Introduction
Testicular germ cell tumors (TGCTs) represent the
most common type of cancer in young men (1). The highest incidence of the disease is
between 15 and 35 years of age (2).
Due to high sensitivity to chemotherapy, the majority of TGCT
patients with metastatic disease may expect to be cured with first
line cisplatin-based chemotherapy. TGCTs are therefore considered
as a model of curable malignancy. However, there are ~20% of
patients who fail to be cured following the first line treatment
(3,4).
Thus, delineation of the molecular basis of insufficient
chemotherapy effect in relapsed patients may enable the
identification of novel biomarkers and prognostic factors, which
may be used as an effective tool for better stratification of
patients prior to treatment.
It is well established that hypoxia and an hypoxic
microenvironment are key factors in cancer pathogenesis (4). Hypoxic microenvironments are frequently
associated with increased disease aggressiveness, tumor
dissemination and poor prognosis (5–9). The main
adaptive response of tumor cells to an hypoxic microenvironment is
mediated by hypoxia inducible factor-1 (HIF-1) transcription
factor, which is stabilized and activated under hypoxic conditions
(10). HIF-1 binds to
hypoxia-responsive elements in its target genes, including those
encoding erythropoietin, vascular endothelial growth factor and
matrix metalloproteinases, and is therefore implicated in several
important processes involved in tumor biology, including cell
proliferation, angiogenesis, cell metabolism, apoptosis and
migration (5,11). One of the HIF-1 transactivated genes
codes for carbonic anhydrase IX (CA IX), a zinc metalloenzyme
catalyzing the reversible hydration of carbon dioxide to carbonic
acid and participating in pH regulation as well as in cell
adhesion-migration-invasion (12).
Thus, CA IX plays a role in maintaining the normal intracellular pH
in tumor tissue under hypoxic conditions. Furthermore, increased CA
IX expression is associated with treatment resistance and cancer
stem cell properties (13–15). As cancer stem cells in TGCTs resemble
embryonic stem cells (16,17), the present authors supposed that CA IX
could be involved in the pathogenesis of TGCTs. Several studies
demonstrated the prognostic value of tissue CA IX in different
types of cancer, including cervical (6,18), ovarian
(19), hepatocellular (20), lung and renal cancer (21,22).
Peripheral blood is easily accessible and enables
repeated examination in the course of the disease. In several
studies evaluating the clinical utility of soluble CA IX levels in
cancer patients, high serum or plasma CA IX levels were associated
with an inferior outcome in cervical and vulvar cancer, oral
squamous cell carcinoma, non-small cell lung cancer and metastatic
breast cancer (23–27). The aim of the present study was to
evaluate CA IX levels in serum and tumor samples from a cohort of
chemotherapy-naive, chemotherapy-pretreated and relapsed TGCTs
patients in order to investigate the potential role of CA IX as a
non-invasive prognostic biomarker in TGCT.
Patients and methods
Study patients
The current prospective translational study
(protocol IZLO1, chair Dr M. Mego) was approved by the
Institutional Review Board (IRB) of the National Cancer Institute
(Bratislava, Slovakia), and was conducted between March 2011 and
April 2013. All consecutive patients with TGCTs treated with ≥1
cycle of cisplatin-based chemotherapy in the National Cancer
Institute or St. Elizabeth Cancer Institute (Bratislava, Slovakia)
were enrolled in the study. Serum samples from 35 healthy
individuals were used as a control group. Data regarding age, tumor
histological subtype, clinical stage, type and number of sites of
metastasis and type of chemotherapy regimen were recorded for all
patients and compared with CA IX expression. TGCTs patients and
controls were recruited and provided informed consent according to
the IRB approved protocol.
Serum samples collection
Peripheral blood samples were collected from all
participants in the present translational study. Samples were
collected into Vacutainer® Rapid Serum Tubes (BD
Biosciences, Franklin Lakes, NJ, USA) containing silica clot
activator polymer gel at baseline in the morning on days 1 or 0 of
the first cycle of chemotherapy (n=73) and prior to the second
cycle of chemotherapy (n=37), or before starting a new line of
salvage chemotherapy in patients with relapsed disease (n=10).
Patients' blood samples (1 ml) were centrifuged at 2,800 × g for 10
min to separate the serum from the blood cells. Serum aliquots were
stored at −80°C until further analysis.
CA IX enzyme-linked immunosorbent
assay (ELISA)
ELISA for the quantitative determination of serum CA
IX concentration was performed at room temperature. The microplate
wells were coated overnight with 100 µl/well of the anti-CA IX
ectodomain (ECD) monoclonal antibody (MAb) that had been purified
in a previous study (28). The MAb
was diluted in phosphate-buffered saline (PBS) to a concentration
of 10 µg/ml. Non-specific binding was blocked with PBS containing
1% bovine serum albumin (Sigma-Aldrich, St. Louis, MO, USA) and
0.05% Tween 20 for 2 h, and washed twice with PBS+0.05% Tween 20
(PBST). Serum samples diluted in PBST were added together with the
peroxidase-conjugated MAb (diluted to 1:10,000 in PBST), which was
created in-house in our previous study (28). Each component was added in a volume of
100 µl/well, and incubated for 3 h. Tested samples were washed
three times with PBST. The bound MAb used for detection was
visualized by addition of 10 mg o-phenylenediamine substrate
(Sigma-Aldrich) with 10 µl H2O2 in citrate
buffer (pH 5) for 5–15 min in the dark. Absorbance was measured at
492 nm. Data were processed, and the CA IX ECD was quantitated
based on a calibration curve obtained using our in-house generated
recombinant CA IX ECD (amino acids 38–406)-polyhistidine-tag fusion
protein, which was used as a standard.
Diagnosis and tumor samples
The study included available tumor specimens
resected from 17 patients prior to the administration of
cisplatin-based chemotherapy, namely from 13 (76.5%) patients with
primary testicular tumors and 4 (23.5%) patients with
retroperitoneal germ cell tumors. All specimens were classified
according to the most recent World Health Organization
classification from 2004 (29).
Tumor pathology
A pathology review was performed at the Department
of Pathology, Faculty of Medicine, Comenius University (Bratislava,
Slovakia), by two pathologists (Z.C. and P.B.) associated with the
study. All specimens were assessed by light microscopy following
hematoxylin and eosin (HE) staining, and with the use of
immunohistochemical markers typical for germ cell tumors.
Tissue microarray construction
From each histological subtype, 1–2 representative
tumor areas were identified on HE-stained sections, according to
tumor histology. If normal testicular tissue samples were present,
they were also marked. Sections were matched to the donor blocks
(corresponding wax blocks). Tumor cores (3-mm diameter) were
removed from the donor blocks using the multipurpose sampling tool
Harris Uni-Core (Sigma-Aldrich) and inserted into the recipient
master block. The recipient block was cut into 5-µm sections, and
sections were transferred to FLEX IHC Microscope slides (cat. no.
K8020; Dako, Glostrup, Denmark).
Immunohistochemistry (IHC)
Deparaffinized slides were rehydrated and immersed
in PBS (10 mM, pH 7.2). Tissue epitopes were demasked using the
automated water bath heating process in PT Link (Dako). Briefly,
the slides were incubated in Tris-ethylenediaminetetraacetic acid
(EDTA) retrieval solution (10 mM Tris, 1 mM, pH 9.0) at 98°C for 20
min. CA IX expression was detected by IHC using the in-house
generated monoclonal antibody M75 against the N-terminal domain of
human CA IX, as described previously (30,31).
Slides were incubated for 60 min at room temperature with the
aforementioned primary antibody diluted to 1:100 in REAL Antibody
Diluent (Dako) and immunostained using an anti-mouse/anti-rabbit
immunoperoxidase polymer (EnVision FLEX/HRP; Dako) for 30 min at
room temperature, according to the manufacturer's protocol. The
reaction was visualized with a 3,3-diaminobenzidine
substrate-chromogen solution (Dako Cytomation; Dako) for 5 min, and
slides were counter-stained with hematoxylin. Clear cell renal cell
carcinoma tissue was used as a positive control. As a negative
control, the same tumor tissue was used, but omitting the primary
antibody from the staining protocol.
IHC scoring
Two observers (Z.C. and P.B.), who were blinded to
the patients clinicopathological data, independently assessed the
tumor cores. In cases of disagreement, the result was reached by
consensus. CA IX expression was stratified as negative or positive
(any staining).
Statistical analyses
Patients' data were tabulated. The patients'
characteristics were summarized using the mean ± standard error of
the mean or median (range) for continuous variables, and frequency
(percentage) for categorical variables. Statistical analysis was
performed using non-parametric tests, as the distribution of CA IX
expression was significantly different from a normal distribution
(Shapiro-Wilk test). The Mann-Whitney U test was used for analysis
of the association between serum CA IX expression and
clinicopathological variables in 2 groups of patients, while the
Kruskal-Wallis test was used to compare >2 groups. The Wilcoxon
test was used to compare the serum CA IX level prior to the first
and second cycles of chemotherapy.
The median follow-up period was calculated as a
median observation time among all patients and among those who were
still alive at the time of their last follow-up. Progression-free
survival (PFS) was calculated from the date of treatment initiation
with cisplatin-based chemotherapy to the date of progression,
mortality or last follow-up. Overall survival (OS) was calculated
from the date of treatment initiation with cisplatin-based
chemotherapy to the date of mortality or last follow-up. Survival
rates were estimated using the Kaplan-Meier product limit method,
and were compared with the log-rank test to determine their
significance. CA IX expression data were dichotomized into high and
low groups based on the CA IX expression median value of all
samples. The Spearman correlation coefficient was used to examine a
potential correlation between CA IX serum concentration and lactate
dehydrogenase (LDH), human chorionic gonadotropin (HCG) and
alpha-fetoprotein (AFP) levels as well as CA IX expression in tumor
specimens. All statistical tests were two-sided, and P<0.05 was
considered to indicate a statistical significant difference.
Statistical analyses were performed using NCSS 2007 software (NCSS,
LLC, Kaysville, UT, USA).
Results
Patients' characteristics
From March 2011 to April 2013, 83 patients,
including 16 non-metastatic patients subjected to orchiectomy with
no evidence of disease (group 1), 57 metastatic chemotherapy-naïve
patients (group 2) and 10 metastatic relapsed
chemotherapy-pretreated patients (group 3), who were starting
adjuvant and/or a new line of chemotherapy were registered to
participate in the present study at the National Cancer Institute
and St. Elizabeth Cancer Institute of Slovakia. The characteristics
of the patients are shown in Table
I.
 | Table I.Patients' characteristics (n=83). |
Table I.
Patients' characteristics (n=83).
|
| Chemotherapy-naïve
TGCTs |
Chemotherapy-pretreated relapsed
TGCTs |
|---|
|
|
|
|
|---|
|
Characteristics | N=73 | % | N=10 | % |
|---|
| Age, years |
|
|
|
|
| Median
(range) | 34 (19–67) | 34 (24–50) |
| Primary tumor |
|
|
|
|
|
Gonadal | 70 | 95.9 | 9 | 90.0 |
|
Retroperitoneal | 3 | 4.1 | 1 | 10.0 |
|
Histologya |
|
|
|
|
|
Seminoma | 12 | 16.4 | 3 | 30.0 |
|
Non-seminoma | 60 | 82.2 | 7 | 70.0 |
| Stage of TGCTs |
|
|
|
|
|
I.A-I.B | 16 | 21.9 |
0c |
0.0 |
|
I.S | 6 | 8.2 | 0 |
0.0 |
|
II.A-III.A | 27 | 37.0 | 2 | 20.0 |
|
III.B | 13 | 17.8 | 2 | 20.0 |
|
III.C | 11 | 15.1 | 6 | 60.0 |
| Sites of
metastasesb |
|
|
|
|
|
Retroperitoneum | 48 | 84.2 | 10 | 100.0 |
|
Mediastinum | 9 | 15.8 | 0 |
0.0 |
|
Lung | 18 | 31.6 | 7 |
70.0 |
|
Liver | 4 | 7.0 | 1 |
10.0 |
|
Brain | 2 | 3.5 | 0 |
0.0 |
|
Other | 5 | 8.8 | 2 |
20.0 |
|
Visceral non-pulmonary | 7 | 12.3 | 1 |
10.0 |
| IGCCCG risk
groupb |
|
|
|
|
| Good
prognosis | 36 | 63.2 | NA | NA |
|
Intermediate prognosis | 11 | 19.3 | NA | NA |
| Poor
prognosis | 10 | 17.5 | NA | NA |
| Mean (range) |
|
|
|
|
| AFP,
mIU/ml | 869.9
(0.9–13,936.0)b | 10,376.2
(1.6–89,954.0) |
| HCG,
IU/ml | 81,602.5
(0.0–1,840,510.0)b | 53,742.2
(0.1–480,259.0) |
| LDH,
mkat/l | 11.8
(1.8–76.0)b | 11.3
(2.3–33.2) |
The majority of patients had non-seminoma histology
and primary testicular cancer. The majority of chemotherapy-naïve
patients (61; 83.6%) were treated with the BEP (bleomycin,
etoposide, cisplatin) regimen; 8 patients (11.0%) received EP
(etoposide, cisplatin) chemotherapy, while 2 patients (2.7%)
received VIP (ifosfamide, etoposide, cisplatin) chemotherapy and 2
patients (2.7%) were treated with dose-dense chemotherapy, as
described previously (32,33). Patients with relapsed disease were
pretreated with ≥2 lines of cisplatin-based chemotherapy, with a
median of 3 lines of treatment. Of the 10 patients, 3 (30.0%) were
platinum resistant, while 7 (70.0%) had platinum-sensitive
disease.
Association between serum CA IX level
and patients/tumor characteristics
The mean serum level of CA IX in TGCT patients was
significantly higher compared with healthy controls (405.2±90.1 vs.
249.6±100.0 pg/ml; P=0.007). Metastatic chemotherapy-naïve patients
had significantly higher mean serum levels of CA IX compared with
serum samples of an independent group of healthy individuals
(490.6±111.8 vs. 249.6±100.0 pg/ml; P=0.005), whereas there was no
significant difference in the mean serum CA IX levels between
metastatic relapsed chemotherapy-pretreated patients and healthy
controls (216.0±170.2 vs. 249.6±100.0 pg/ml; P=0.370). Similarly,
the mean serum levels of CA IX in non-metastatic upon orchiectomy
with no evidence of disease patients were not significantly
different compared with those in healthy donors (218.9±128.8 vs.
249.6±100.0 pg/ml; P=0.080).
There was no significant difference in the mean CA
IX serum levels between non-metastatic, metastatic
chemotherapy-naïve and chemotherapy-pretreated TGCT patients
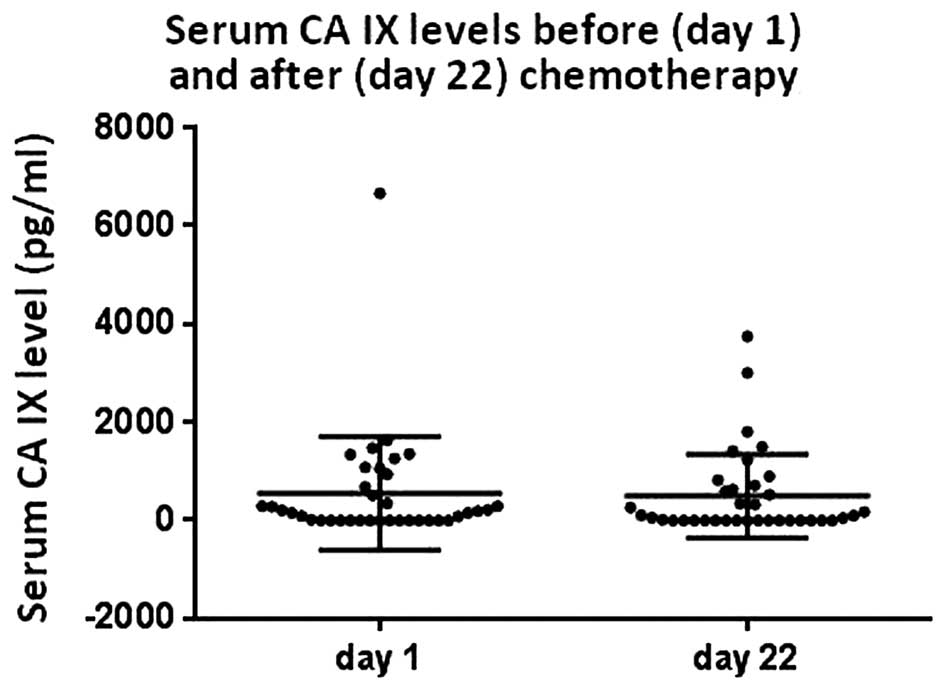
(Fig. 1). For 37 chemotherapy-naïve
patients, the serum was available for the CA IX measurement on days
1 and 2. There was no significant difference in the median CA IX
level in serum prior to chemotherapy (157.8 pg/ml) and prior to the
second cycle of chemotherapy (52.1 pg/ml, P=0.360) (Fig. 2).
 | Figure 1.Serum CA IX in different groups of
TGCT patients (group 1, adjuvant chemotherapy-naïve patients, n=16;
group 2, metastatic chemotherapy-naïve patients, n=57; group 3,
relapsed chemotherapy-pretreated patients, n=10). The median level
of CA IX in serum in groups 1, 2 and 3 was 93.7, 186.7 and 141.7
pg/ml, respectively (P=0.630). CA IX, carbonic anhydrase IX; TGCT,
testicular germ cell tumor. |
The associations between the histological subtypes
of the primary germ cell tumors and the mean CA IX levels detected
by ELISA in the patients' serum samples are summarized in Table II. No significant correlation was
observed between the mean serum CA IX levels and
clinicopathological variables (Table
III). The Spearman's correlation coefficient (rho) between the
mean serum CA IX concentration and LDH, HCG and AFP levels was
−0.11 (P=0.440), −0.20 (P=0.180) and 0.12 (P=0.430),
respectively.
 | Table II.CA IX concentration in serum of
metastatic chemotherapy-naïve patients with different histological
subtypes of primary germ cell tumors (n=55). |
Table II.
CA IX concentration in serum of
metastatic chemotherapy-naïve patients with different histological
subtypes of primary germ cell tumors (n=55).
|
| Serum CA IX level
(pg/ml) |
|---|
|
|
|
|---|
| Histological
subtypea | N | Mean | SEM | Median | P-value |
|---|
| Seminoma | 25 | 417.5 | 196.3 | 207.8 | 0.830 |
| Embryonal
carcinoma | 26 | 644.8 | 189.7 | 198.7 | 0.570 |
| Yolk sac tumor | 25 | 372.1 | 195.7 | 186.7 | 0.570 |
|
Choriocarcinoma | 12 | 261.7 | 281.8 | 152.3 | 0.410 |
| Teratoma | 20 | 304.1 | 217.9 |
77.9 | 0.510 |
 | Table III.Association between serum CA IX level
and patients/tumor characteristics in metastatic chemotherapy-naïve
testicular germ cell tumor patients (n=57). |
Table III.
Association between serum CA IX level
and patients/tumor characteristics in metastatic chemotherapy-naïve
testicular germ cell tumor patients (n=57).
|
| Serum CA IX level
(pg/ml) |
|---|
|
|
|
|---|
| Variable | N | Mean | SEM | Median | P-value |
|---|
| All patients | 57 | 490.6 | 127.9 | 186.7 | NA |
| Primary
tumora |
|
|
|
| 0.750 |
|
Seminoma | 12 | 315.0 | 282.6 | 142.2 |
|
|
Non-seminoma | 43 | 510.4 | 149.3 | 157.8 |
|
| IGCCCG risk
group |
|
|
|
| 1.000 |
| Good
prognosis | 36 | 590.4 | 162.2 | 169.4 |
|
|
Intermediate prognosis | 11 | 382.6 | 231.7 | 231.7 |
|
| Poor
prognosis | 10 | 250.1 | 78.9 |
78.9 |
|
| Number of
metastatic sites |
|
|
|
| 0.200 |
|
0–1 | 31 | 409.5 | 174.3 |
86.7 |
|
|
>2 | 26 | 587.3 | 190.3 | 219.8 |
|
| Retroperitoneal
lymph nodes metastases |
|
|
|
| 0.420 |
|
Present | 9 | 266.1 | 323.1 | 221.3 |
|
|
Absent | 48 | 532.7 | 139.9 | 209.3 |
|
| Mediastinal lymph
nodes metastases |
|
|
|
| 0.770 |
|
Present | 48 | 534.6 | 139.9 | 172.2 |
|
|
Absent | 9 | 255.9 | 323.0 | 231.7 |
|
| Lung
metastases |
|
|
|
| 0.670 |
|
Present | 39 | 419.1 | 155.1 | 210.8 |
|
|
Absent | 18 | 645.7 | 228.3 | 149.4 |
|
| Non-pulmonary
visceral metastases |
|
|
|
| 0.310 |
|
Present | 50 | 494.8 | 137.8 | 149.4 |
|
|
Absent | 7 | 460.7 | 368.3 | 286.9 |
|
| S-stage |
|
|
|
| 0.460 |
| 0 | 11 | 378.1 | 290.7 | 285.7 |
|
| 1 | 22 | 773.6 | 205.6 | 197.3 |
|
| 2 | 16 | 290.0 | 241.1 | 221.3 |
|
| 3 | 8 | 268.3 | 341.0 |
0.0 |
|
Correlation between CA IX in serum
samples and tumor specimens
Intratumoral CA IX expression was evaluated by IHC
in 17 tumor tissue specimens collected on tissue microarray. CA IX
protein was detected in 8 specimens (47.1%) and exhibited mostly a
focal expression pattern. CA IX staining was present in cancer
cells, but in certain specimens, it was visible in the stroma
(Fig. 3). Using Spearman correlation
analysis, a significant association was identified between CA IX
expression in tumor specimens and CA IX values in the corresponding
serum samples from 17 patients (Spearman's rho=0.51, P=0.040).
Prognostic value of serum CA IX
In the median follow-up time of 31.5 months (range,
0.3–46.8 months), 11 patients (13.3%) experienced disease
progression (4 in the chemotherapy-naïve group and 7 in the
chemotherapy-pretreated group) and 10 patients (12.0%) succumbed to
the disease (4 in the chemotherapy-naïve group and 6 in the
chemotherapy-pretreated group).
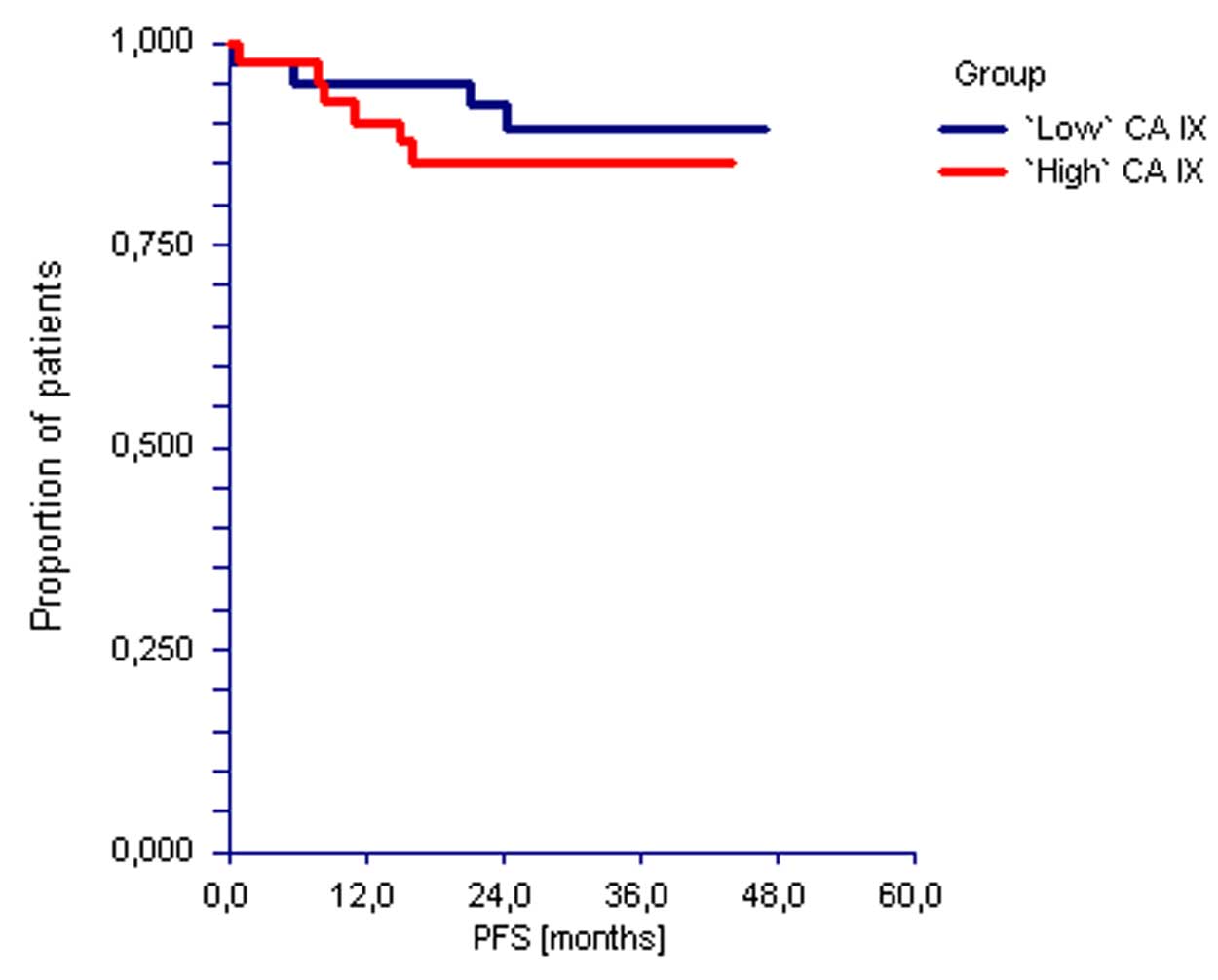
No significant differences in PFS were observed
based on the CA IX levels in serum of the TGCT patients enrolled in
the present study [hazard ratio (HR)=0.81, 95% confidence interval
(CI)=0.25–2.65, P=0.730). Similarly, there was no difference in OS
according to the serum CA IX level (HR=0.64, 95% CI=0.18–2.20,
P=0.48) (Figs. 4 and 5). Of 37 patients who exhibited CA IX
overexpression prior to the second cycle of chemotherapy, 3
patients experienced disease progression and succumbed to the
disease. All these patients had ‘high’ CA IX level in serum;
however this difference did not reach statistical significance
(P=0.070).
Discussion
TGCTs belong to the most chemosensitive group of
tumors, and represent a model for the cure of cancer (34). However, a small number of patients
fail to achieve complete remission with initial cisplatin-based
chemotherapy (35). Biomarkers and
pathways involved in the treatment failure in TGCT patients remain
poorly defined. Emerging data suggest that microenvironmental
factors such as hypoxia may play a role in chemoresistance and in
the acquisition of an aggressive phenotype by TGCT cells (36,37). In
the present prospective translational study, serum CA IX, which was
used as a marker of tumor tissue hypoxia, was significantly
increased in TGCT patients compared with healthy controls. However,
serum CA IX failed to display a prognostic value in TGCTs. This
observation was consistent for chemotherapy-naïve non-metastatic
and metastatic patients, as well as for relapsed
chemotherapy-pretreated TGCT patients. In addition, there was no
significant difference in the mean serum CA IX level between
different groups of patients and between CA IX levels prior to the
first and second cycles of chemotherapy. By contrast, there was a
significant association between CA IX expression in tumor samples
and CA IX levels in serum.
The absence of a significant correlation between CA
IX levels in the serum of the analyzed TGCT patients and their
treatment outcome is not an unusual finding. Recently published
data suggest that the potential clinical value of the CA IX ECD
shed from the surface of tumor cells to the serum of patients is
inconsistent. While the studies by Műller et al (25), Ostheimer et al (38) and Kock et al (24) support the prognostic role of CA IX in
metastatic breast cancer, non-small cell lung cancer and vulvar
cancer, respectively, the study by Hyrsl et al (39) fails to identify any significant
correlation between CA IX EDC values and clinicopathological
features. Based on the present findings, it may be supposed that
the CA IX EDC level cannot reliably reflect the expression of CA IX
or the activation of the HIF-1 signaling pathway in TGCT.
For survival analysis, the CA IX level was
dichotomized based on the median values of all analyzed samples.
Although it cannot be excluded that using different cut-off values
may be able to discriminate good vs. poor prognosis patients, there
was no correlation between serum CA IX level and disease stage,
International Germ Cell Consensus Classification Group prognostic
group, disease burden or any other known prognostic factors, thus
supporting the limited prognostic value of serum CA IX in TGCTs. To
increase the statistical power, chemotherapy-naïve and
chemotherapy-pretreated patients were combined. However, when
separate analyses of these subgroups were performed, the study
results remained the same. It is also possible that, due to the
good prognosis of TGCTs, all pathophysiological aspects associated
with the activation of CA IX and HIF pathways do not affect the
chemosensitivity of TGCTs. This would be consistent with the
findings of Vranic et al, who observed low frequency of
HIF-1α overexpression in TGCTs (40).
A significant association between CA IX expression
in tumor samples and CA IX values in serum was observed. However,
there were several cases of CA IX positivity in serum samples and
CA IX negativity in the corresponding tumors. Since the
hypoxia-related expression of CA IX in tumors is generally very
heterogeneous and often focal, it is probable that the available
region encompassed in the tissue microarray subjected to IHC
staining was actually devoid of CA IX, but this does not exclude
the presence of CA IX in another tumor region. Thus, the
correlation observed in the present study may actually be an
underestimation of the real association. The limited sample size
(n=17) and time period between orchiectomy and administration of
chemotherapy could represent limitations that may affect the study
results. The association between CA IX expression in tumor samples
and the CA IX values in serum could be explained by intratumoral
hypoxia, which leads to the release of the soluble form of CA IX
into the bloodstream. Studies on the association of intratumoral CA
IX expression and detection of its soluble isoform are limited. A
study by Zhou et al demonstrated an association between CA
IX serum level and tumor size, but not between intratumoral CA IX
expression and tumor size in renal cell cancer (41). In contrast to these results, a study
on urinary CA IX in renal cell cancer patients identified a
coherence of soluble CA IX and intratumoral CA IX expression
(39).
In a previous study, CA IX expression was identified
in the flat surface epithelium (modified mesothelium) of all male
and female genital organs (42).
These findings indicated that, during human development, all CA
IX-expressing cells have a mesodermal origin and thus, one would
have expected higher CA IX expression in teratoma and
teratocarcinoma compared with other TGCT histological subtypes. The
presents results revealed no correlation between primary germ cell
tumors histological subtypes and CA IX levels detected in serum,
despite the fact that an independent analysis of a much larger
collection of TGCT tissue specimens revealed a significant
correlation between CA IX expression in tumors and patients'
prognosis (data not shown). The reasons for the lack of
tissue-serum CA IX correlation remain unknown, but they may be
linked to the fact that the activation of the HIF-1 signaling
pathway is relatively rare in TGCTs, and CA IX expression may also
be driven by factors other than hypoxia (40). In addition, CA IX is often visible in
the stroma of TGCTs; thus, it is conceivable that the ECD of CA IX
could remain deposited in the extracellular matrix surrounding the
stromal cells and act locally rather then being released into the
circulation.
In the present study, there were no differences in
serum CA IX levels between non-metastatic, metastatic
chemotherapy-naïve and chemotherapy-pretreated TGCT patients, but
significant differences were observed between CA IX serum levels in
TGCT patients and healthy controls. Unexpectedly, there was no
difference when comparing only relapsed chemotherapy-pretreated
patients, suggesting a different biology and role of hypoxia in
relapsed TGCTs compared with chemotherapy-naïve patients. Analysis
of serum CA IX levels revealed no significant changes during
therapy (prior to chemotherapy and prior to the initiation of the
second cycle of chemotherapy). However, in the cohort of 37
patients prior to receiving the second line of chemotherapy, 3
patients experienced disease progression and succumbed to the
disease. All these patients had CA IX levels above the median
values. However, the limited sample size precludes the drawing of
any definitive conclusions from these data.
In summary, the present study is the first aimed to
assess the prognostic role of serum CA IX level in TGCTs. The
present data suggest that there is an increased level of serum CA
IX in metastatic chemotherapy-naïve TGCTs compared with healthy
controls, and that a weak correlation exists between serum CA IX
levels and tissue CA IX expression. These data suggest neither a
prognostic value for serum CA IX levels nor an association between
serum CA IX levels and patients/tumor characteristics. Despite the
limited clinical utility of serum CA IX, the biological and
clinical value of CA IX expression in TCGT tissues cannot be
excluded, and therefore, further research into this area is
warranted.
Acknowledgements
The authors would like to acknowledge Mrs. Daniela
Jantekova from the Population Registry of Slovak Republic
(Bratislava, Slovakia) for helping to update the patient database;
Dr Maria Reckova from the 2nd Department of Oncology,
Faculty of Medicine, Comenius University for discussion and
critical input; Mrs. Zlatica Pekova from the Department of
Oncology, National Cancer Institute for administration support; and
Mrs. Emilia Klincova from the Department of Pathology, Faculty of
Medicine, Comenius University for the excellent technical
assistance. The present study was supported by the Slovak Research
and Development Agency (Bratislava, Slovakia; contract nos.
APVV-0016-11 and APVV-15-0086), the European Regional Development
Fund (Brussels, Belgium), the State Budget of the Slovak Republic
(Bratislava, Slovakia; project no. ITMS 26240220071) and the Slovak
Scientific Grant Agency (Bratislava, Slovakia; grant no.
VEGA-2/0108/16).
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rijlaarsdam MA and Looijenga LHJ: An
oncofetal and developmental perspective on testicular germ cell
cancer. Semin Cancer Biol. 29:59–74. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Feldman DR, Bosl GJ, Sheinfeld J and
Motzer RJ: Medical treatment of advanced testicular cancer. JAMA.
299:672–684. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Einhorn LH: Clinical trials in testicular
cancer. Cancer. 71:3182–3184. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wykoff CC, Beasly NJP, Watson PH, Turner
KJ, Pastorek J, Sibtain A, Wilson GD, Turley H, Talks KL, Maxwell
PH, et al: Hypoxia-inducible expression of tumor-associated
carbonic anhydrases. Cancer Res. 60:7075–7083. 2000.PubMed/NCBI
|
|
6
|
Loncaster JA, Harris AL, Davidson SE,
Logue JP, Hunter RD, Wycoff CC, Pastorek J, Ratcliffe PJ, Stratford
IJ and West CM: Carbonic anhydrase IX (CA IX) expression, a
potential new intrinsic marker of hypoxia: Correlations with tumor
oxygen measurements and prognosis in locally advanced carcinoma of
the cervix. Cancer Res. 61:6394–6397. 2001.PubMed/NCBI
|
|
7
|
Vordermark D, Kaffer A, Riedl S, Katzer A
and Flentje M: Characterisation of carbonic anhydrase IX (CAIX) as
an andogenous marker of chronic hypoxia in live human tumor cells.
Int J Radiant Oncol Biol Phys. 61:1197–1207. 2005. View Article : Google Scholar
|
|
8
|
Kim SJ, Shin HJ, Jung KY, Baek SK, Shin
BK, Choi J, Kim BS, Shin SW, Kim YH, Kim JS, et al: Prognostic
value of carbonic anhydrase IX and Ki-67 expression in squamous
cell carcinoma of the tongue. Jpn J Clin Oncol. 37:812–819. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Harris AL: Hypoxia-a key regulatory factor
in tumour growth. Nat Rev Cancer. 2:38–47. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ratcliffe PJ: Oxygen sensing and hypoxia
signalling pathways in animals: The implications of physiology for
cancer. J Physiol. 591:2027–2042. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Moon EJ, Brizel DM, Chi JT and Dewhirst
MW: The potential role of intrinsic hypoxia markers as prognostic
variables in cancer. Antioxid Redox Signal. 9:1237–1294. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pastorek J and Pastorekova S:
Hypoxia-induced carbonic anhydrase IX as a target for cancer
therapy: From biology to clinical use. Semin Cancer Biol. 31:52–64.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
McCord AM, Jamal M, Shankavarum UT, Lang
FF, Camphausen K and Tofilon PJ: Physiologic oxygen concentration
enhances the stem-like properties of CD133+ human glioblastoma
cells in vitro. Mol Cancer Res. 7:489–497. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lock FE, McDonald PS, Lou Y, Serrano I,
Chafe SC, Ostlund C, Aparicio S, Winum JY, Supuran CT and Dedhar S:
Targeting carbonic anhydrase IX depletes breast cancer stem cells
within the hypoxic niche. Oncogene. 32:5210–5219. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ledaki I, McIntyre A, Wigfield S, Buffa F,
McGowan S, Baban D, Li JL and Harris AL: Carbonic anhydrase IX
induction defines a heterogenous cancer cell response to hypoxia
and mediates stem cell-like properties and sensitivity to HDAC
inhibition. Oncotarget. 6:19413–194127. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
McIntyre A, Gilbert D, Goddard N,
Looijenga L and Shipley J: Genes, chromosomes and the development
of testicular germ cell tumors of adolescents and adults. Genes
Chromosomes Cancer. 47:547–557. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sheikine Y, Gebega E, Melamed J, Lee P,
Reuter VE and Ye H: Molecular genetics of testicular germ cell
tumors. Am J Cancer Res. 2:153–167. 2012.PubMed/NCBI
|
|
18
|
Liao SY, Darcy KM, Randall LM, Tian C,
Monk BJ, Burger RA, Fruehauf JP, Peters WA, Stock RJ and Stanbridge
EJ: Prognostic relevance of carbonic anhydrase-IX in high-risk,
early-stage cervical cancer: A gynecologic oncology group study.
Gynecol Oncol. 116:452–458. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Choschizick M, Oosterwijk E, Müller V,
Simon R, Moch H and Tennstedt P: Overexpression of carbonic
anhydrase IX (CAIX) is an independent unfavorable prognostic marker
in endometrioid ovarian cancer. Virchows Arch. 459:193–200. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kang HJ, Kim IH, Sung CO, Shim JH and Yu
E: Expression of carbonic anhydrase 9 is a novel prognostic marker
in resectable hepatocellular carcinoma. Virchows Arch. 466:403–413.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Steward GD, O'Mahony FC, Laird A, Rashid
S, Martin SA, Eory L, Lubbock AL, Nanda J, O'Donnell M, Mackay A,
et al: Carbonic anhydrase 9 expression increases with vascular
endothelial growth factor-targeted therapy and is predictive of
outcome in metastatic clear cell renal cancer. Eur Urol.
66:956–963. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhao Z, Liao G, Li Y, Zhou S, Zou H and
Fernando S: Prognostic value of carbonic anhydrase IX
immunohistochemical expression in renal cell carcinoma: A
metaanalysis of the literature. PLoS One. 9:e1140962014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ilie M, Mazure NM, Hofman V, Ammadi RE,
Ortholan C, Bonnetaud C, Havet K, Venissac N, Mograbi B, Mouroux J,
et al: High levels of carbonic anhydrase IX in tumour tissue and
plasma are biomarkers of poor prognostic in patients with non-small
cell lung cancer. Br J Cancer. 102:1627–1635. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kock L, Mahner S, Choschzick M, Eulenburg
C, Milde-Langosch K, Schwarz J, Jaenicke F, Müller V and Woelber L:
Serum carbonic anhydrase IX and its prognostic relevance in vulvar
cancer. Int J Gynecol Cancer. 21:141–148. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Müller V, Riethdorf S, Rack B, Janni W,
Fasching PA, Solomayer E, Aktas B, Kasimir-Bauer S, Zeitz J, Pantel
K, et al: Prospective evaluation of serum tissue inhibitor of
metalloproteinase 1 and carbonic anhydrase IX in correlation to
circulating tumor cells in patients with metastatic breast cancer.
Breast Cancer Res. 13:R712011. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Woelber L, Kress K, Kersten JF, Choschzick
M, Kilic E, Herwig U, Lindner C, Schwarz J, Jaenicke F, Mahner S,
et al: Carbonic anhydrase IX in tumor tissue and sera of patients
with primary cervical cancer. BMC Cancer. 11:122011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yang JS, Chen MK, Yang SF, Chang YC, Su
SC, Chiou HL, Chien MH and Lin CW: Increased expression of carbonic
anhydrase IX in oral submucous fibrosis and oral squamous cell
carcinoma. Clin Chem Lab Med. 52:1367–1377. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zat'ovicová M, Tarábková K, Svastová E,
Gibadulinová A, Mucha V, Jakubícková L, Biesová Z, Rafajová M, Gut
M Ortova, Parkkila S, et al: Monoclonal antibodies generated in
carbonic anhydrase IX-deficient mice recognize different domains of
tumour-associated hypoxia-induced carbonic anhydrase IX. J Immunol
Methods. 282:117–134. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mostofi FK and Sesterhenn IA: Tumours of
the testis and paratesticular tissuePathology and Genetics of
Tumours of the Urinary System and Male Genital Organs (IARC WHO
Classification of Tumours). Eble JN, Sauter G, Epstein JI and
Sesterhenn IA: IARC Press; Lyon: pp. 216–278. 2004
|
|
30
|
Pastoreková S, Závadová Z, Kostál M,
Babusíková O and Závada J: A novel quasi-viral agent, MaTu, is a
two-component system. Virology. 187:620–626. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Takacova M, Bullova P, Simko V, Skvarkova
L, Poturnajova M, Feketeova L, Babal P, Kivela AJ, Kuopio T,
Kopacek J, et al: Expression pattern of carbonic anhydrase IX in
Medullary thyroid carcinoma supports a role for RET-mediated
activation of the HIF pathway. Am J Pathol. 184:953–965. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
deWit R, Roberts JT, Wilkinson PM, de
Mulder PH, Mead GM, Fossa SD, Cook P, de Prijck L, Stenning S and
Collette L: Equivalence of three or four cycles of bleomycin,
etoposide, and cisplatin chemotherapy and of a 3- or 5-day schedule
in good-prognosis germ cell cancer: A randomized study of the
European Organization for Research and Treatment of Cancer
Genitourinary Tract Cancer Cooperative Group and the Medical
Research Council. J Clin Oncol. 19:1629–1640. 2001.PubMed/NCBI
|
|
33
|
Bokenmeyer C, Kollmannsberger C, Stenning
S, Hartmann JT, Horwich A, Clemm C, Gerl A, Meisner C, Ruckerl P,
Schmoll HJ, et al: Metastatic seminoma treated with either single
agent carboplatin or cisplatin-based combination chemotherapy: A
pooled analysis of two randomised trials. Br J Cancer. 91:683–687.
2004.PubMed/NCBI
|
|
34
|
Einhorn LH: Treatment of testicular
cancer: A new and improved model. J Clin Oncol. 8:1777–1781.
1990.PubMed/NCBI
|
|
35
|
Kelland L: The resurgence of
platinum-based cancer chemotherapy. Nat Rev Cancer. 7:573–584.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Rofstad EK: Microenviroment-induced cancer
metastasis. Int J Radiat Biol. 76:589–605. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ljungkvist AS, Bussink J, Kaanders JH and
van der Kogel AJ: Dynamics of tumor hypoxia measured with
bioreductive hypoxic cell markers. Radiat Res. 167:127–145. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ostheimer C, Bache M, Güttler A, Kotzsch M
and Vordermark D: A pilot study on potential plasma hypoxia markers
in the radiotherapy of non-small cell lung cancer. Osteopontin,
carbonic anhydrase IX and vascular endothelial growth factor.
Strahlenther Onkol. 190:276–282. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hyrsl L, Zavada J, Zavadova Z, Kawaciuk I,
Vesely S and Skapa P: Soluble form of carbonic anhydrase IX (CAIX)
in transitional cell carcinoma of urinary tract. Neoplasma.
56:298–302. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Vranic S, Hes O, Grossmann P and Gatalica
Z: Low frequency of HIF-1α overexpression in germ cell tumors of
the testis. Appl Immunohistochem Mol Morphol. 21:165–169.
2013.PubMed/NCBI
|
|
41
|
Zhou GX, Ireland J, Rayman P, Finke J and
Zhou M: Quantification of carbonic anhydrase IX expression in serum
and tissue of renal cell carcinoma patients using enzyme-linked
immunosorbent assy: Prognostic and diagnostic potentials. Urology.
75:257–261. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Suzuki J Jr, Therrien J, Filion F,
Lefebvre R, Goff AK and Smith LC: In vitro culture and somatic cell
nuclear transfer affect imprinting of SNRPN gene in pre- and
post-implantation stages of development in cattle. BMC Dev Biol.
9:92009. View Article : Google Scholar : PubMed/NCBI
|