|
<0.001a |
|
Single | 47 | 95.7 | 58.3 |
| 93.6 | 56.3 |
|
|
Multiple | 27 | 48.4 | 36.3 |
| 44.9 | 23.0 |
|
| Tumor capsule |
|
|
| 0.739 |
|
| 0.715 |
|
Complete | 52 | 76.5 | 44.0 |
| 72.6 | 44.3 |
|
|
Incomplete | 22 | 85.7 | 66.0 |
| 85.7 | 45.7 |
|
| Vascular
infiltration |
|
|
| 0.035a |
|
| 0.049a |
| No | 54 | 92.4 | 54.1 |
| 90.5 | 50.2 |
|
|
Yes | 20 | 45.0 | 40.0 |
| 45.0 | 30.0 |
|
| Edmondson
grade |
|
|
|
<0.001a |
|
|
<0.001a |
|
I/II | 48 | 93.7 | 56.4 |
| 93.7 | 54.5 |
|
|
III/IV | 26 | 46.3 | 37.9 |
| 42.6 | 25.6 |
|
| TNM Stage |
|
|
| 0.020a |
|
| 0.027a |
|
I/II | 45 | 93.3 | 54.1 |
| 91.1 | 47.3 |
|
|
III/IV | 29 | 48.5 | 40.7 |
| 45.2 | 32.9 |
|
| BCAT1 protein
level |
|
|
| 0.004a |
|
| 0.004a |
|
Low | 37 | 94.6 | 66.8 |
| 94.6 | 58.5 |
|
|
|
High | 37 | 60.3 | 33.2 |
| 57.7 | 30.5 |
|
Stratified univariate and multivariate
analysis
In a multivariate analysis model, the expression
levels of BCAT1 were significantly associated with the overall (HR,
2.546; 95% CI, 1.427–4.543; P=0.002) and disease-free (HR, 2.443;
95% CI, 1.392–4.290; P=0.002) survival rates (Table III). Multivariate analysis indicated
that the expression level of BCAT1, Edmonson-Steiner classification
and tumor number were all independent prognostic factors of HCC
(Table III).
 | Table III.Multivariate analysis of factors
contributing to overall and disease-free survival rates in patients
with hepatocellular carcinoma. |
Table III.
Multivariate analysis of factors
contributing to overall and disease-free survival rates in patients
with hepatocellular carcinoma.
|
| Overall survival
rate | Disease-free
survival rate |
|---|
|
|
|
|
|---|
| Variable | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Vascular
infiltration | 0.796
(0.300–2.116) | 0.648 | 0.785
(0.298–2.070) | 0.624 |
| Tumor number | 3.745
(1.162–12.064) | 0.027a | 3.337
(1.032–10.794) | 0.044a |
| TNM stage | 0.232
(0.052–1.044) | 0.057 | 0.252
(0.056–1.126) | 0.071 |
| Edmondson
grade | 4.321
(1.074–17.379) | 0.039a | 4.101
(1.026–16.382) | 0.046a |
| BCAT1 protein
level | 2.546
(1.427–4.543) | 0.002a | 2.443
(1.392–4.290) | 0.002a |
Association between BCAT1 and c-Myc
protein expression levels
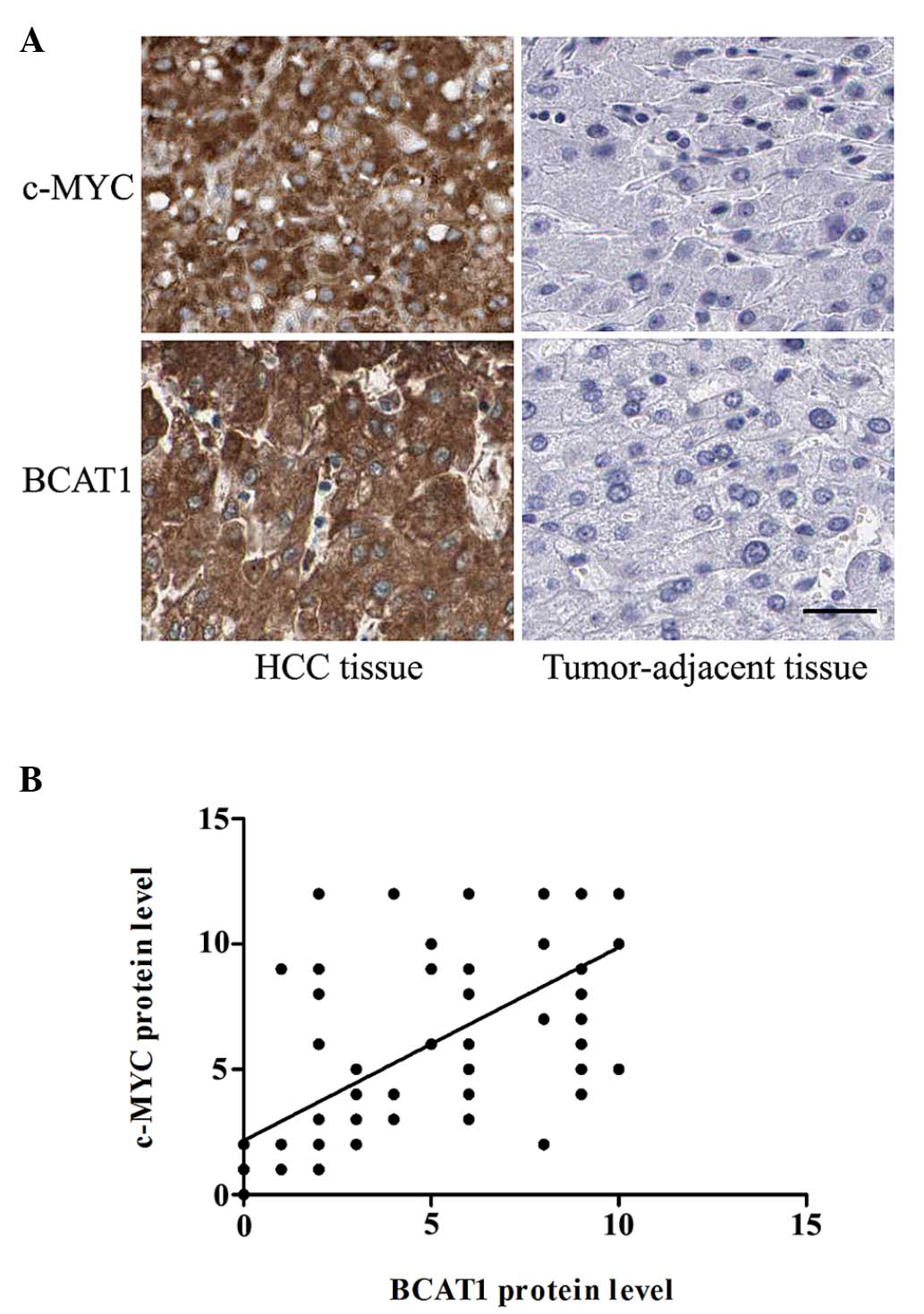
To determine whether the expression level of BCAT1
was correlated with c-Myc expression in patients with HCC,
immunohistochemical staining was performed. The protein expression
levels of c-Myc protein level were significantly higher in the HCC
tissues compared with the corresponding adjacent non-tumorous
tissues (P<0.001; Fig. 3A). In
addition, the correlation between BCAT1 and C-Myc protein
expression levels was analyzed. Notably, there was a significant
positive correlation between the protein expression levels of c-Myc
and BCAT1 (r=0.706; P<0.001; Fig.
3B).
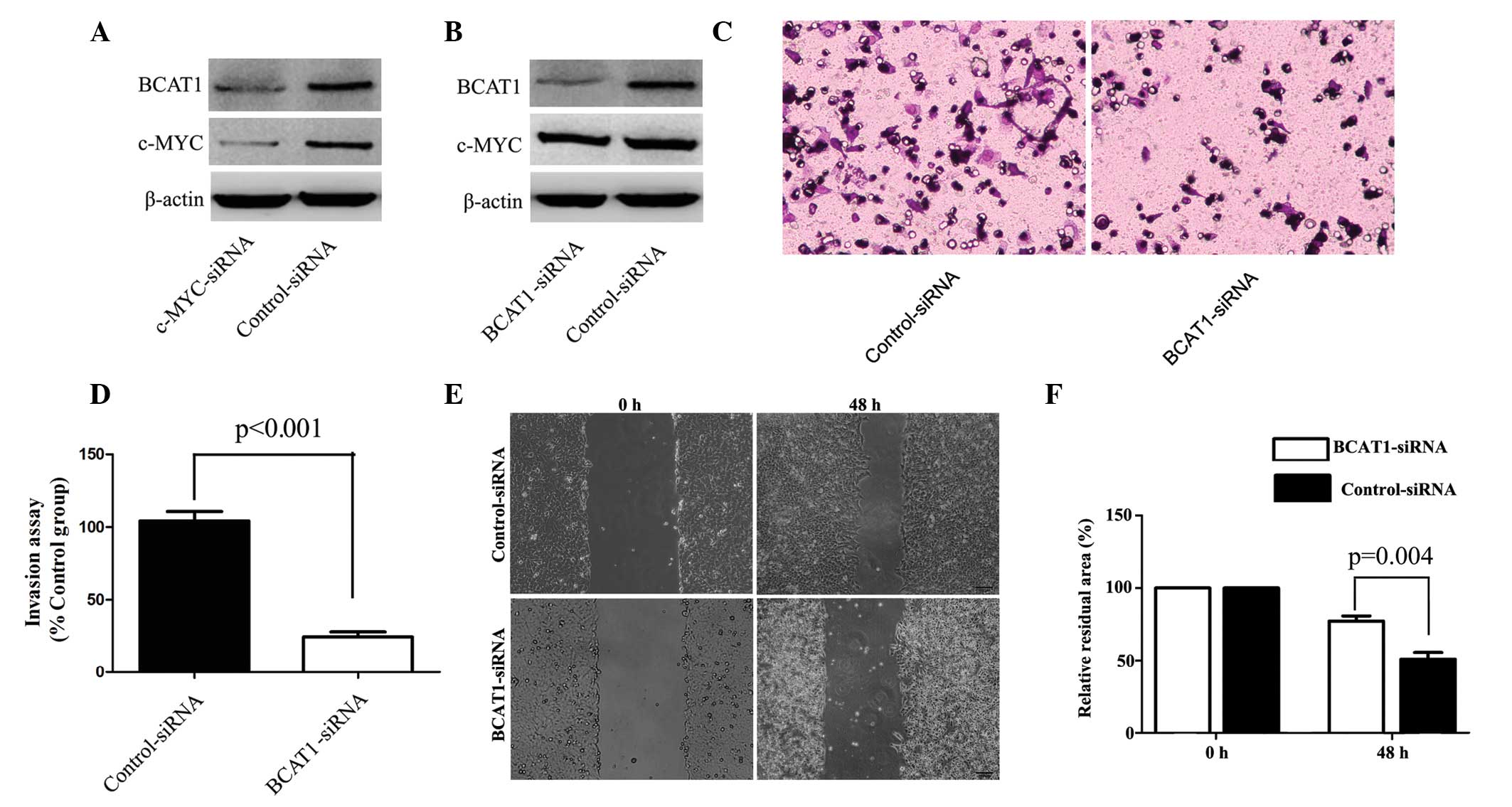
c-Myc-knockdown reduces BCAT1
expression
A previous study reported that c-Myc was able to
upregulate BCAT1 expression in nasopharyngeal carcinoma (11). Therefore, to further elucidate the
underlying mechanism of BCAT1 in HCC cells, MHCC-97H cells were
transfected with c-Myc-specific siRNA. Silencing of c-Myc
expression was shown to significantly downregulate the expression
of BCAT1 in MHCC-97H cells (P=0.005; Fig.
4A). Furthermore, BCAT1-specific siRNA was used to knockdown
the expression of BCAT1 in MHCC-97H cells. Compared with the
control group, the silencing of BCAT1 did not significantly alter
the protein expression levels of c-Myc (Fig. 4B).
BCAT1-knockdown suppresses cell
invasion and migration
To further investigate the underlying mechanism of
BCAT1 in HCC, the effect of BCAT1 on MHCC-97H cell migration and
invasion was investigated using BCAT1-specific and control siRNA.
Compared with the control group, BCAT1-knockdown significantly
repressed cell migration and invasion (Fig. 4C-F).
Discussion
At present, liver transplantation and radical liver
resection are the main curative treatments for patients with HCC
(24). However, the prognosis of HCC
remains unsatisfactory and the overall survival rate of affected
patients remains markedly low (25).
Therefore, elucidation of the mechanisms underlying the
pathogenesis of HCC is important in order to identify effective
prognostic and therapeutic biomarkers for the disease.
Increasingly, targeted therapy has exhibited encouraging outcomes
for numerous tumors (26–29); thus, efforts should be made to
identify novel tumor biomarkers with clinical utility for the
treatment of HCC.
BCAT1, which is the cytosolic form of the enzyme
BCAT, catalyzes the reversible transamination of branched-chain
α-keto acids to branched-chain L-amino acids essential for cell
growth (6–10). Previous studies identified positive
BCAT1 expression in c-Myc-induced tumors, and demonstrated that
BCAT1 was directly regulated by c-Myc through its binding to the
specific DNA sequence, CACGTG. c-Myc is an oncogene and
transcription factor involved in the tumorigenesis of numerous
cancers, including Burkitt's lymphoma and breast cancer.
Furthermore, it was reported that c-Myc upregulated BCAT1
expression, which in turn led to the proliferation, migration and
invasion of nasopharyngeal carcinoma (11). Although BCAT1 has been investigated in
several tumor types, including colorectal cancer (6), few previous studies have evaluated the
role of BCAT1 in HCC (30).
Considering that c-Myc regulates BCAT1 expression, the present
study aimed to verify whether c-Myc is involved in the expression
of BCAT1 in HCC tissues and cell lines. Since carcinogenesis is
characterized by uncontrolled cell growth and alterations in the
expression patterns of various molecules associated with the
modulation of cell migration and invasion (8,11–13), the identification of these molecules
is valuable for the development of effective therapeutic
strategies.
In the present study, the expression levels of BCAT1
in several cell lines were initially detected, and it was
demonstrated that the expression levels of BCAT1 in HCC cell lines
were significantly higher compared within the L-02 immortalized
normal human liver cell line. In addition, the expression levels of
BCAT1 in tumor tissues derived from a relatively large population
of HCC patients were determined, and it was shown that the
expression levels of BCAT1 were upregulated in HCC tissue compared
with tumor-adjacent tissues. Further studies demonstrated that the
expression levels of BCAT1 protein were positively correlated with
those of c-Myc, which indicated that c-Myc may be partially
responsible for the high expression levels of BCAT1 in HCC tissues
and cell lines. For better elucidation of the role and underlying
mechanisms of BCAT1 in HCC cells, the effects of c-Myc- and
BCAT1-knockdown on MHCC-97H cells were investigated. As was
expected, c-Myc-knockdown was found to downregulate BCAT1
expression in MHCC-97H cells. Furthermore, the expression of BCAT1
was associated with the biological characteristics of HCC cells,
since it was demonstrated that knockdown of BCAT1 expression
repressed the migration and invasion of MHCC-97H cells. Taken
together, these results support the hypothesis that BCAT1 has a
critical role in the migration and invasion of HCC, and that its
expression may be regulated by c-Myc. Therefore, BCAT1 may serve as
a potential biomarker for the diagnosis and treatment of HCC.
In the present study, the associations between the
expression levels of BCAT1 and the clinicopathological parameters
and prognosis of patients with HCC were analyzed. It was
demonstrated that the upregulation of BCAT1 was significantly
correlated with lower overall and disease-free survival rates, and
other clinicopathological parameters, including the
Edmondson-Steiner grade, tumor number, vascular invasion and TNM
stage.
In conclusion, the present study demonstrated that
BCAT1 was upregulated in HCC tissue samples and cell lines compared
with normal adjacent tissue samples and the L-02 immortalized
normal human liver cell line, respectively. Furthermore, the BCAT1
expression level was positively correlated with c-Myc expression,
and knockdown of c-Myc in HCC cells resulted in the downregulation
of BCAT1. In addition, knockdown of BCAT1 expression was shown to
repress the migration and invasion of an HCC cell line. The results
of the present study suggested that BCAT1 is important for the
migration and invasion of HCC and may represent a novel prognostic
biomarker for the disease.
Acknowledgements
The present study was supported by grants from the
National Natural Science Foundation of China (nos. 81402039,
81272645 and 81301743).
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
El-Serag HB, Davila JA, Petersen NJ and
McGlynn KA: The continuing increase in the incidence of
hepatocellular carcinoma in the United States: An update. Ann
Intern Med. 139:817–823. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shariff MI, Cox IJ, Gomaa AI, Khan SA,
Gedroyc W and Taylor-Robinson SD: Hepatocellular carcinoma: Current
trends in worldwide epidemiology, risk factors, diagnosis and
therapeutics. Expert Rev Gastroenterol Hepatol. 3:353–367. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dhanasekaran R, Limaye A and Cabrera R:
Hepatocellular carcinoma: Current trends in worldwide epidemiology,
risk factors, diagnosis, and therapeutics. Hepat Med. 4:19–37.
2012.PubMed/NCBI
|
|
5
|
Zheng X, Song T, Dou C, Jia Y and Liu Q:
CtBP2 is an independent prognostic marker that promotes GLI1
induced epithelial-mesenchymal transition in hepatocellular
carcinoma. Oncotarget. 6:3752–3769. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yoshikawa R, Yanagi H, Shen CS, Fujiwara
Y, Noda M, Yagyu T, Gega M, Oshima T, Yamamura T, Okamura H, et al:
ECA39 is a novel distant metastasis-related biomarker in colorectal
cancer. World J Gastroenterol. 12:5884–5889. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Schuldiner O, Eden A, Ben-Yosef T, Yanuka
O, Simchen G and Benvenisty N: ECA39, a conserved gene regulated by
c-Myc in mice, is involved in G1/S cell cycle regulation in yeast.
Proc Natl Acad Sci USA. 93:7143–7148. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Eden A and Benvenisty N: Involvement of
branched-chain amino acid aminotransferase (Bcat1/Eca39) in
apoptosis. FEBS Lett. 457:255–261. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bledsoe RK, Dawson PA and Hutson SM:
Cloning of the rat and human mitochondrial branched chain
aminotransferases (BCATm). Biochim Biophys Acta. 1339:9–13. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Eden A, Simchen G and Benvenisty N: Two
yeast homologs of ECA39, a target for c-Myc regulation, code for
cytosolic and mitochondrial branched-chain amino acid
aminotransferases. J Biol Chem. 271:20242–20245. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhou W, Feng X, Ren C, Jiang X, Liu W,
Huang W, Liu Z, Li Z, Zeng L, Wang L, et al: Over-expression of
BCAT1, a c-Myc target gene, induces cell proliferation, migration
and invasion in nasopharyngeal carcinoma. Mol Cancer. 12:532013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bible E: Neuro-oncology: BCAT1 promotes
cell proliferation in aggressive gliomas. Nat Rev Neurol.
9:4202013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zerban H, Radig S, Kopp-Schneider A and
Bannasch P: Cell proliferation and cell death (apoptosis) in
hepatic preneoplasia and neoplasia are closely related to
phenotypic cellular diversity and instability. Carcinogenesis.
15:2467–2473. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tönjes M, Barbus S, Park YJ, Wang W,
Schlotter M, Lindroth AM, Pleier SV, Bai AH, Karra D, Piro RM, et
al: BCAT1 promotes cell proliferation through amino acid catabolism
in gliomas carrying wild-type IDH1. Nat Med. 19:901–908. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Greco D, Kotronen A, Westerbacka J, Puig
O, Arkkila P, Kiviluoto T, Laitinen S, Kolak M, Fisher RM, Hamsten
A, et al: Gene expression in human NAFLD. Am J Physiol Gastrointest
Liver Physiol. 294:G1281–G1287. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Matsumura T, Morinaga Y, Fujitani S,
Takehana K, Nishitani S and Sonaka I: Oral administration of
branched-chain amino acids activates the mTOR signal in cirrhotic
rat liver. Hepatol Res. 33:27–32. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Honda M, Takehana K, Sakai A, Tagata Y,
Shirasaki T, Nishitani S, Muramatsu T, Yamashita T, Nakamoto Y,
Mizukoshi E, et al: Malnutrition impairs interferon signaling
through mTOR and FoxO pathways in patients with chronic hepatitis
C. Gastroenterology. 141:128–140, 140.e1-e2. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ben-Yosef T, Yanuka O, Halle D and
Benvenisty N: Involvement of Myc targets in c-myc and N-myc induced
human tumors. Oncogene. 17:165–171. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ben-Yosef T, Eden A and Benvenisty N:
Characterization of murine BCAT genes: Bcat1, a c-Myc target, and
its homolog, Bcat2. Mamm Genome. 9:595–597. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Peng SY, Lai PL and Hsu HC: Amplification
of the c-myc gene in human hepatocellular carcinoma: Biologic
significance. J Formos Med Assoc. 92:866–870. 1993.PubMed/NCBI
|
|
21
|
Liu Q, Tu K, Zhang H, Zheng X, Yao Y and
Liu Q: TPX2 as a novel prognostic biomarker for hepatocellular
carcinoma. Hepatol Res. 45:906–18. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Edmondson HA and Steiner PE: Primary
carcinoma of the liver: A study of 100 cases among 48,900
necropsies. Cancer. 7:462–504. 1954. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Colombo M and Sangiovanni A: Treatment of
hepatocellular carcinoma: Beyond international guidelines. Liver
Int. 35(Suppl 1): S129–S138. 2015. View Article : Google Scholar
|
|
25
|
Bruix J and Sherman M: American
Association for the Study of Liver Diseases: Management of
hepatocellular carcinoma: An update. Hepatology. 53:1020–1022.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Roberts LR and Gores GJ: Hepatocellular
carcinoma: Molecular pathways and new therapeutic targets. Semin
Liver Dis. 25:212–225. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Scaggiante B, Kazemi M, Pozzato G, Dapas
B, Farra R, Grassi M, Zanconati F and Grassi G: Novel
hepatocellular carcinoma molecules with prognostic and therapeutic
potentials. World J Gastroenterol. 20:1268–1288. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Satow R, Shitashige M, Kanai Y, Takeshita
F, Ojima H, Jigami T, Honda K, Kosuge T, Ochiya T, Hirohashi S and
Yamada T: Combined functional genome survey of therapeutic targets
for hepatocellular carcinoma. Clin Cancer Res. 16:2518–2528. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Schattenberg JM, Schuchmann M and Galle
PR: Cell death and hepatocarcinogenesis: Dysregulation of apoptosis
signaling pathways. J Gastroenterol Hepatol. 26(Suppl 1):
S213–S219. 2011. View Article : Google Scholar
|
|
30
|
Elsemman IE, Mardinoglu A, Shoaie S,
Solima TH and Nielsen J: Systems biology analysis of hepatitis C
virus infection reveals the role of copy number increases in
regions of chromosome 1q in hepatocellular carcinoma metabolism.
Mol Biosyst. 12:1496–1506. 2016. View Article : Google Scholar : PubMed/NCBI
|