Introduction
Cancer of the cervix occurs in ~500,000 women
worldwide each year, with an increased prevalence in relatively
young women (1). An essential feature
of the progression of cervical cancer is stromal invasion, which is
the result of complex, multifactorial processes involving matrix
metalloproteinases (MMPs), a closely related multigene family of
zinc-dependent proteolytic enzymes (2). Among MMPs, MMP-1 (collagenase-1),
together with MMP-8 and MMP-13, are known as the interstitial
collagenases, and are capable of initiating the degradation of
fibrillar-type collagens by cleaving their N-terminus (3). MMP-1 presents specific substrates for
collagenases I, II, III, VII, VIII and X as well as for
proteoglycans (4,5). Increased MMP-1 expression has been
associated with the incidence or invasiveness of various types of
cancer, including colorectal, esophageal, pancreatic, gastric and
breast cancer (6–9). Recent studies have demonstrated that the
collagenase activity of MMP-1 may be associated with tumor cell
invasion and increased angiogenesis in xenograft models of
malignant melanomas such as breast cancer (9). Furthermore, MMP-1 has also been shown to
liberate signaling molecule precursors, including epidermal growth
factor (EGF)-like ligands and transforming growth factor-β, from
cell surfaces or the extracellular matrix (ECM) (10,11).
The EGF receptor (EGFR) has been shown to be
expressed at moderate-to-high levels in carcinoma of the cervix in
addition to a wide variety of other solid tumors (12). Furthermore, an increase in EGFR
expression with an increase in disease stage has been observed, and
EGFR expression has been associated with poor prognosis (13). EGFR is a receptor tyrosine kinase
whose function has been implicated in regulating nuclear and
cytoplasmic events, including proliferation, survival,
differentiation and migration. Autophosphorylation of EGFR to
phosphorylated (phospho)-EGFR leads to the activation of two
downstream pathways: The mitogen-activated protein kinase (MAPK)
pathway and the phosphatidylinositol 3-kinase (PI3-K)/AKT pathway.
The major MAPK pathways consist of extracellular signal-regulated
kinase (ERK)1/2, p38 and c-Jun N-terminal kinase (JNK) (14). The sub-cellular localization of EGFR
determines the signaling pathways stimulated by EGFR activation
(15). The most well-known
localization of EGFR is to lipid rafts, which are enriched in
cholesterol, sphingolipids and gangliosides, and are less fluid
than the surrounding bulk plasma membrane (16). Lipid rafts may act as platforms to
facilitate the crosstalk between different components of various
signaling pathways that are in close proximity or as sequestering
regions to prevent the association of components of signaling
events (17,18). However, the effect of EGFR
localization to lipid rafts is not well understood. While it has
been noted that lipid raft localization of EGFR inhibits ligand
binding and subsequent downstream signaling (19,20), other
studies have shown that lipid rafts promote EGFR signaling
(21).
Our analyses previously demonstrated that EGFR
regulates melatonin receptor type 1A (MT1)-MMP and MMP-2 synthesis
in the SiHa cervical cancer cell line via both the PI3-K/AKT and
MAPK/ERK pathways (22). However, the
effects of EGFR localization to lipid rafts on signaling pathways
involved in the regulation of MMP-1 expression are unknown. In the
present study, it was concluded that lipid raft localization of
EGFR alters MMP-1 expression in SiHa cells via the MAPK/ERK
signaling pathway.
Materials and methods
Cell culture
SiHa cells were purchased from the Chinese Academy
of Sciences cell bank (Shanghai, China). Cells were plated in
6-well plates at ~2×106 viable cells per well in 1 ml of
Dulbecco's modified Eagle medium (Solarbio Science and Technology
Co., Ltd., Beijing, China) containing 10% fetal bovine serum
(Zhejiang Tianhang Biotechnology Co., Ltd., Hangzhou, China), and
cultured at 37°C in an atmosphere of 5% CO2 and 95% air.
Cells were allowed to attach for 24 h and then incubated in
serum-free medium for 16–18 h. Subsequently, the cells were treated
with EGF (PeproTech Inc., Rocky Hill, NJ, USA) in the presence or
absence of methyl-β-cyclodextrin (MβCD; 0.5 nM; Sigma-Aldrich;
Merck Millipore, Darmstadt, Germany), cholesterol (10 µM;
Sigma-Aldrich; Merck Millipore) and inhibitors of EGFR (ZD1839; 10
nM; AstraZeneca, London, UK), PI3-K (LY294002; 20 nM; and
wortmannin; 5 nM), MAPK kinase (MEK) (PD98059; 20 nM; and U0126; 10
nM), p38 (SB203580; 10 nM) and JNK (SP600125; 10 nM; all
Sigma-Aldrich; Merck Millipore) for 1 h prior to exposure to EGF.
Cells were harvested after incubation with EGF for the indicated
length of time.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total cellular RNA was extracted using TRIzol
reagent (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) according to the manufacturer's protocol. Total RNA was
reverse-transcribed into single strand complementary DNA using the
First Strand cDNA Synthesis kit (Takara Biotechnology Co., Ltd.,
Dalian, China). qPCR was performed using the Power SYBR®
Green PCR Master Mix on an ABI 7500 Real Time PCR System (both
Applied Biosystems; Thermo Fisher Scientific, Inc.). The cycling
conditions were as follows: 95°C for 10 min, followed by 40 cycles
at 95°C for 15 sec, 60°C for 30 sec and 72°C for 30 sec. The
sequences of the RT primers were as follows: Human (h) MMP-1 (346
bp) sense 5′-CATCGTGTTGCGGCTCAT-3′ and antisense
5′-GCCCATTTGGCAGTTGTG-3′ (59.4°C); h tissue inhibitor of matrix
metalloproteinase-1 (TIMP-1) (285 bp) sense
5′-TCCTGTTGTTGCTGTGGCTGAT-3′ and antisense
5′-ACTCCTCGCTGCGGTTGTG-3′ (59.4°C); and hGAPDH (502 bp) sense
5′-GGTGAAGGTCGGTGTGAACGGATTT-3′ and antisense
5′-AATGCCAAAGTTGTCATGGATGACC-3′ (58.0°C). The relative expression
level was calculated using the ΔΔCq method (23).
Biochemical lipid raft isolation
Biochemical lipid raft isolation was performed
following established protocols (24). Briefly, cells in 6-well plates were
scraped in buffer [20 mM Tris (pH 7.8), 250 mM sucrose, 1 mM
MgCl2, 1 mM CaCl2 and 100 M sodium
orthovanadate] and then lysed in buffer containing 1X protease
inhibitor cocktail (Solarbio Science and Technology Co., Ltd.) by
passing through a 22-gauge needle (Sigma-Aldrich; Merck Millipore)
20 times. Lysates were centrifuged as described (24), and the first and second post-nuclear
supernatants were combined and frozen at −20°C. Samples were thawed
and combined with an equal volume of 50% Opti-Prep (Greiner
Bio-One, Monroe, NC, USA), and 0–20% Opti-Prep gradient was then
applied. Gradients were centrifuged for 90 min at 52,000 × g
and then fractionated into 12 0.74-ml fractions. Fractions were
either dot blotted with cholera toxin subunit B-horseradish
peroxidase (1:100 dilution; cat. no. C34780; Invitrogen; Thermo
Fisher Scientific, Inc.) for 30 min on ice, and then for 20 min at
37°C, to determine monosialotetrahexosylganglioside (GM1)
expression or subjected to western blotting with antibodies.
Western blotting
Cells were lysed in radioimmunoprecipitation assay
lysis buffer plus protease inhibitors (Solarbio Science and
Technology Co., Ltd.) and phosphatase inhibitor cocktail
(Sigma-Aldrich; Merck Millipore). The concentration of protein in
each sample was measured with a BCA Protein Assay kit (Pierce;
Thermo Fisher Scientific, Inc.). Aliquots of protein (40 µg) were
subjected to western blotting as described previously (25). The following primary antibodies were
used: Anti-EGFR (1:1,000 dilution; cat. no. 2239),
anti-phospho-EGFR (1:1,000 dilution; cat. no. 2641), anti-AKT
(1:2,000 dilution; cat. no. 2920), anti-phospho-AKT (Ser473)
(1:1,000 dilution; cat. no. 12694), anti-ERK1/2 (1:1,000 dilution;
cat. no. 4348), anti-phospho-ERK1/2 (Thr202/Tyr204) (1:1,000
dilution; cat. no. 14227), anti-p38 (1:1,000 dilution; cat. no.
14451), anti-phospho-p38 (Thr180/Tyr182) (1:1,000 dilution; cat.
no. 4092), anti-JNK (1:1,000 dilution; cat. no. 3708),
anti-phospho-JNK (Thr183/Tyr185) (1:1,000 dilution; cat. no. 4671;
all Cell Signaling Technology, Inc., Danvers, MA, USA),
anti-β-actin (1:1,000 dilution; cat. no. SC-130300) and anti-MMP-1
(1:1,000 dilution; cat. no. SC-8836-R; both Santa Cruz
Biotechnology Inc., Dallas, TX, USA). The bound antibodies were
detected with the appropriate secondary antibodies (1:2,000
dilution; cat. nos. 7074, 7076 and 7077; Cell Signaling Technology,
Inc.) and the protein bands were visualized with a
3,3′-diaminobenzidine staining kit (Beijing Zhongshan Goldenbridge
Biotechnology Co., Ltd., Beijing, China). The bands were quantified
using ImageJ 1.37 software (National Institutes of Health,
Bethesda, MD, USA).
Immunostaining
Cells were plated on coverslips at a density of
2.0×105 cells per 35-mm dish and grown for 48 h in
growth medium. Coverslips containing cells were then incubated with
1 mg/ml Alexa Fluor 594-labeled cholera toxin subunit B (red; cat.
no. C-34777; Thermo Fisher Scientific, Inc.) for 10 min on ice.
Following incubation, cells were fixed with formalin, permeabilized
with 0.1% Triton X-100, blocked in 20% goat serum (Zhejiang
Tianhang Biotechnology Co., Ltd.) for 1 h and incubated with
anti-EGFR labeled with Alexa Fluor 488 (green; 1:100 dilution; cat.
no. A-11008; Invitrogen; Thermo Fisher Scientific, Inc.) for 1 h at
37°C. Nuclei were stained with DAPI (blue; Invitrogen; Thermo
Fisher Scientific, Inc.). Imaging was performed via confocal
microscopy using an Axioplan 2 Apotome microscope (Zeiss GmbH,
Jena, Germany) fitted with a 63×1.25 oil immersion lens.
Adenoviral transfections
Cells were plated in 6-well plates at a density of
200,000 cells/ml in triplicates for each condition. The pCMV
adenoviruses constitutively active (CA)-MEK and dominant negative
(DN)-MEK were purchased from Shanghai GenePharma Co., Ltd.
(Shanghai, China). The cells were infected with recombinant
adenoviruses to overexpress CA-MEK and DN-MEK at a multiplicity of
infection of 25 for 48 h. The medium was then aspirated and
replaced with serum-free medium containing MβCD (0.5 mM) for 1 h.
Upon incubation, the cells were treated with EGF (10 ng/ml) for 24
h before protein collection.
Statistical analyses
The data were presented as the mean ± standard
deviation and subjected to analysis of variance with the
Student-Newman-Keuls test using the statistical software package
SPSS 11.0 (SPSS Inc., Chicago, IL, USA). P<0.05 was considered
to indicate a statistically significant difference.
Results
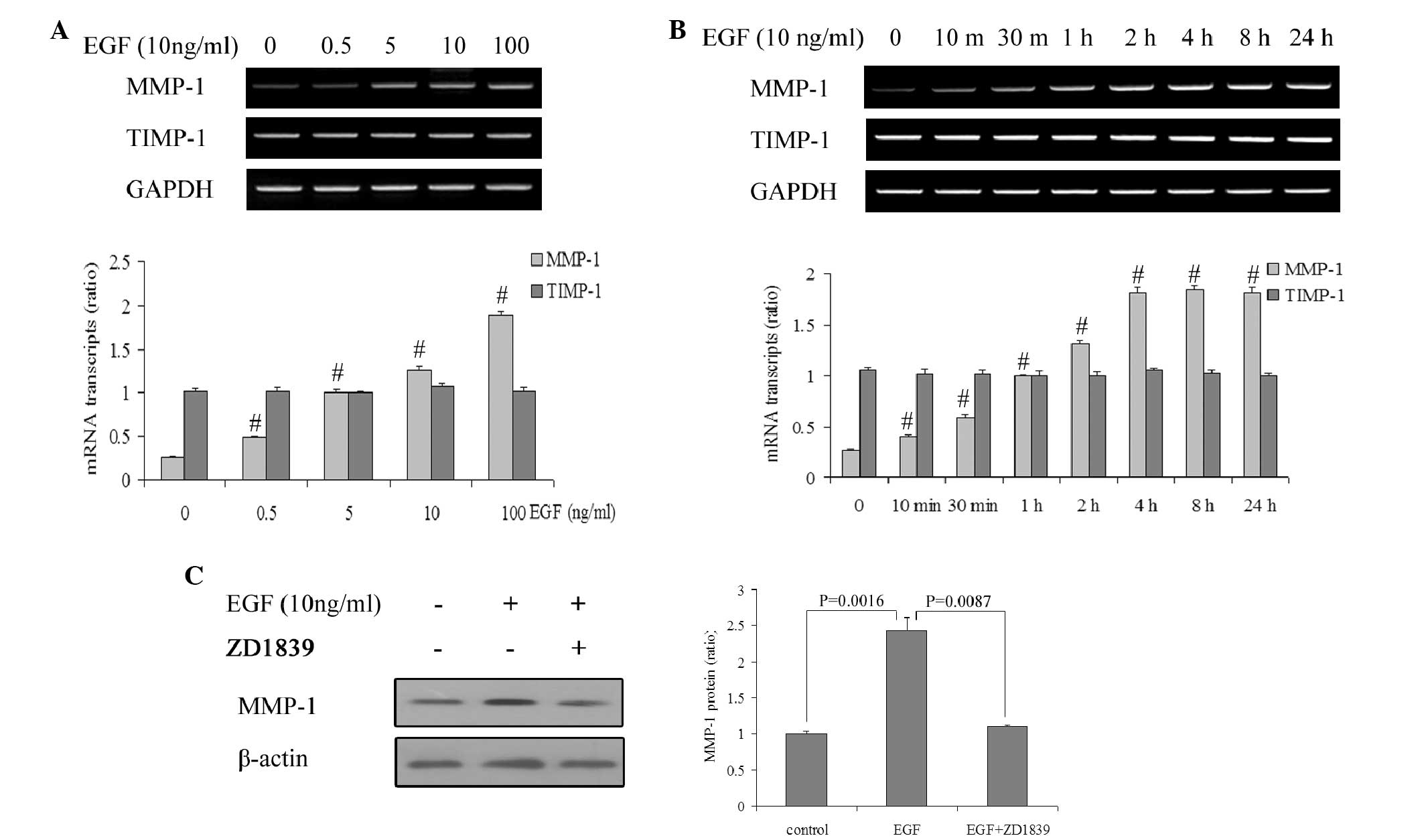
EGF upregulates MMP-1 expression at
the messenger RNA (mRNA) and protein levels
It was previously demonstrated by the present
authors that EGFR regulates MT1-MMP and MMP-2 synthesis in SiHa
cells via both the PI3-K/AKT and MAPK/ERK pathways in SiHa cells
(22). To further indicate a role for
EGFR in the synthesis and function of other MMP members in SiHa
cells, the changes in MMP-1 expression were investigated at the
mRNA and protein levels following EGF treatment in the present
study. The RT-qPCR results demonstrated that EGF induced an
increase in MMP-1 mRNA in SiHa cells in a concentration-dependent
manner (P<0.05; Fig. 1A).
Additional analysis revealed that the MMP-1 mRNA expression
commenced to increase in response to 10 ng/ml EGF (P<0.05), and
that it reached maximal levels at 4 h and remained high for ≤24 h
(Fig. 1B). However, TIMP-1 mRNA
levels remained unchanged by EGF (P>0.05). Furthermore,
increased MMP-1 mRNA synthesis was reflected in increased protein
levels (P<0.05) that were detectable 24 h after EGF regulation
(Fig. 1C).
To demonstrate that EGFR activation by EGF
specifically regulates MMP-1 expression, the activation of EGFR was
inhibited using the small molecule inhibitor ZD1839. Addition of
ZD1839 (10 nM) 1 h prior to treatment with EGF completely inhibited
the EGF-induced increase in MMP-1 at the protein level (P<0.05;
Fig. 1C).
EGFR localizes to lipid rafts in SiHa
cells
Previous studies have shown that EGFR localizes to
lipid rafts in CHO and HeLa cells (24). To determine whether EGFR localizes
specifically to lipid rafts in SiHa cells, two methods were used to
identify these structures: Biochemical raft isolation and confocal
microscopy. First, a detergent-free Opti-Prep gradient was used to
isolate lipid rafts (26). Dot
blotting for the lipid raft-specific glycosphingolipid GM1
identified fractions 1–6 as lipid raft fractions. When these
fractions were immunoblotted using anti-EGFR antibodies, EGFR
localization to lipid raft fractions was observed to be most
prominent in SiHa cells. When MβCD, a cytotoxic
cholesterol-sequestering agent, was used to pharmacologically
deplete cholesterol from the cells, the protein levels of EGFR were
decreased in the lipid raft fractions (Fig. 2A). Quantitative analysis demonstrated
that the lipid raft fractions contained significantly more EGFR
compared with the non-lipid raft fractions in SiHa cells
(P<0.05; Fig. 2B). Cells were
stained with Alexa Fluor 488-labeled anti-EGFR antibodies (green)
and Alexa Fluor 594-labeled cholera toxin subunit B (red), which
binds specifically to GM1 (27), to
detect localization of lipid rafts. Using confocal microscopy, it
was observed that EGFR (green) co-localized (yellow/orange) with
GM1 (red) at the plasma membrane of SiHa cells (Fig. 2C). Taken together, these data
suggested that EGFR localizes within lipid rafts in SiHa cells.
Lipid raft disruption reinforces
EGFR-induced upregulation of MMP-1 expression
As previously reported, lipid raft localization of
EGFR inhibits ligand binding in certain types of cancers, and lipid
rafts promote EGFR signaling in other types of cancer (19–21). Since
it was noticed that EGFR localizes to lipid rafts in SiHa cells,
the present study examined whether the redistribution of EGFR
induced by lipid raft disruption reinforces the EGFR-induced
upregulation of MMP-1 expression in SiHa cells. Lipid raft
disruption by MβCD enhanced the EGF-induced increase in MMP-1
synthesis at both the mRNA and protein levels (P<0.05), and
cholesterol post-treatment reversed this change. To investigate
whether EGFR activation by lipid raft disruption specifically
regulates MMP-1 expression, the activation of EGFR was inhibited
with ZD1839. The results revealed that ZD1839 completely inhibited
the MβCD-reinforced MMP-1 synthesis induced by EGF at both the mRNA
and protein levels (Fig. 3). These
data indicate that lipid raft localization of EGFR inhibits
EGFR-induced upregulation of MMP-1 expression.
MAPK/ERK signaling is involved in the
regulation of MMP-1 expression
Localization of EGFR to lipid rafts has variable
effects on signaling pathways downstream of EGFR (19–21). Thus,
the effect of cholesterol depletion on EGFR signaling was examined
in SiHa cells. Cells were treated with MβCD, and western blotting
was performed to determine whether EGFR induced the phosphorylation
of key mediators, including AKT, MAPK, p38 and JNK. As expected,
lipid raft disruption resulted in increased AKT, MAPK, p38 and JNK
phosphorylation, while cholesterol addition abrogated the
MβCD-induced increase in phosphorylation of these kinases (Fig. 4A). To determine the downstream EGFR
signaling pathways involved in the increase of MMP-1 expression,
SiHa cells were treated with selective PI3-K inhibitors (LY294002
or wortmannin), MEK inhibitors (PD98059 or U0126), a p38 inhibitor
(SB203580) or a JNK inhibitor (SP600125) for 2 h before the
addition of EGF. The results demonstrated that treatment of SiHa
cells with PD98059 or U0126 reduced MMP-1 mRNA expression, which
was mediated by both EGF and MβCD (P<0.05). By contrast,
application of LY294002, wortmannin, SB203580 or SP600125 had no
effect on MMP-1 mRNA synthesis (P>0.05). Western blot analysis
revealed that the impact of these inhibitors on MMP-1 mRNA
synthesis induced by both EGF and MβCD was similar to the impact on
MMP-1 protein synthesis (P<0.05; Fig.
4B). To more closely examine the involvement of the MAPK
signaling pathway in the induction of MMP-1 by both EGF and MβCD,
adenoviral constructs targeting MEK were employed. The levels of
phospho-ERK1/2, total ERK1/2 and MMP-1 were examined following
transfection of SiHa cells with a CA-MEK adenoviral construct
(Ad-CA-MEK), a DN-MEK adenoviral construct (Ad-DN-MEK) or pCMV
control. In the presence of DN-MEK, the phospho-ERK and MMP-1
levels were reduced. In contrast, MMP-1 protein expression was
increased in cells transfected with the CA-MEK construct (Fig. 4C). These results indicated that the
MAPK/ERK signaling pathway is involved in the regulation of MMP-1
expression by lipid raft disruption.
 | Figure 4.The MAPK/ERK signaling pathway is
involved in the regulation of MMP-1 expression. (A) Serum-deprived
SiHa cells were incubated for 1 h in the presence or absence of
MβCD and cholesterol, and were then incubated with 10 ng/ml EGF for
2 h. The cell lysates were analyzed by western blotting with
anti-AKT, anti-phospho-AKT, anti-ERK1/2, anti-phospho-ERK1/2,
anti-p38, anti-phospho-p38, anti-JNK, anti-phospho-JNK and
anti-β-actin antibodies. The results shown are from representative
experiments performed in triplicate. (B) Serum-deprived SiHa cells
were incubated with 10 ng/ml EGF in the presence of the
corresponding pharmacological inhibitors for 1 h before exposure to
EGF. Total RNA was extracted 2 h later and then analyzed by reverse
transcription-quantitative polymerase chain reaction, in which the
MMP-1 or GAPDH mRNA levels were measured. Cell lysates were
analyzed 24 h later by western blotting, in which anti-MMP-1 or
anti-β-actin antibodies were used as probes. The laser densitometry
results (the data are the mean of three independent experiments
±SD) are shown in the bar graph and are expressed as a ratio.
#P<0.05 compared with EGF-stimulated cells with MβCD
treatment. *P<0.05 compared with EGF-stimulated cells with MβCD
treatment. (C) Cells were infected with recombinant adenoviruses
for expression of CA-MEK and DN-MEK for 48 h, and then treated with
EGF (10 ng/ml) for 24 h in the absence of MβCD treatment for 1 h
before exposure to EGF. Cell lysates were analyzed by western
blotting, in which anti-ERK1/2, anti-phospho-ERK1/2, anti-MMP-1 or
anti-β-actin antibodies were used as probes. The laser densitometry
results (the data are the mean of three independent experiments ±
SD) are shown in the bar graphs and are expressed as a ratio.
P<0.05 compared with each other. Phospho, phosphorylated; MMP,
matrix metalloproteinase; EGF, epidermal growth factor; MβCD,
methyl-β-cyclodextrin; mRNA, messenger RNA; ERK, extracellular
signal-regulated kinase; JNK, c-Jun N-terminal kinase; MAPK,
mitogen-activated protein kinase; MEK, MAPK kinase; CA,
constitutively active; DN, dominant negative; SD, standard
deviation. |
Discussion
The present study provides evidence describing a
role for lipid rafts in the resistance to EGFR-induced MMP-1
expression in SiHa cells. The results demonstrated that EGFR
activation by EGF specifically regulates MMP-1 expression at the
mRNA and protein levels. Additionally, the current study provided
evidence that EGFR localizes to lipid rafts in SiHa cells. In our
study, MβCD, a cytotoxic cholesterol-sequestering agent that
pharmacologically depletes cholesterol from the cells, decreased
EGFR protein levels in lipid raft fractions. Importantly,
redistribution of EGFR induced by lipid raft disruption altered
MMP-1 expression levels. Furthermore, lipid raft disruption
resulted in increased phosphorylation of AKT, MAPK, p38 and JNK.
Thus, the MAPK/ERK signaling pathway may be involved in the
regulation of MMP-1 expression. Our data suggest that lipid rafts
provide a platform to inhibit EGFR regulation of MMP-1 in SiHa
cells through the MAPK/ERK signaling pathway.
Invasion and distant metastasis are important events
that affect the prognosis and treatment of cervical cancer patients
(28). Patients in the later stages
of cervical cancer with invasion or metastasis have a significantly
worse prognosis (29). Fewer than 20%
of women with stage IV cervical cancer survive for ≥5 years
(30). Thus, understanding the
molecular and cellular mechanisms of cell invasion and metastasis
is critical for developing effective cervical cancer therapies and
improving patient survival. Cell invasion and metastasis have been
associated primarily with degradation of ECM components (26,31). MMP-1
is important in malignant processes of cervical cancer (32). Furthermore, it has become increasingly
clear in the past years that MMP-1 substrates extend to numerous
non-matrix extracellular and membrane-bound proteins, including
protease precursors, protease inhibitors, cytokines, latent growth
factors, growth factor-binding proteins and adhesion molecules
(9,10,33). Thus,
understanding how MMP-1 is regulated in cervical cancer may be
crucial for developing more effective therapies for metastatic
cancer. The present study examined the influence of EGFR in the
regulation of MMP-1 expression. By perturbing EGFR using EGF
stimulation in SiHa cervical cancer cells, MMP-1 synthesis was
increased. In the same model system, inhibition of EGFR by ZD1839
led to decreased MMP-1 levels.
Cervical carcinoma is associated primarily with
high-risk human papillomaviruses (HR-HPVs), including HPV-16 and
HPV-18, which encode the E6 and E7 oncogenes (34). The E6 and E7 proteins are considered
to immortalize cervical epithelial cells by interfering with the
function of the tumor suppressor proteins p53 and retinoblastoma
protein, respectively (35). The
expression of HR-HPV E6 has been linked to an increase in EGFR
levels (13,36), and changes in the functional levels of
the HPV E6/E7 proteins may alter the growth rate of cervical cancer
cell lines by reducing the stability of EGFR at the
post-transcriptional level (37).
Thus, it is reasonable to infer that E6/E7 proteins could
upregulate the expression of MMP-1 by inducing high levels of EGFR
in SiHa cells.
The present data indicate that the localization of
EGFR specifically to lipid rafts contributes to the inhibition of
the EGFR-induced MMP-1 expression in SiHa cells. EGFR co-localizes
with lipid rafts in SiHa cells, and the lipid environment of
EGFR-overexpressing cells influences the dimerization properties
and signaling functions of EGFR (38). Of note, the
3-hydroxy-3-methyl-glutaryl-coenzyme A-reductase inhibitor statin
has been commonly used to deplete cells of lipid rafts for various
years (39). Preclinical data have
demonstrated that lipid raft depletion by statins can reduce cell
growth and sensitize cells to apoptotic stimuli in a number of
cancers, including prostate, melanoma and EGFR-overexpressing
breast cancer (40,41). Epidemiologic data have demonstrated
that the use of statins as single agents in breast cancer is
beneficial (42,43). Furthermore, in vitro studies
combining statins along with other therapies suggest that statins
may have a greater clinical benefit when used as part of
combinatorial therapies (39).
However, the present results indicated that cholesterol depletion
synergizes with the activation of EGFR and results in increased
phosphorylation of AKT, MAPK, p38 and JNK. It is well known that
the activation of two downstream pathways of EGFR, the
Ras/Raf/MAPK/ERK pathway and the PI3-K/AKT pathway, can induce cell
proliferation and decrease cell apoptosis (44). Importantly, the mechanism of action of
statin drugs is not solely through the reduction of cholesterol but
also via the inhibition of geranylgeranylated and farnesylated
small GTPases, which suppress the activation of small G proteins
(45). Therefore, it is difficult to
infer whether statins would be beneficial as a part of cervical
cancer therapies.
The associations between different signaling
pathways and MMPs have been investigated in a number of cell
culture systems (46–48). Using selective pharmaceutical
inhibitors of EGFR downstream signaling pathway effectors, the
present study revealed that MMP-1 expression was upregulated
through the MAPK/ERK signaling pathway in SiHa cells treated with
EGF and MβCD, and that the PI3-K/AKT, p38 MAPK and JNK/MAPK
signaling pathways were not involved in this process. These
observations were further supported by the fact that the
transfection of MEK-CA led to increased ERK phosphorylation and
MMP-1 levels, as well as the fact that the transfection of MEK-DN
abolished the basal expression of MMP-1. However, our previous
studies indicated that EGFR upregulates MT1-MMP and downregulates
MMP-2 through the MAPK/ERK pathway, while concomitantly
transmitting a mild positive regulatory signal to the expression of
MMP-2 via the PI3-K/AKT pathway in SiHa cells (22). Thus, the signaling pathways involved
in the regulation of different MMPs by EGFR are varied.
In conclusion, the present study demonstrated that
lipid raft localization of EGFR repressed EGFR-induced MMP-1
expression in SiHa cells, and that the MAPK/ERK signaling pathway
was involved in this process. Since MMP-mediated ECM remodeling and
invasion of tumors are tightly linked, the regulation of MMP-1 may
contribute to the central role that EGFR and lipid rafts have in
the development and metastasis of cervical cancer. However, the
data presented herein were based on in vitro experiments;
thus, additional in vivo studies are required to obtain a
better understanding of MMP-1 involvement in cervical
tumorigenesis. Experiments focusing on manipulating MMP-1
expression using an in vivo model are in progress and will
provide further information regarding the influence of MMP-1 on
cervical carcinomas.
Acknowledgements
The present study was supported by the Natural
Science Foundation of Heilongjiang Province (Harbin, China; grant
no. 200906), the Foundation of Heilongjiang Provincial Educational
Department (Harbin, China; grant no. 11551163) and the Foundation
of Heilongjiang Provincial Health Department (Harbin, China; grant
no. 2009–112).
Glossary
Abbreviations
Abbreviations:
|
MMPs
|
matrix metalloproteinases
|
|
EGFR
|
epidermal growth factor receptor
|
|
ERK
|
extracellular signal-regulated
kinase
|
|
MAPK
|
mitogen-activated protein kinase
|
|
PI3-K
|
phosphatidylinositol 3-kinase
|
|
JNK
|
c-Jun N-terminal kinase
|
|
MβCD
|
methyl-β-cyclodextrin
|
|
HR-HPVs
|
high-risk human papillomaviruses
|
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pei D: Matrix metalloproteinases target
protease-activated receptors on the tumor cell surface. Cancer
Cell. 7:207–208. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pittayapruek P, Meephansan J, Prapapan O,
Komine M and Ohtsuki M: Role of Matrix Metalloproteinases in
Photoaging and Photocarcinogenesis. Int J Mol Sci. 17:8682016.
View Article : Google Scholar
|
|
4
|
Poola I, DeWitty RL, Marshalleck JJ,
Bhatnagar R, Abraham J and Leffall LD: Identification of MMP-1 as a
putative breast cancer predictive marker by global gene expression
analysis. Nat Med. 11:481–483. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yang R, Xu Y, Li P, Zhang X, Wang J, Gu D
and Wang Y: Combined upregulation of matrix metalloproteinase-1 and
proteinase-activated receptor-1 predicts unfavorable prognosis in
human nasopharyngeal carcinoma. Onco Targets Ther. 6:1139–1146.
2013.PubMed/NCBI
|
|
6
|
Guan X, Wang X, Luo H and Wu J, Zhang X
and Wu J: Matrix metalloproteinase 1, 3 and 9 polymorphisms and
esophageal squamous cell carcinoma risk. Med Sci Monit.
20:2269–2274. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Botta GP, Reginato MJ, Reichert M, Rustgi
AK and Lelkes PI: Constitutive K-RasG12D activation of ERK2
specifically regulates 3D invasion of human pancreatic cancer cells
via MMP-1. Mol Cancer Res. 10:183–196. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cai QW, Li J, Li XQ, Wang JQ and Huang Y:
Expression of STAT3, MMP-1 and TIMP-1 in gastric cancer and
correlation with pathological features. Mol Med Rep. 5:1438–1442.
2012.PubMed/NCBI
|
|
9
|
Liu H, Kato Y, Erzinger SA, Kiriakova GM,
Qian Y, Palmieri D, Steeg PS and Price JE: The role of MMP-1 in
breast cancer growth and metastasis to the brain in a xenograft
model. BMC Cancer. 12:5832012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hua H, Li M, Luo T, Yin Y and Jiang Y:
Matrix metalloproteinases in tumorigenesis: An evolving paradigm.
Cell Mol Life Sci. 68:3853–3868. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Iida J and McCarthy JB: Expression of
collagenase-1 (MMP-1) promotes melanoma growth through the
generation of active transforming growth factor-beta. Melanoma Res.
17:205–213. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ma D, Hovey RL, Zhang Z, Fye S, Huettner
PC, Borecki IB and Rader JS: Genetic variations in EGFR and ERBB4
increase susceptibility to cervical cancer. Gynecol Oncol.
131:445–450. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Schrevel M, Gorter A, Kolkman-Uljee SM,
Trimbos JB, Fleuren GJ and Jordanova ES: Molecular mechanisms of
epidermal growth factor receptor overexpression in patients with
cervical cancer. Mod Pathol. 24:720–728. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wagner EF and Nebreda AR: Signal
integration by JNK and p38 MAPK pathways in cancer development. Nat
Rev Cancer. 9:537–549. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sadowski L, Pilecka I and Miaczynska M:
Signaling from endosomes: Location makes a difference. Exp Cell
Res. 315:1601–1609. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Guéguinou M, Gambade A, Félix R, Chantôme
A, Fourbon Y, Bougnoux P, Weber G, Potier-Cartereau M and Vandier
C: Lipid rafts, KCa/ClCa/Ca2+ channel complexes and EGFR signaling:
Novel targets to reduce tumor development by lipids? Biochim
Biophys Acta. 1848:2603–2620. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Staubach S and Hanisch FG: Lipid rafts:
Signaling and sorting platforms of cells and their roles in cancer.
Expert Rev Proteomics. 8:263–277. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mollinedo F and Gajate C: Lipid rafts as
major platforms for signaling regulation in cancer. Adv Biol Regul.
57:130–146. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen X and Resh MD: Cholesterol depletion
from the plasma membrane triggers ligand-independent activation of
the epidermal growth factor receptor. J Biol Chem. 277:49631–49637.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Roepstorff K, Thomsen P, Sandvig K and van
Deurs B: Sequestration of epidermal growth factor receptors in
non-caveolar lipid rafts inhibits ligand binding. J Biol Chem.
277:18954–18960. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Irwin ME, Bohin N and Boerner JL: Src
family kinases mediate epidermal growth factor receptor signaling
from lipid rafts in breast cancer cells. Cancer Biol Ther.
12:718–726. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang Z, Song T, Jin Y, Pan J, Zhang L,
Wang L and Li P: Epidermal growth factor receptor regulates MT1-MMP
and MMP-2 synthesis in SiHa cells via both PI3-K/AKT and MAPK/ERK
pathways. Int J Gynecol Cancer. 19:998–1003. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pugniere P, Banzet S, Chaillou T, Mouret C
and Peinnequin A: Pitfalls of reverse transcription quantitative
polymerase chain reaction standardization: Volume-related
inhibitors of reverse transcription. Anal Biochem. 415:151–157.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Macdonald JL and Pike LJ: A simplified
method for the preparation of detergent-free lipid rafts. J Lipid
Res. 46:1061–1067. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang D and Brodt P: Type 1 insulin-like
growth factor regulates MT1-MMP synthesis and tumor invasion via PI
3-kinase/Akt signaling. Oncogene. 22:974–982. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Egeblad M and Werb Z: New functions for
the matrix metalloproteinases in cancer progression. Nat Rev
Cancer. 2:161–174. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Janes PW, Ley SC and Magee AI: Aggregation
of lipid rafts accompanies signaling via the T cell antigen
receptor. J Cell Biol. 147:447–461. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhou X, Xu CJ, Wang JX, Dai T, Ye YP, Cui
YM, Liao WT, Wu XL and Ou JP: Metastasis-Associated in Colon
Cancer-1 Associates With Poor Prognosis and Promotes Cell Invasion
and Angiogenesis in Human Cervical Cancer. Int J Gynecol Cancer.
25:1353–1363. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lee JY, Lee C, Hahn S, Kim MA, Kim HS,
Chung HH, Kim JW, Park NH and Song YS: Prognosis of adenosquamous
carcinoma compared with adenocarcinoma in uterine cervical cancer:
A systematic review and meta-analysis of observational studies. Int
J Gynecol Cancer. 24:289–294. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Song C, Zhu S, Wu C and Kang J: Histone
deacetylase (HDAC) 10 suppresses cervical cancer metastasis through
inhibition of matrix metalloproteinase (MMP) 2 and 9 expression. J
Biol Chem. 288:28021–28033. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bonnans C, Chou J and Werb Z: Remodelling
the extracellular matrix in development and disease. Nat Rev Mol
Cell Biol. 15:786–801. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lai HC, Chu CM, Lin YW, Chang CC, Nieh S,
Yu MH and Chu TY: Matrix metalloproteinase 1 gene polymorphism as a
prognostic predictor of invasive cervical cancer. Gynecol Oncol.
96:314–319. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
McCawley LJ and Matrisian LM: Matrix
metalloproteinases: They're not just for matrix anymore! Curr Opin
Cell Biol. 13:534–540. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tan S, de Vries EG, van der Zee AG and de
Jong S: Anticancer drugs aimed at E6 and E7 activity in
HPV-positive cervical cancer. Curr Cancer Drug Targets. 12:170–184.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Saha SK and Khuda-Bukhsh AR: Berberine
alters epigenetic modifications, disrupts microtubule network, and
modulates HPV-18 E6-E7 oncoproteins by targeting p53 in cervical
cancer cell HeLa: A mechanistic study including molecular docking.
Eur J Pharmacol. 744:132–146. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Soonthornthum T, Arias-Pulido H, Joste N,
Lomo L, Muller C, Rutledge T and Verschraegen C: Epidermal growth
factor receptor as a biomarker for cervical cancer. Ann Oncol.
22:2166–2178. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Mathur RS and Mathur SP: Vascular
endothelial growth factor (VEGF) up-regulates epidermal growth
factor receptor (EGF-R) in cervical cancer in vitro: This action is
mediated through HPV-E6 in HPV-positive cancers. Gynecol Oncol.
97:206–213. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Suprynowicz FA, Disbrow GL, Krawczyk E,
Simic V, Lantzky K and Schlegel R: HPV-16 E5 oncoprotein
upregulates lipid raft components caveolin-1 and ganglioside GM1 at
the plasma membrane of cervical cells. Oncogene. 27:1071–1078.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Irwin ME, Mueller KL, Bohin N, Ge Y and
Boerner JL: Lipid raft localization of EGFR alters the response of
cancer cells to the EGFR tyrosine kinase inhibitor gefitinib. J
Cell Physiol. 226:2316–2328. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Roy M, Kung HJ and Ghosh PM: Statins and
prostate cancer: Role of cholesterol inhibition vs. prevention of
small GTP-binding proteins. Am J Cancer Res. 1:542–561.
2011.PubMed/NCBI
|
|
41
|
Herrero-Martin G and López-Rivas A:
Statins activate a mitochondria-operated pathway of apoptosis in
breast tumor cells by a mechanism regulated by ErbB2 and dependent
on the prenylation of proteins. FEBS Lett. 582:2589–2594. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kwan ML, Habel LA, Flick ED, Quesenberry
CP and Caan B: Post-diagnosis statin use and breast cancer
recurrence in a prospective cohort study of early stage breast
cancer survivors. Breast Cancer Res Treat. 109:573–579. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Cardwell CR, Hicks BM, Hughes C and Murray
LJ: Statin use after diagnosis of breast cancer and survival: A
population-based cohort study. Epidemiology. 26:68–78. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhang Y, Wang L, Zhang M, Jin M, Bai C and
Wang X: Potential mechanism of interleukin-8 production from lung
cancer cells: An involvement of EGF-EGFR-PI3K-Akt-Erk pathway. J
Cell Physiol. 227:35–43. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Vallianou NG, Kostantinou A, Kougias M and
Kazazis C: Statins and cancer. Anticancer Agents Med Chem.
14:706–712. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Hsieh SC, Tsai JP, Yang SF, Tang MJ and
Hsieh YH: Metformin inhibits the invasion of human hepatocellular
carcinoma cells and enhances the chemosensitivity to sorafenib
through a downregulation of the ERK/JNK-mediated NF-κB-dependent
pathway that reduces uPA and MMP-9 expression. Amino Acids.
46:2809–2822. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wang F, Ke ZF, Wang R, Wang YF, Huang LL
and Wang LT: Astrocyte elevated gene-1 (AEG-1) promotes
osteosarcoma cell invasion through the JNK/c-Jun/MMP-2 pathway.
Biochem Biophys Res Commun. 452:933–939. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Liu B, Li G, Wang X and Liu Y: A furin
inhibitor downregulates osteosarcoma cell migration by
downregulating the expression levels of MT1-MMP via the Wnt
signaling pathway. Oncol Lett. 7:1033–1038. 2014.PubMed/NCBI
|