Introduction
Meningeal metastasis is a metastatic carcinoma of
the central nervous system caused by diffuse dissemination or focal
infiltration of malignant tumor cells in meninges and spinal
subarachnoid space, secondary to leukemia, lymphoma, lung cancer
and breast cancer (1). The disease is
characterized by rapid progression and poor prognosis (2). The median survival time of patients with
meningeal metastasis, who did not undergo treatment, was only 4–6
weeks (2). Therefore, to seek a
sensitive method in the detection of potential meningeal metastasis
is extremely necessary. Previous studies have identified that blood
circulating tumor cells (CTCs), namely solid tumor cells falling
into the blood, have a high correlation with tumor diagnosis
(3), metastasis and prognosis
(4,5).
In the present study, CTCs in the cerebrospinal fluid (CSF) of
patients with meningeal metastasis from lung cancer were detected
using immunofluorescence in situ hybridization (immuno-FISH)
technology and the detection value of the CTCs were evaluated in
the adjuvant diagnosis of meningeal metastasis from lung cancer.
The judging criterion for CTCs in the optimal CSF was selected to
improve the early diagnostic rate of meningeal metastasis from lung
cancer.
Materials and methods
General patient data
Patients in the present study were enrolled at
Tianjin Huanhu Hospital (Tianjin, China). The inclusion criteria
was as follows: i) Patients with non-small cell lung cancer
definitely diagnosed by histology or cytology; ii) patients
diagnosed with meningeal metastasis*; iii) no intracranial
metastatic lesions with a diameter >1 cm determined via magnetic
resonance imaging (MRI); iv) no history of encephalitis and
craniocerebral trauma within 6 months, and no brain surgery or
radiotherapy; v) controllable intracranial hypertension following
treatment with dehydrated drugs; vi) tolerable to lumber puncture
in the collection of CSF; vii) exclusion of patients complicated by
cerebrospinal lesions, including intracranial meningioma,
ependymoma and spinal meningioma; and viii) providing informed
consent. According to the aforementioned inclusion criteria, a
total of 16 patients were enrolled, in which there were 2 males and
14 females with a median age of 62 years (range, 46–76 years). They
all pertained to lung adenocarcinoma, and 5 cases were complicated
by brain parenchyma metastasis. *Diagnostic criteria for meningeal
metastasis: i) Specific tumor history; ii) presence of newly-onset
nervous system symptoms and signs; iii) typical enhanced MRI
manifestations in the brain; and iv) identification of tumor cells
in CSF by cytological examination. The diagnosis could be made if
the patient had the former two and the third or the fourth items
(6).
The inclusion criteria for the patients with
non-tumor diseases in the brain was as follows: i) Patients with
non-tumor diseases in the brain, who were admitted to hospital at
the same time as those in the experimental group; ii) Patients who
required treatment with extra ventricular drainage, a
ventriculoperitoneal shunt, lumbar cistern drainage and lumber
puncture for CSF examination during hospitalization; iii) providing
informed consent. The study was approved by the Ethics Committee of
Tianjin Huanhu Hospital (Tianjin, China). According to the above
inclusion criteria, a total of 8 patients were enrolled, in which
there were 6 males and 2 females with the median age of 40 (35~60
years old). They were all admitted into hospital due to
communicating hydrocephalus.
Immuno-FISH
CSF (7.5 ml) was collected from the patients with
meningeal metastasis via lumber puncture and stored in the
specialized tube for immuno-FISH detection at room temperature. CSF
(7.5 ml) was also collected from the patients with non-tumor
diseases in the brain through an external ventricular drainage tube
and preserved in a specialized tube for immuno-FISH detection at
room temperature. The tumor cells were detected within 72 h through
immuno-FISH technology. The detection steps were as follows: i) A
negative screening method with immunomagnetic beads was used to
enrich the cells: Immunomagnetic beads coated with anti-cluster of
differentiation (CD)45 antibody (part of the Human Circulating Rare
Cell Subtraction Enrichment kit; Cytelligen, Inc., San Diego, CA,
USA) were adopted to remove CD45-positive white cells, and 7.5 ml
CSF was added to 100 µl cell suspension. ii) Using cell fixative
(20% acetic acid in methanol mixed solution) 100 µl of cell
suspension was fixed on the slide. Subsequently, centromeric probe
8 (CEP8) was used to detect certain factors for tumor marker-iFISH,
including the numbers of chromosome 8, and the expression of
PAN-cytokeratin (CK) (which is present in cells derived from the
epithelium) and CD45 (which demonstrates that cells are
non-leukocytes. The cells were stained with DAPI as they were
karyocytes. Cell count was performed using an Olympus-BX53
fluorescence microscope (Olympus Corporation, Tokyo, Japan) and
repeated 5 times. The mean was used as the final value.
Judging criteria for CTCs
DAPI staining was observed under the blue channel
using a fluorescence microscope, and blue fluorescence represented
karyocytes. CEP8 FISH signals were observed under the orange
channel, and the number of orange light spots reflected chromosome
8 number. The majority of chromosome 8 copies in the CTCs were
polyploidy, identified by positive CEP8. Chromosome 8 in
hematogenous white cells was diploid, identified by negative CEP8.
Expression of PAN-CK and CD45 were observed under the green and red
channels, respectively, and both green and red fluorescences were
identified as being positive. The tumor cells from non-hematogenous
epithelial cells were also detected under various channels. CTCs
were evaluated according to whether chromosome 8 originated from
diploid nucleate, non-hematogenous cells, namely DAPI+,
CD45− and PAN-CK+ or PAN-CK− and
CEP8−.
The judging criteria for CTCs included the
following: i) DAPI+, CD45− and
PAN-CK+ or PAN-CK− and CEP8+ or
CEP8−; and ii) DAPI+, CD45− and
PAN-CK+ or PAN-CK− and CEP8+. The
cells pertained to white cells when DAPI+,
CD45+, PAN-CK− and CEP8− were all
present (Fig. 1).
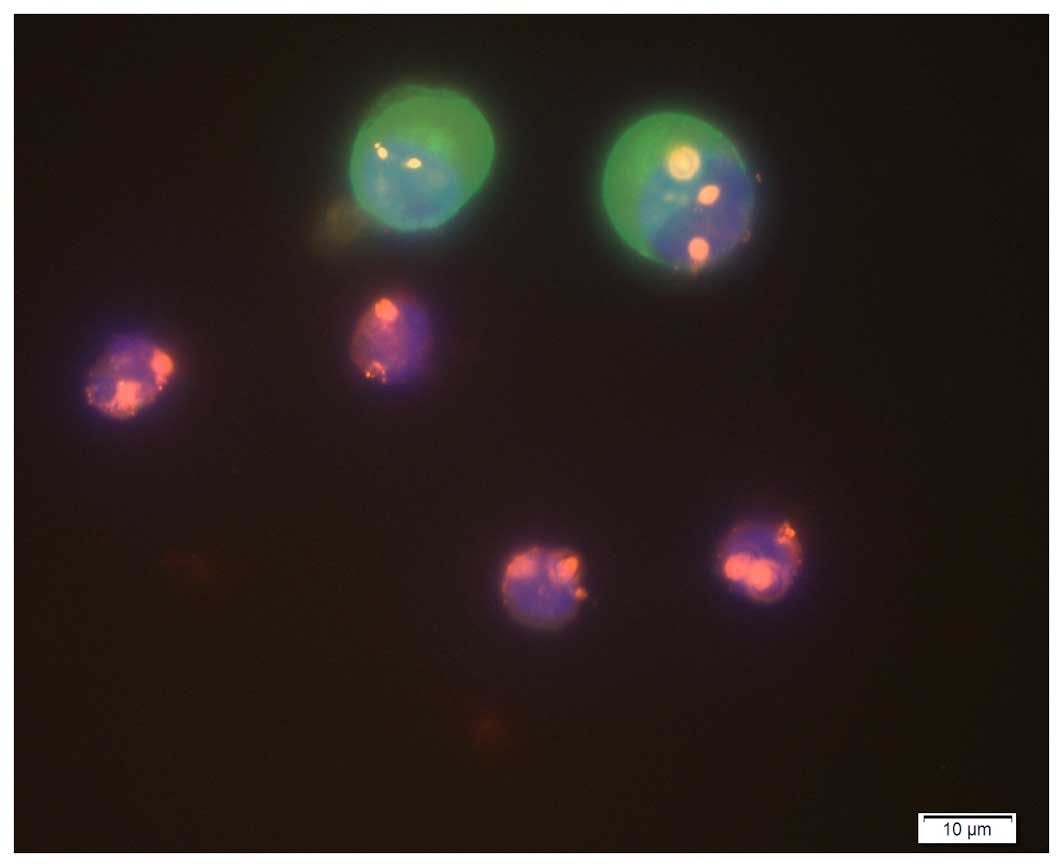 | Figure 1.Image of enriched cells under a
fluorescence microscope (magnification, ×400). Circulating tumor
cells: Positive DAPI staining, blue fluorescence; highly expressed
PAN-CK, green fluorescence; CEP8, polyploidy; and CD45, no
expression. White cells: Positive DAPI staining, blue fluorescence;
PAN-CK, no expression; CEP8, diploid; and CD45, red fluorescence.
CK, cytokeratin; CD, cluster of differentiation. |
Statistical analysis
SPSS v17.0 (SPSS, Inc., Chicago, IL, USA) was used
for data analysis. Measurement data were compared using the
rank-sum test and were expressed as the mean ± standard deviation
(x±s). According to receiver operating characteristic (ROC)
curves, the diagnostic sensitivity, specificity, effectiveness, and
positive and negative predictive values were calculated. P<0.05
was considered to indicate a statistically significant
difference.
Results
Detection results of CTCs based on
different judging criteria
According to criterion 1, CTCs were identified in
12/16 CSF samples from patients with meningeal metastasis and in
2/8 CSF samples from the patients with non-tumor diseases in the
brain. According to criterion 2, CTCs were detected in 12/16 CSF
samples from the patients with meningeal metastasis, while no CTCs
were identified in the 8 CSF samples from patients with non-tumor
diseases in the brain. Based on the aforementioned criteria, the
number of tumor cells in the CSF of patients with meningeal
metastasis was significantly higher than those with non-tumor
diseases in the brain (P=0.009; P=0.002) (Table I). The number of tumor cells in the
patients with meningeal metastasis and non-tumor diseases in the
brain based on criterion 1 was more than that on criterion 2, but
was not statistically significant (P=0.531; P=0.062) (Table I).
 | Table I.Detection results of circulating tumor
cells based on different judging criteria (x±s)
(number of tumor cells/7.5 ml cerebrospinal fluid). |
Table I.
Detection results of circulating tumor
cells based on different judging criteria (x±s)
(number of tumor cells/7.5 ml cerebrospinal fluid).
| Judging criteria | Patients with
meningeal metastasis | Patients with
non-tumor diseases in the brain | Z | P-value |
|---|
| Criterion 1 | 277.81±523.21 | 0.88±1.25 | −2.612 | 0.009 |
| Criterion 2 | 243.25±489.67 | 0.00 | −3.142 | 0.002 |
| Z | −0.267 | −1.852 | – | – |
| P |
0.531 | 0.062 | – | – |
Diagnostic critical values of CTCs
based on different judging criteria
At present, there are no unified evaluation
standards or diagnostic critical values regarding the detection of
tumor cells in CSF for the diagnosis of meningeal metastasis from
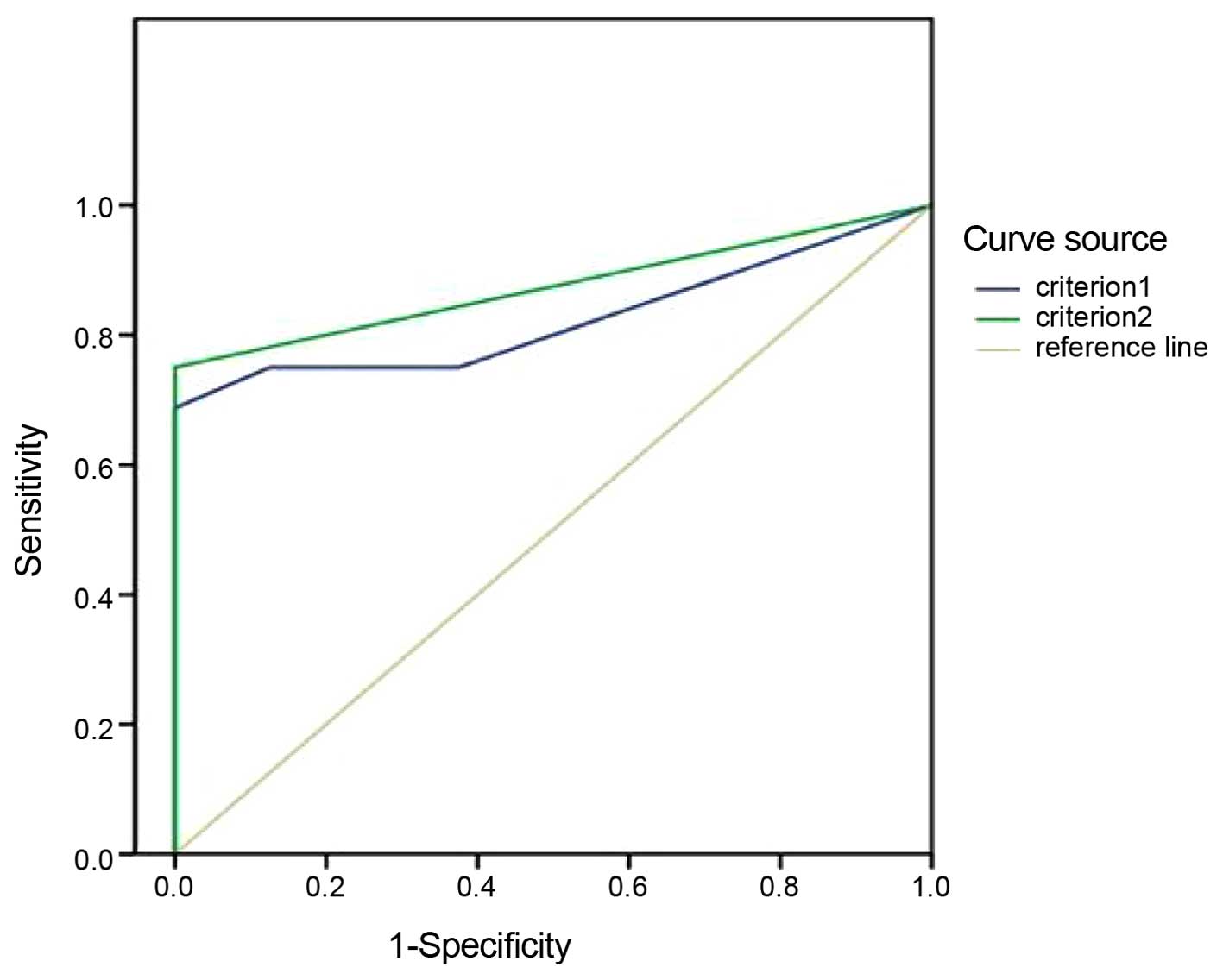
lung cancer. According to the aforementioned criteria, the AUCs and
critical points of tumor cell detection indexes in the CSF samples
were confirmed based on the ROC curves, and the results indicated
that the AUC based on criterion 1 was less than that of criterion 2
(Table II; Fig. 2).
 | Table II.AUC, standard deviation, P-value and
95% CI of circulating tumor cells in the diagnosis of meningeal
metastasis by immunofluorescence in situ hybridization. |
Table II.
AUC, standard deviation, P-value and
95% CI of circulating tumor cells in the diagnosis of meningeal
metastasis by immunofluorescence in situ hybridization.
| Judging criteria | AUC | Standard
deviation | P-value | 95% CI |
|---|
| Criterion 1 | 0.824 | 0.085 | 0.011 | 0.657–0.991 |
| Criterion 2 | 0.875 | 0.072 | 0.003 | 0.705–1.000 |
The critical point of the maximum correct diagnostic
index (Youden index) was regarded as the positive judgement value
in the ROC curves. When criterion 1 was used for judging CTCs in
the diagnosis of meningeal metastasis, the maximum Youden index was
0.688, and the diagnostic critical value of CTCs, sensitivity and
specificity were 3 tumor cells/7.5 ml CSF, 68.8 and 100.0%,
respectively. When criterion 2 was adopted to judge CTCs in the
diagnosis of meningeal metastasis, the maximum Youden index was
0.750, and diagnostic critical value of CTCs, sensitivity and
specificity were 1 tumor cell/7.5 ml CSF, 75.0 and 100.0%,
respectively (Table III).
 | Table III.Lower co-ordinate points of ROC curves
corresponding to CTCs based on two judging criteria. |
Table III.
Lower co-ordinate points of ROC curves
corresponding to CTCs based on two judging criteria.
| Variables | Diagnostic
points | Sensitivity | 1-specificity | Youden
indexa |
|---|
| CTCs based | 1 | 0.750 | 0.625 | 0.375 |
| on criterion 1 | 2 | 0.750 | 0.125 | 0.625 |
|
| 3 | 0.688 | 0.000 | 0.688 |
|
| 32 | 0.500 | 0.000 | 0.500 |
|
| 197 | 0.250 | 0.000 | 0.250 |
|
| 1,824 | 0.000 | 0.000 | 0.000 |
| CTCs based | 1 | 0.750 | 0.000 | 0.750 |
| on criterion 2 | 6 | 0.625 | 0.000 | 0.625 |
|
| 12 | 0.500 | 0.000 | 0.500 |
|
| 150 | 0.250 | 0.000 | 0.250 |
|
| 1,639 | 0.000 | 0.000 | 0.000a |
Sensitivity, specificity,
effectiveness, and positive and negative predictive values of CTCs
in CSF in the diagnosis of meningeal metastasis
The specificity, effectiveness, and positive and
negative predictive values of CTCs in CSF in the diagnosis of
meningeal metastasis based on criterion 2 were all higher than
criterion 1, while sensitivity was the same (Table IV).
 | Table IV.Relevant indexes of circulating tumor
cells in cerebrospinal fluid in the diagnosis of meningeal
metastasis (%). |
Table IV.
Relevant indexes of circulating tumor
cells in cerebrospinal fluid in the diagnosis of meningeal
metastasis (%).
| Judging criteria | Sensitivity | Specificity | Effectiveness | Positive predictive
value | Negative predictive
value |
|---|
| Criterion 1 | 75.0 | 75.0 | 75.0 | 85.7 | 60.0 |
| Criterion 2 | 75.0 | 100.0 | 83.3 | 100.0 | 66.7 |
Discussion
Meningeal metastasis from lung cancer is
characterized by rapid progression of the pathological condition
and poor prognosis (2). Early
diagnosis and treatment may effectively alleviate neurological
impairment due to progression (2). At
present, the following criteria are primarily adopted to diagnose
meningeal metastasis: i) Definite history of tumors; ii) presence
of newly-onset nervous system symptoms and signs; iii) typical
manifestations of enhanced MRI; and iv) presence of tumor cells via
CSF cytology (7). Diagnosis may be
made immediately when the former two and the third or fourth items
are present. Nevertheless, typical symptoms and signs associated
with the central nervous system do not manifest in >90% of
patients with meningeal metastatic carcinoma (6). Due to the different invasive sites of
tumor cells, the clinical manifestations of meningeal metastatic
carcinoma are complicated, varied and short of specificity, which
makes identification difficult when symptoms caused by brain
parenchyma and spinal cord metastases are present, in addition to
adverse reactions to treatment for primary tumors. The positive
rate of CSF cytology examination was only 55% through CSF cytology,
and a second examination found a rate of 80%; however, performing a
third examination did not increased the positive rate any further
(8). The specificity of enhanced
brain MRI is close to 100%, however, a 65% false-negative rate and
10% false-positive rate still exists (9). Therefore, the aforementioned diagnostic
methods cannot meet the demand of clinicians in the diagnosis and
efficacy evaluation of meningeal metastatic carcinoma. It is thus
necessary to identify a detection method with higher sensitivity
and specificity. Immuno-FISH detection is able to effectively
identify various non-hematogenous epithelial tumor cells in
biological fluids by enrichment and analysis techniques. Previous
studies have demonstrated that detection of CTCs in CSF samples of
patients with meningeal metastasis from lung cancer had higher
sensitivity compared to cytology examination (10,11).
Therefore, in the present study, immuno-FISH technology was used to
detect CTCs in patients with meningeal metastasis and non-tumor
diseases in the brain, and its value was investigated in the
adjuvant diagnosis of meningeal metastasis from lung cancer.
Clinically, an ideal detection method should possess
100% specificity and sensitivity, and reliable predictive value;
however, it is challenging to apply these methods in clinical
settings due to the complicated processes of tumorigenesis,
progression and prognosis. Selection of the indexes with higher
sensitivity and specificity must respectively lead to increase of
false-positive and -negative rates. Positive predictive value
refers to the proportion of really-positive patients in the total
number of positive cases detected by screening tests. Positive
predictive value may reflect the possibility that patients may
develop the disease, based on screening results. Negative
predictive value refers to the proportion of really-negative
population in the total number of negative cases detected by
screening tests. The predictive value of diagnostic trials is
impacted by sensitivity, specificity and morbidity among the
subjects. ROC curves are able to control the false-positive and
-negative rates under a small range if it is used to confirm the
critical value of diagnostic trials. In the present study, the
critical point of the maximum correct diagnostic index (Youden
index) was regarded as the judging criterion for positive tumor
cells in CSF, and sensitivity and specificity were taken into
account to the greatest extent (12).
AUC has been widely recognized as a fixed accuracy index that is
able to correctly evaluate the diagnostic trials. The AUCs of
completely valueless and ideal diagnostic trials are 0.5 and 1,
respectively (13). Nevertheless, it
is generally considered that the diagnostic value is lower,
moderate and higher if the AUC of this diagnostic trial is 0.5–0.7,
0.7–0.9 and >0.9, respectively (13).
In the current study, the difference between
criterion 1 and 2 was whether non-hematogenous cells with negative
chromosome 8 copies were determined as CTCs. The number of tumor
cells in the patients with meningeal metastasis and non-tumor
diseases in the brain based on criterion 1 was more than that of
criterion 2, but was not statistically significant (P>0.05). In
the diagnosis of meningeal metastasis, each index of tumor cells in
CSF based on criterion 2 was superior to criterion 1. The
sensitivities of the CTCs were 75% in the diagnosis of meningeal
metastasis according to two detection criteria, but based on
criterion 2, the diagnostic specificity, effectiveness, and
positive and negative predictive values of the CTCs in CSF were
higher in the diagnosis of meningeal metastasis from lung cancer.
Therefore, it is recommended to use criterion 2 (DAPI+,
CD45− and PAN-CK+ or PAN-CK− and
CEP8+) to evaluate CTCs in CSF. Additionally,
non-hematogenous cells with negative chromosome 8 may be deciduous
meningocytes or epidermal cells, arising as a result particular
clinical operations, including lumber puncture and extra
ventricular drainage, that enter into the CSF samples and
consequently lead to an increase in the diagnostic false-positive
rate. However, whether removal of these types of cells is able to
increase the false-negative rate due to a small sample size, as in
the present study, remains to be elucidated.
In conclusion, when criterion 2 was adopted by the
current study to judge CTCs in the diagnosis of meningeal
metastasis, the AUC, 95% CI, P-value and maximum of Youden index
were 0.875, 0.705–1.000, 0.003 and 0.750, respectively. The
diagnostic critical value of CTCs was 1 tumor cell/7.5 ml CSF.
Namely, when one CTC was identified in 7.5 ml CSF from patients
with meningeal metastasis, the sensitivity, specificity,
effectiveness, and positive and negative predictive values of
diagnosing meningeal metastasis were 75.0, 100.0, 83.3, 100.0 and
66.7%, respectively. Therefore, detection of tumor cells in CSF has
better clinical value in the diagnosis of meningeal metastasis from
lung cancer via immuno-FISH technology. As a small sample size was
used in the current study, further clinical experiments are
required to verify whether multiple detections of one sample is
able to increase the diagnostic sensitivity. Further study is of
great importance for the detection of CTCs in CSF in the evaluation
of efficacy and prognosis.
Acknowledgements
The present study received funding from the Tianjin
Municipal Health Bureau, which supports science and technology
projects (no. 2014kz042).
References
|
1
|
Leal T, Chang JE, Mehta M and Robins HI:
Leptomeningeal metastasis: Challenges in diagnosis and treatment.
Curr Cancer Ther Rev. 7:319–327. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nagpal S, Riess J and Wakelee H: Treatment
of leptomeningeal spread of NSCLC: A continuing challenge. Curr
Treat Options Oncol. 13:491–504. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yu Y, Chen Z, Dong J, Wei P, Hu R, Zhou C,
Sun N, Luo M, Yang W, Yao R, et al: Folate receptor-positive
circulating tumor cells as a novel diagnostic biomarker in
non-small cell lung cancer. Transl Oncol. 6:697–702. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Igawa S, Gohda K, Fukui T, Ryuge S, Otani
S, Masago A, Sato J, Murakami K, Maki S, Katono K, et al:
Circulating tumor cells as a prognostic factor in patients with
small cell lung cancer. Oncol Lett. 7:1469–1473. 2014.PubMed/NCBI
|
|
5
|
Romiti A, Raffa S, Di Rocco R, Roberto M,
Milano A, Zullo A, Leone L, Ranieri D, Mazzetta F, Medda E, et al:
Circulating tumor cells count predicts survival in colorectal
cancer patients. J Gastrointestin Liver Dis. 23:279–284.
2014.PubMed/NCBI
|
|
6
|
Ma C, Jiang R, Li J, Wang B, Sun L and Lv
Y: Research progress of lung cancer with leptomeningeal metastasis.
Zhongguo Fei Ai Za Zhi. 17:695–700. 2014.(In Chinese). PubMed/NCBI
|
|
7
|
Wang Y, Gao Y, Zhu YF and Tao RJ: The
diagnosis and treatments progress of meningeal carcinomatosis.
Zhongguo Lin Chuang Shen Jing Wai Ke Za Zhi. 18:760–762. 2013.
|
|
8
|
Le Rhun E, Massin F, Tu Q, Bonneterre J,
Mde C Bittencourt and Faure GC: Development of a new method for
identification and quantification in cerebrospinal fluid of
malignant cells from breast carcinoma leptomeningeal metastasis.
BMC Clin Pathol. 12:212012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Clarke JL, Perez HR, Jacks LM, Panageas KS
and Deangelis LM: Leptomeningeal metastases in the MRI era.
Neurology. 74:1449–1454. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jiang R, Ma CH, Zhu ZL, Li JD, Wang B, Sun
LW and Lv Y: Application of circulating tumor cell detection in
cerebrospinal fluid in the diagnosis of meningeal metastasis from
non-small cell lung cancer. Chin J Contemp Neurol Neurosurg.
14:698–701. 2014.
|
|
11
|
Ma CH, Jiang R, Li JD, Wang B, Sun LW and
Lv Y: A new method for enrichment and calculation of malignant
cells in cerebrospinal fluid of patients with meningeal metastasis
from lung cancer. Tianjin Medical Journal. 43:419–421. 2015.
|
|
12
|
Wang P, She CH, Li P, Pu YZ, Wang XG and
Li WL: Application of tumor markers in the adjuvant diagnosis of
meningeal metastasis from lung cancer. Chin J Clin Oncol. 35:61–64.
2008.
|
|
13
|
Wang JH: Application of ROC curve in
clinical diagnostic experiments. Chin J Hypertens. 16:175–177.
2008.
|