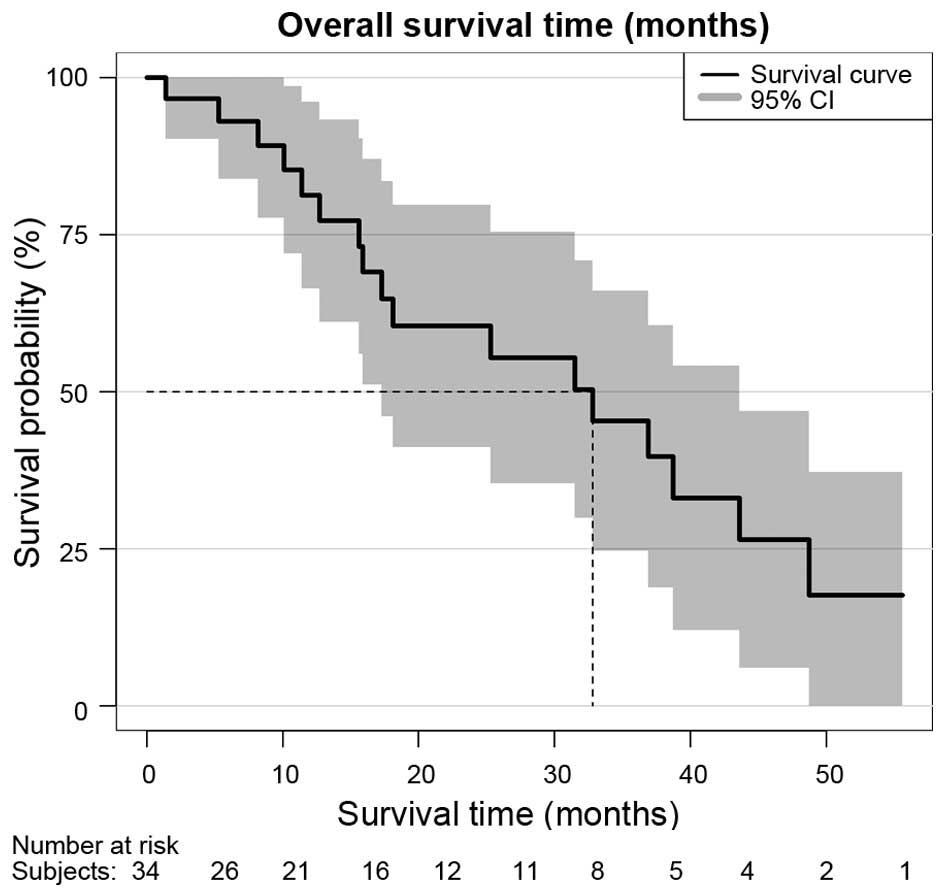
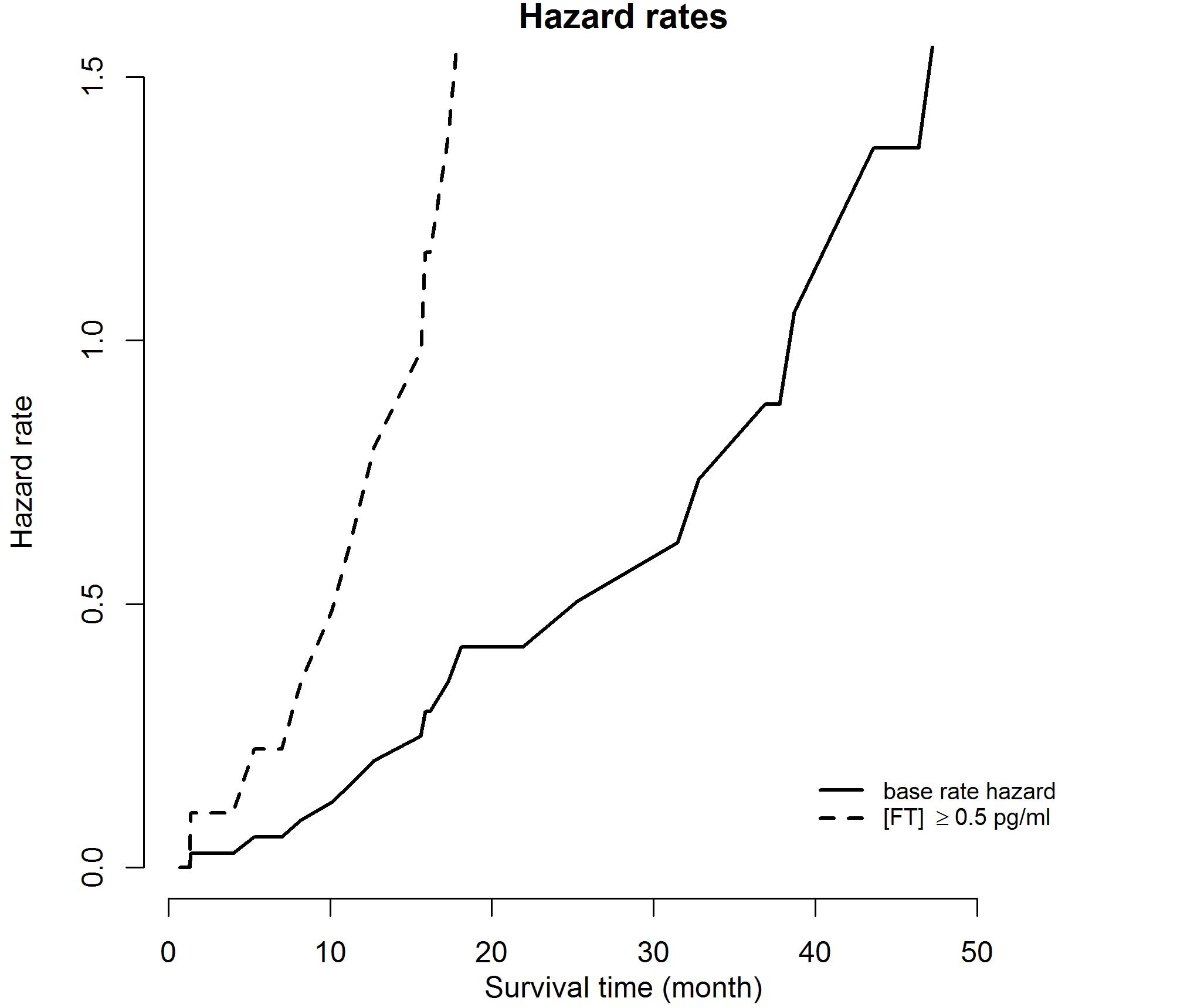
|
1
|
Malvezzi M, Bertuccio P, Levi F, La
Vecchia C and Negri E: European cancer mortality predictions for
the year 2013. Ann Oncol. 24:792–800. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Huggins C, Stevens RE and Hodges CV:
Studies on prostate cancer. II. The effects of castration on
advanced carcinoma of the prostate gland. Arch Surg. 43:209–223.
1941. View Article : Google Scholar
|
|
3
|
Walsh PC: Physiologic basis for hormonal
therapy in carcinoma of the prostate. Urol Clin North Am.
2:125–140. 1975.PubMed/NCBI
|
|
4
|
Merseburger AS, Hammerer P, Rozet F,
Roumeguère T, Caffo O, da Silva FC and Alcaraz A: Androgen
deprivation therapy in castrate-resistant prostate cancer: How
important is GnRH agonist backbone therapy? World J Urol.
33:1079–1085. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Faris JE and Smith MR: Metabolic sequelae
associated with androgen deprivation therapy for prostate cancer.
Curr Opin Endocrinol Diabetes Obes. 17:240–246. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Heidenreich A, Bastian PJ, Bellmunt J,
Bolla M, Joniau S, van der Kwast T, Mason M, Matveev V, Wiegel T,
Zattoni F, et al: EAU guidelines on prostate cancer. Part II:
Treatment of advanced, relapsing and castration-resistant prostate
cancer. Eur Urol. 65:467–479. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Scher HI, Halabi S, Tannock I, Morris M,
Sternberg CN, Carducci MA, Eisenberger MA, Higano C, Bubley GJ,
Dreicer R, et al: Design and end points of clinical trials for
patients with progressive prostate cancer and castrate levels of
testosterone: Recommendations of the prostate cancer clinical
trials working group. J Clin Oncol. 26:1148–1159. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fizazi K, Scher HI, Molina A, Logothetis
CJ, Chi KN, Jones RJ, Staffurth JN, North S, Vogelzang NJ, Saad F,
et al: Abiraterone acetate for treatment of metastatic
castration-resistant prostate cancer: Final overall survival
analysis of the COU-AA-301 randomised, double-blind,
placebo-controlled phase 3 study. Lancet Oncol. 13:983–992. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ryan CJ, Smith MR, de Bono JS, Molina A,
Logothetis CJ, de Souza P, Fizazi K, Mainwaring P, Piulats JM, Ng
S, et al: Abiraterone in metastatic prostate cancer without
previous chemotherapy. N Engl J Med. 368:138–148. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
de Bono JS, Oudard S, Ozguroglu M, Hansen
S, Machiels JP, Kocak I, Gravis G, Bodrogi I, Mackenzie MJ, Shen L,
et al: Prednisone plus cabazitaxel or mitoxantrone for metastatic
castration-resistant prostate cancer progressing after docetaxel
treatment: A randomised open-label trial. Lancet. 376:1147–1154.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mendel CM: The free hormone hypothesis: A
physiologically based mathematical model. Endocr Rev. 10:232–274.
1989. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Moreno SA, Shyam A and Morgentaler A:
Comparison of free testosterone results by analog radioimmunoassay
and calculated free testosterone in an ambulatory clinical
population. J Sex Med. 7:1948–1953. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Trial of Abiraterone Acetate Plus
LHRH-therapy Versus Abiraterone Acetate Sparing LHRH-therapy in
Patients With Progressive Chemotherapy-naïve Castration-resistant
Prostate Cancer (SPARE) (SPARE). https://clinicaltrials.gov/ct2/show/NCT02077634Accessed
June 12, 2016.
|
|
14
|
Oken MM, Creech RH, Tormey DC, Horton J,
Davis TE, McFadden ET and Carbone PP: Toxicity and response
criteria of the Eastern Cooperative Oncology Group. Am J Clin
Oncol. 5:649–655. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Reuter CWM, Morgan MA, Ivanyi P, Fenner M,
Ganser A and Grünwald V: Carboplatin plus weekly docetaxel as
salvage chemotherapy in docetaxel-resistant and
castration-resistant prostate cancer. World J Urol. 28:391–398.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gleason DF and Mellinger GT: Prediction of
prognosis for prostatic adenocarcinoma by combined histological
grading and clinical staging. J Urol. 111:58–64. 1974.PubMed/NCBI
|
|
17
|
Sobin LH and Compton CC: TNM seventh
edition: What's new, what's changed: Communication from the
International Union Against Cancer and the American Joint Committee
on Cancer. Cancer. 116:5336–5339. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Huggins C and Hodges CV: Studies on
prostatic cancer. I. The effect of castration, of estrogen and of
androgen injection on serum phosphatases in metastatic carcinoma of
the prostate. 1941. J Urol. 167:948–952. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Attard G, Belldegrun AS and de Bono JS:
Selective blockade of androgenic steroid synthesis by novel lyase
inhibitors as a therapeutic strategy for treating metastatic
prostate cancer. BJU Int. 96:1241–1246. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tran C, Ouk S, Clegg NJ, Chen Y, Watson
PA, Arora V, Wongvipat J, Smith-Jones PM, Yoo D, Kwon A, et al:
Development of a second-generation antiandrogen for treatment of
advanced prostate cancer. Science. 324:787–790. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Perachino M, Cavalli V and Bravi F:
Testosterone levels in patients with metastatic prostate cancer
treated with luteinizing hormone-releasing hormone therapy:
Prognostic significance? BJU Int. 105:648–651. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Morote J, Orsola A, Planas J, Trilla E,
Raventós CX, Cecchini L and Catalán R: Redefining clinically
significant castration levels in patients with prostate cancer
receiving continuous androgen deprivation therapy. J Urol.
178:1290–1295. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Byar DP: Proceedings: The veterans
administration cooperative urological research group's studies of
cancer of the prostate. Cancer. 32:1126–1130. 1973. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hussain M, Wolf M, Marshall E, Crawford ED
and Eisenberger M: Effects of continued androgen-deprivation
therapy and other prognostic factors on response and survival in
phase II chemotherapy trials for hormone-refractory prostate
cancer: A southwest oncology group report. J Clin Oncol.
12:1868–1875. 1994.PubMed/NCBI
|
|
25
|
Taylor CD, Elson P and Trump DL:
Importance of continued testicular suppression in
hormone-refractory prostate cancer. J Clin Oncol. 11:2167–2172.
1993.PubMed/NCBI
|
|
26
|
de Bono JS, Logothetis CJ, Molina A,
Fizazi K, North S, Chu L, Chi KN, Jones RJ, Goodman OB Jr, Saad F,
et al: COU-AA-301 Investigators: Abiraterone and increased survival
in metastatic prostate cancer. N Engl J Med. 364:1995–2005. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ryan CJ, Peng W, Kheoh T, Welkowsky E,
Haqq CM, Chandler DW, Scher HI and Molina A: Androgen dynamics and
serum PSA in patients treated with abiraterone acetate. Prostate
Cancer Prostatic Dis. 17:192–198. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Oefelein MG and Cornum R: Failure to
achieve castrate levels of testosterone during luteinizing hormone
releasing hormone agonist therapy: The case for monitoring serum
testosterone and a treatment decision algorithm. J Urol.
164:726–729. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sharifi R and Browneller R: Leuprolide
Study Group: Serum testosterone suppression and potential for
agonistic stimulation during chronic treatment with monthly and
3-month depot formulations of leuprolide acetate for advanced
prostate cancer. J Urol. 168:1001–1004. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Smith MR: Obesity and sex steroids during
gonadotropin-releasing hormone agonist treatment for prostate
cancer. Clin Cancer Res. 13:241–245. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Colombel M, Symmans F, Gil S, O'Toole KM,
Chopin D, Benson M, Olsson CA, Korsmeyer S and Buttyan R: Detection
of the apoptosis-suppressing oncoprotein bc1-2 in
hormone-refractory human prostate cancers. Am J Pathol.
143:390–400. 1993.PubMed/NCBI
|
|
32
|
Sun M, Yang L, Feldman RI, Sun XM, Bhalla
KN, Jove R, Nicosia SV and Cheng JQ: Activation of
phosphatidylinositol 3-kinase/Akt pathway by androgen through
interaction of p85alpha, androgen receptor, and src. J Biol Chem.
278:42992–43000. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Craft N, Shostak Y, Carey M and Sawyers
CL: A mechanism for hormone-independent prostate cancer through
modulation of androgen receptor signaling by the hER-2/neu tyrosine
kinase. Nat Med. 5:280–285. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
Antonarakis ES, Lu C, Wang H, Luber B,
Nakazawa M, Roeser JC, Chen Y, Mohammad TA, Chen Y, Fedor HL, et
al: AR-v7 and resistance to enzalutamide and abiraterone in
prostate cancer. N Engl J Med. 371:1028–1038. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Egan A, Dong Y, Zhang H, Qi Y, Balk SP and
Sartor O: Castration-resistant prostate cancer: Adaptive responses
in the androgen axis. Cancer Treat Rev. 40:426–433. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Merseburger AS, Kuczyk MA and Wolff JM:
Pathophysiology and therapy of castration-resistant prostate
cancer. Urologe A. 52:219–225. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Pinski J, Xiong S, Wang Q, Stanczyk F,
Hawes D and Liu SV: Effect of luteinizing hormone on the
steroidogenic pathway in prostate cancer. Prostate. 71:892–898.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Mostaghel EA, Nelson PS, Lange P, Lin DW,
Taplin ME, Balk S, Ellis W, Kantoff P, Marck B, Tamae D, et al:
Targeted androgen pathway suppression in localized prostate cancer:
A pilot study. J Clin Oncol. 32:229–237. 2014. View Article : Google Scholar : PubMed/NCBI
|