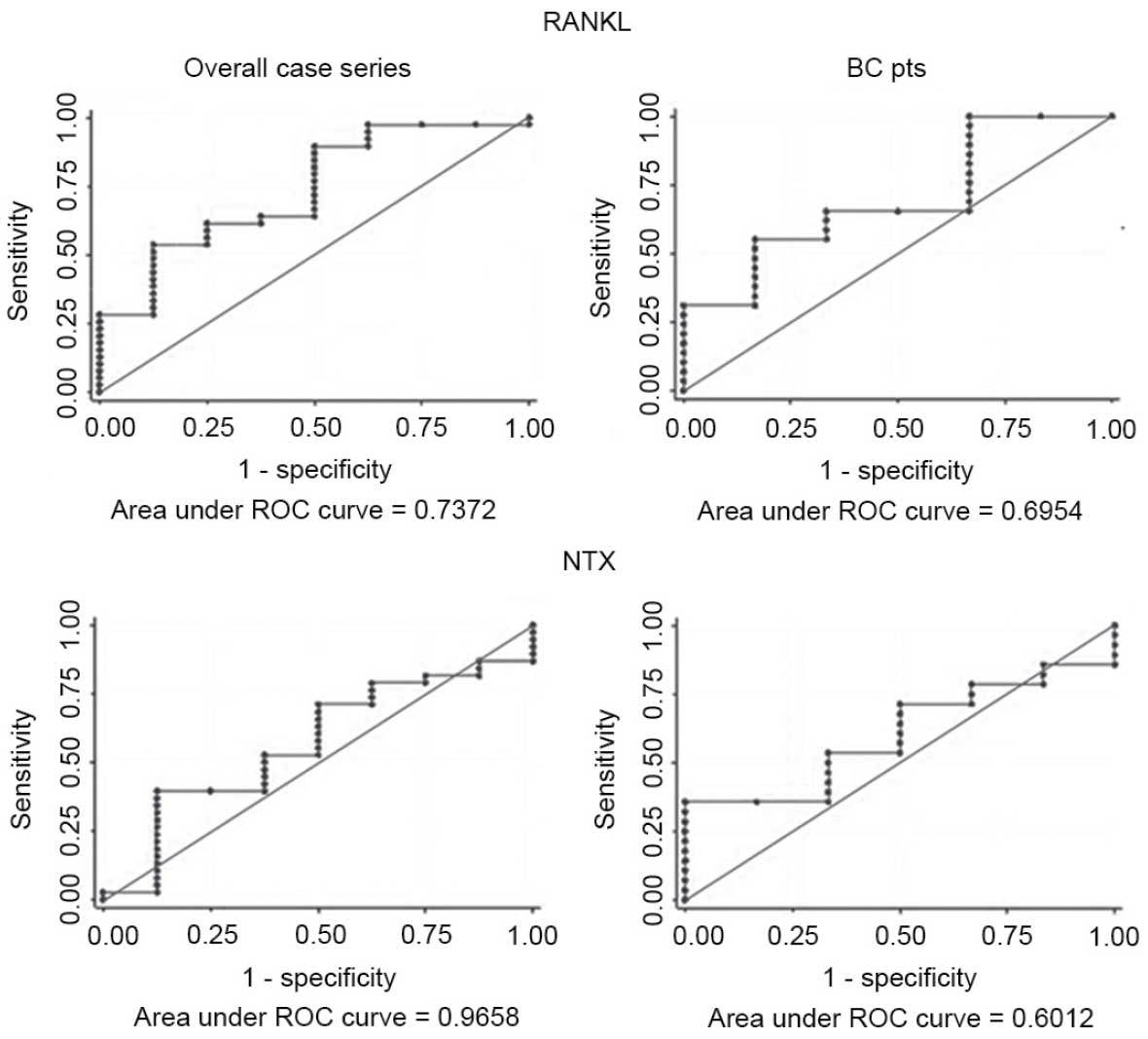
|
1
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J and
Thun MJ: Cancer statistics, 2009. CA Cancer J Clin. 59:225–249.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Harries M, Taylor A, Holmberg L, Agbaje O,
Garmo H, Kabilan S and Purushotham A: Incidence of bone metastases
and survival after a diagnosis of bone metastases in breast cancer
patients. Cancer Epidemiol. 38:427–434. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ibrahim T, Mercatali L and Amadori D: Bone
and cancer: The osteoncology. Clin Cases Miner Bone Metab.
10:121–123. 2013.PubMed/NCBI
|
|
4
|
Ibrahim T, Mercatali L, Casadei R and
Sabbatini R: Clinical manifestationOsteoncology textbook. Amadori
D, Cascinu S, Conte P and Ibrahim T: Poletto editore; Milan: pp.
pp258–pp276. 2010
|
|
5
|
Mundy GR: Metastasis to bone: Causes,
consequences and therapeutic opportunities. Nat Rev Cancer.
2:584–593. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ibrahim T, Flamini E, Fabbri L, Serra P,
Mercatali L, Ricci R, Sacanna E, Falasconi MC, Casadei R, Galassi
R, et al: Multidisciplinary approach to the treatment of bone
metastases: Osteo-oncology center, a new organizational model.
Tumori. 95:291–297. 2009.PubMed/NCBI
|
|
7
|
Lipton A, Theriault RL, Hortobagyi GN,
Simeone J, Knight RD, Mellars K, Reitsma DJ, Heffernan M and Seaman
J: Pamidronate prevents skeletal complications and its effective
palliative treatment in women with breast carcinoma and osteolytic
bone metastases: Long term follow-up of two randomized,
placebo-controlled trials. Cancer. 88:1082–1090. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Saad F: Clinical benefit of zoledronic
acid for the prevention of skeletal complications in advanced
prostate cancer. Clin Prostate Cancer. 4:31–37. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Berenson JR, Rosen LS, Howell A, Porter L,
Coleman RE, Morley W, Dreicer R, Kuross SA, Lipton A and Seaman JJ:
Zoledronic acid reduces skeletal-related events in patients with
osteolytic metastases. Cancer. 91:1191–2000. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Amadori D, Aglietta M, Alessi B, Gianni L,
Ibrahim T, Farina G, Gaion F, Bertoldo F, Santini D, Rondena R, et
al: Efficacy and safety of 12-weekly versus 4-weekly zoledronic
acid for prolonged treatment of patients with bone metastases from
breast cancer (ZOOM): A phase 3, open-label, randomised,
non-inferiority trial. Lancet Oncol. 14:663–670. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lipton A, Fizazi K, Stopeck AT, Henry DH,
Brown JE, Yardley DA, Richardson GE, Siena S, Maroto P, Clemens M,
et al: Superiority of denosumab to zoledronic acid for prevention
of skeletal-related events: A combined analysis of 3 pivotal,
randomised, phase 3 trials. Eur J Cancer. 48:3082–3092. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Costelloe CM, Chuang HH, Madewell JE and
Ueno NT: Cancer response criteria and bone metastases: RECIST 1.1,
MDA and PERCIST. J Cancer. 1:80–92. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Coleman R, Brown J, Terpos E, Lipton A,
Smith MR, Cook R and Major P: Bone markers and their prognostic
value in metastatic bone disease: Clinical evidence and future
directions. Cancer Treat Rev. 34:629–639. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Aktas B, Kasimir-Bauer S, Lehmann N,
Kimmig R and Tewes M: Validity of bone marker measurements for
monitoring response to bisphosphonate therapy with zoledronic acid
in metastatic breast cancer. Oncol Rep. 30:441–447. 2013.PubMed/NCBI
|
|
15
|
Mountzios G, Terpos E, Syrigos K,
Papadimitriou C, Papadopoulos G, Bamias A, Mavrikakis M and
Dimopoulos MA: Markers of bone remodeling and skeletal morbidity in
patients with solid tumors metastatic to the skeleton receiving the
biphosphonate zoledronic acid. Transl Res. 155:247–255. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ulrich U, Rhiem K, Schmolling J, Flaskamp
C, Paffenholz I, Sälzer H, Bauknecht T and Schlebusch H:
Cross-linked type I collagen C- and N-telopeptides in women with
bone metastases from breast cancer. Arch Gynecol Obstet.
264:186–190. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lipton A, Cook R, Saad F, Major P, Garnero
P, Terpos E, Brown JE and Coleman RE: Normalization of bone markers
is associated with improved survival in patients with bone
metastases from solid tumors and elevated bone resorption receiving
zoledronic acid. Cancer. 113:193–201. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ibrahim T, Sacanna E, Gaudio M, Mercatali
L, Scarpi E, Zoli W, Serra P, Ricci R, Serra L, Kang Y and Amadori
D: Role of RANK, RANKL, OPG and CXCR4 tissue markers in predicting
bone metastases in breast cancer patients. Clin Breast Cancer.
11:369–375. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mercatali L, Ricci M, Scarpi E, Serra P,
Fabbri F, Ricci R, Liverani C, Zanoni M, Zoli W, Maltoni R, et al:
RANK/RANK-L/OPG in patients with bone metastases treated with
anticancer agents and zoledronic acid: A prospective study. Int J
Mol Sci. 14:10683–10693. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ibrahim T, Flamini E, Mercatali L, Sacanna
E, Serra P and Amadori D: Pathogenesis of osteoblastic bone
metastases from prostate cancer. Cancer. 116:1406–1418. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ibrahim T, Mercatali L and Amadori D: A
new emergency in oncology: Bone metastases in breast cancer
patients (Review). Oncol Lett. 6:306–310. 2013.PubMed/NCBI
|
|
22
|
Drooger JC, van der Padt A, Sleijfer S and
Jager A: Denosumab in breast cancer treatment. Eur J Pharmacol.
717:12–19. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hayashi N, Costelloe CM, Hamaoka T, Wei C,
Niikura N, Theriault RL, Hortobagyi GN, Madewell JE and Ueno NT: A
prospective study of bone tumor response assessment in metastatic
breast cancer. Clin Breast Cancer. 13:24–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Amadori D, Mercatali L, Nanni O, Aglietta
M, Alessi B, Gianni L, Farina G, Gaion F, Bertoldo F, Santini D, et
al: What can we learn from the ZOOM trial?-Authors' reply. Lancet
Oncol. 14:e388–e390. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Coleman RE, Major P, Lipton A, Brown JE,
Lee KA, Smith M, Saad F, Zheng M, Hei YJ, Seaman J, et al:
Predictive value of bone resorption and formation markers in cancer
patients with bone metastases receiving the bisphosphonate
zoledronic acid. J Clin Oncol. 23:4925–4935. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Brown JE, Cook RJ, Major P, Lipton A, Saad
F, Smith M, Lee KA, Zheng M, Hei YJ and Coleman RE: Bone turnover
markers as predictors of skeletal complications in prostate cancer,
lung cancer and other solid tumors. J Natl Cancer Inst. 97:59–69.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Coleman R, Costa L, Saad F, Cook R, Hadji
P, Terpos E, Garnero P, Brown J, Body JJ, Smith M, et al: Consensus
on the utility of bone markers in the malignant bone disease
setting. Crit Rev Oncol Hematol. 80:411–432. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Weilbaecher KN, Guise TA and McCauley LK:
Cancer to bone: A fatal attraction. Nat Rev Cancer. 11:411–425.
2011. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tamiya M, Tokunaga S, Okada H, Suzuki H,
Kobayashi M, Sasada S, Okamoto N, Morishita N, Matsuura Y, Miyamoto
N, et al: Prospective study of urinary and serum cross-linked
N-telopeptide of type I collagen (NTx) for diagnosis of bone
metastasis in patients with lung cancer. Clin Lung Cancer.
14:364–369. 2013. View Article : Google Scholar : PubMed/NCBI
|