Introduction
Endometrial carcinoma is one of the most common
gynecological malignancies, and its incidence is rising (1). Despite improved surgical treatment and
the development of adjuvant therapy, the prognosis of endometrial
carcinoma has not improved significantly (2,3). Thus, it
is important to identify molecular mediators conferring the
malignant potential to endometrial carcinoma cells that may be used
as tumor markers for predicting the risk of endometrial carcinoma
progression (4–6).
V-Crk avian sarcoma virus CT10 oncogene homolog-like
(CRKL) is a member of the CRK family of adapter proteins, which
have a variety of biological roles, including cell proliferation,
adhesion and migration (7). CRKL was
considered to be a key substrate of the break point cluster-Abelson
murine leukemia viral oncogene homolog 1 fusion protein in chronic
myeloid leukemia (8–10). The CRKL protein has been reported to
be upregulated in several malignant cancers (11). CRKL amplification and protein
overexpression was observed in lung cancer, pancreatic cancer and
colorectal cancer (12–14). In addition, CRKL was demonstrated to
facilitate cell malignant invasion, and correlated with poor
patient prognosis (15,16). Taken together, these findings suggest
that CRKL may serve as an important oncoprotein in cancer
development. However, its expression pattern and biological roles
in endometrial carcinoma remain unexplored.
In the present study, endogenous CRKL protein
expression was examined in 87 endometrial carcinoma specimens. In
addition, CRKL expression was upregulated in the Ishikawa cell
line, and its effect on cell proliferation and apoptosis was
examined. Furthermore, the molecular signaling pathways underlying
the biological effects of CRKL were investigated.
Materials and methods
Patients and specimens
The study protocol was approved by the Institutional
Review Board of Shengjing Hospital of China Medical University
(Shenyang, China) and written informed consent was obtained from
all patients. Primary tumor specimens were obtained from patients
diagnosed with endometrioid adenocarcinoma who underwent resection
in the First Affiliated Hospital (Shenyang, China) and the
Shengjing Hospital of China Medical University between January 2008
and December 2010. The histological diagnosis was evaluated for
sections stained with hematoxylin and eosin according to the World
Health Organization classification guidelines (17). Clinical and histopathological data
were obtained from medical records.
Immunohistochemistry
Surgically excised tumor specimens were fixed with
10% neutral formalin and embedded in paraffin, and 4-µm-thick
sections were prepared. Immunostaining was performed using the
avidin-biotin-peroxidase complex method (Ultra Sensitive™; Maixin,
Fuzhou, China). The sections were deparaffinized in xylene,
rehydrated with graded alcohol, and then boiled in 0.01 M citrate
buffer (pH 6.0) for 2 min in an autoclave. Hydrogen peroxide (0.3%)
was applied to block the endogenous peroxide activity, and the
sections were then incubated with normal goat serum (Invitrogen;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) to reduce
nonspecific binding. Tissue sections were incubated with an
anti-CRKL rabbit polyclonal antibody (1:400; cat. no. ABC242; EMD
Millipore, Billerica, MA, USA) at 4°C overnight. Rabbit
immunoglobulin (Ig) (1:300; cat. no. HPA009178; Sigma-Aldrich, St.
Louis, MO, USA) was used as a negative control, by applying the
same concentration as that used for the above antigen-specific
antibody. Biotinylated goat anti-rabbit serum IgG (1:200; cat. no.
A0545; Sigma-Aldrich) was used applied as the secondary antibody at
room temperature for 10 min. Upon washing with phosphate-buffered
saline (PBS), the sections were incubated with streptavidin-biotin
conjugated with horseradish peroxidase, and the peroxidase reaction
was developed with 3,3′-diaminobenzidine tetrahydrochloride.
Counterstaining with hematoxylin was performed, and the sections
were dehydrated in ethanol prior to mounting.
Two independent blinded investigators examined all
tumor slides randomly. Five views were examined per slide, and 100
cells were observed per view at ×400 magnification. Immunostaining
of CRKL was scored on a semiquantitative scale by evaluating the
staining intensity and the percentage of positively stained tumor
cells. Cytoplasmic and nuclear immunostaining in tumor cells was
considered as positive staining. In total, 400 tumor cells were
counted, and the percentage of positively stained cells was
calculated. The intensity of CRKL staining was scored as 0 (no
signal), 1 (moderate) and 2 (strong). The percentage scores were
assigned as 1 (1–25%), 2 (26–50%), 3 (51–75%) and 4 (76–100%). The
scores of each tumor sample were multiplied to obtain a final score
of 0–8. Tumor samples scoring 4–8 were considered to exhibit CRKL
overexpression.
Cell culture and transfection
The Ishikawa cell line was obtained from the
American Type Culture Collection (Manassas, VA, USA). Cells were
cultured in Dulbecco's modified Eagle medium (Invitrogen; Thermo
Fisher Scientific, Inc.) containing 10% fetal calf serum
(Invitrogen; Thermo Fisher Scientific, Inc.), 100 IU/ml penicillin
(Sigma-Aldrich) and 100 µg/ml streptomycin (Sigma-Aldrich) at 37°C
in an atmosphere of 5% CO2. Cells were grown on sterilized culture
dishes and were passaged every 2 days with 0.25% trypsin
(Invitrogen; Thermo Fisher Scientific, Inc.).
The pCMV6-CRKL plasmid was purchased from OriGene
Technologies, Inc. (Rockville, MD, USA), and was transfected into
the cells using Lipofectamine 2000 reagent (Invitrogen; Thermo
Fisher Scientific, Inc.). The pCMV6 empty vector was used as a
negative control. Cells were harvested 48 h after transfection and
subjected to various analyses.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
RT-qPCR was performed using SYBR® Green
PCR Master Mix (Applied Biosystems; Thermo Fisher Scientific, Inc.)
in a total volume of 20 µl on a 7500 Real-Time PCR System (Applied
Biosystems; Thermo Fisher Scientific, Inc.) as follows: 95°C for 30
sec, and 40 cycles of 95°C for 5 sec and 60°C for 30 sec. A
dissociation step was performed to generate a melting curve to
confirm the specificity of the amplification. β-actin was used as
the reference gene. The relative levels of gene expression were
represented as ΔCq=Cq gene - Cq reference, and the fold-change of
gene expression was calculated by the 2-ΔΔCq method (18). Experiments were conducted in
triplicate. The primer sequences were as follows: CRKL forward,
5′-CCTTTGCCATCCACACAGAAT-3′ and reverse,
5′-TTTCACGATGTCACCAACCTCTA-3′; and β-actin forward,
5′-ATAGCACAGCCTGGATAGCAACGTAC-3′ and reverse,
5′-CACCTTCTACAATGAGCTGCGTGTG-3′.
Western blot analysis
Total proteins from cells were extracted in lysis
buffer (Pierce; Thermo Fisher Scientific, Inc.) and quantified
using the Bradford method. Proteins (50 µg) were separated by 10%
sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Samples
were transferred to polyvinylidene fluoride membranes (EMD
Millipore) and incubated overnight at 4°C with antibodies against
CRKL (rabbit polyclonal; 1:1,000; cat. no. ABC242; EMD Millipore),
B cell lymphoma (Bcl)-2 (rabbit monoclonal; 1:1,000; cat. no. 3948;
Cell Signaling Technology, Inc., Danvers, MA, USA), Bcl-2
associated X protein (Bax) (rabbit monoclonal; 1:1,000; cat. no.
4223; Cell Signaling Technology, Inc.), survivin (rabbit
monoclonal; 1:1,000; cat. no. 2808; Cell Signaling Technology,
Inc.), caspase-3 (rabbit monoclonal; 1:1,000; cat. no. 9665; Cell
Signaling Technology, Inc.), cleaved caspase-3 (rabbit monoclonal;
1:1,000; cat. no. 9664; Cell Signaling Technology, Inc.), caspase-9
(rabbit monoclonal; 1:1,000; cat. no. 9502; Cell Signaling
Technology, Inc.), cleaved caspase-9 (rabbit monoclonal; 1:1,000;
cat. no. 9501; Cell Signaling Technology, Inc.), cyclin D1 (rabbit
monoclonal; 1:1,000; cat. no. 2978; Cell Signaling Technology,
Inc.), cyclin E (rabbit monoclonal; 1:1,000; cat. no. 20808; Cell
Signaling Technology, Inc.) and glyceraldehyde 3-phosphate
dehydrogenase (GAPDH) (rabbit polyclonal; 1:1,000; cat. no.
sc-25778; Santa Cruz Biotechnology, Inc., Dallas, TX, USA). After
incubation with peroxidase-coupled monoclonal mouse anti-rabbit IgG
(1:1,000; cat. no. 5127; Cell Signaling Technology, Inc.) at 37°C
for 2 h, the bound proteins were visualized by enhanced
chemiluminescence (Pierce; Thermo Fisher Scientific, Inc.) and
detected using an imaging system (DNR Bio-Imaging Systems Ltd.,
Jerusalem, Israel). Relative protein levels were quantified using
GAPDH as the loading control.
Colony formation and
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assays
For the colony formation assay, cells were
transfected with the aforementioned plasmids for 48 h, and then
plated into three 6-cm cell culture dishes (~2,000 cells/dish).
Cells were incubated for 12 days in medium containing 10% fetal
bovine serum. Plates were next washed with PBS and stained with
Giemsa. The number of colonies containing >50 cells was counted
manually using a microscope.
For the MTT assay, 24 h after transfection, cells
were plated in 96-well plates at a density of ~3,000 cells/well,
and were cultured for 5 days. For quantitation of cell viability,
20 µl of 5 mg/ml MTT (thiazolyl blue) solution was added to each
well, and incubated for 4 h at 37°C. Subsequently, the medium was
removed from each well, and the resulting MTT formazan was
solubilized in 150 µl of dimethyl sulfoxide. Each solution was
measured spectrophotometrically at 490 nm.
Cell cycle and apoptosis analysis
Cells (100,000) were seeded into 6-cm tissue culture
dishes, and 12 h later were transfected with the indicated amounts
of plasmid (1.2 µg/dish). After 48 h, cells were harvested, fixed
in 1% paraformaldehyde, washed with PBS and stained in 5 mg/ml
propidium iodide (PI) in PBS supplemented with RNase A (Roche
Diagnostics, Indianapolis, IN, USA) for 30 min at room temperature.
Data were collected using a BD system (BD Biosciences, Franklin
Lakes, NJ, USA).
Cell apoptosis detection was performed with Annexin
V/PI double staining. Briefly, 48 h after transfection, cells were
harvested by 0.25% trypsin, washed twice with chilled PBS and
resuspended in 250 µl of binding buffer. Staining solution
containing Annexin V/fluorescein isothiocyanate and PI was added to
the cell suspension. After incubation in the dark for 30 min, cells
were analyzed by FACSCalibur flow cytometer (BD Biosciences).
Statistical analysis
SPSS version 11.5 (SPSS, Inc., Chicago, IL, USA) was
used for all statistical analyses. The χ2 test was used to examine
possible correlations between CRKL expression and
clinicopathological factors. The Student's t-test was used to
compare densitometry data between the control and CRKL-transfected
cells. All P-values are based on a two-sided statistical analysis,
and P<0.05 was considered to indicate a statistically
significant difference.
Results
Expression of CRKL in human
endometrial carcinoma
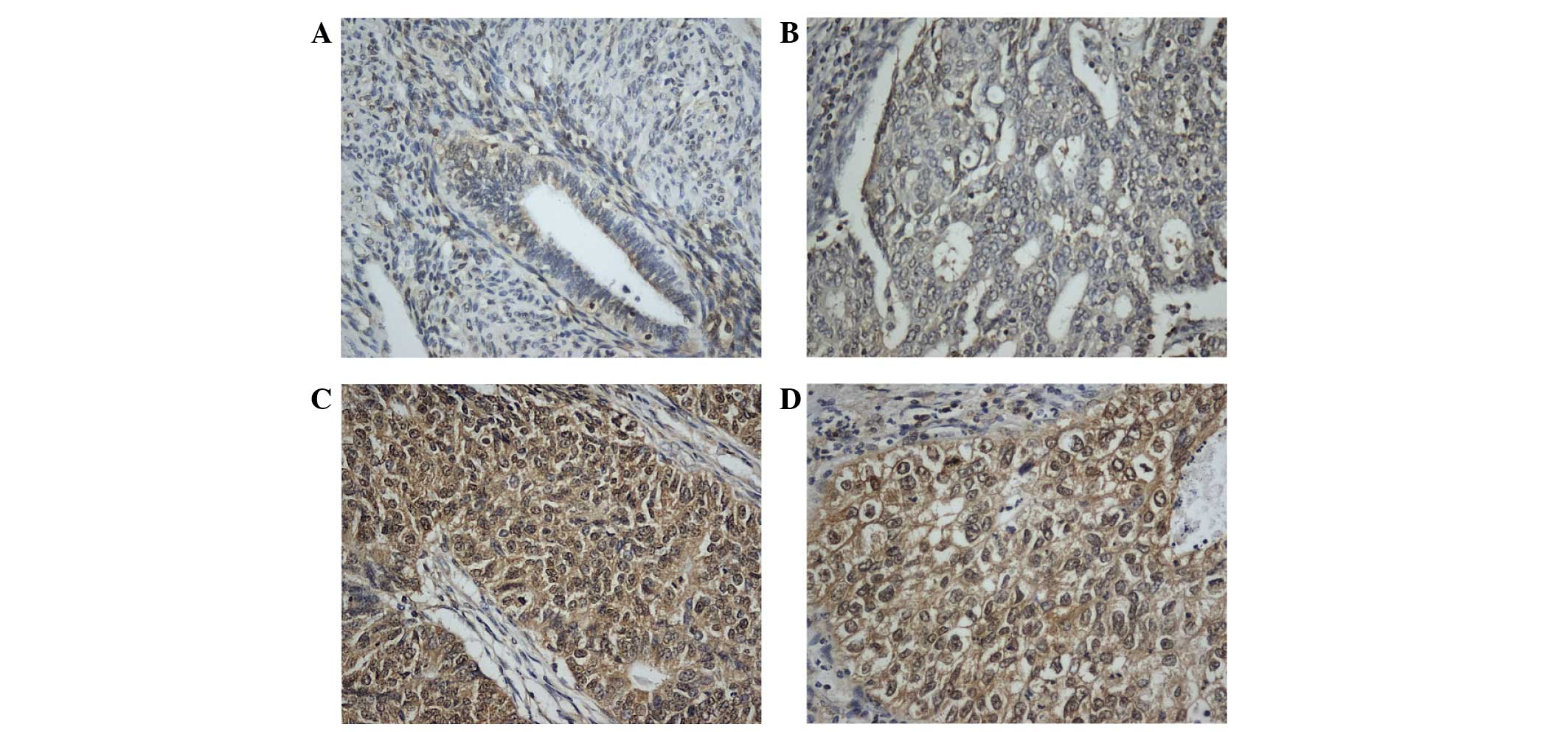
CRKL immunostaining was examined in 87 cases of
endometrial carcinoma. In normal endometrial tissues, stromal and
glandular tissues exhibited weak or negative staining (Fig. 1A). In total, 44 of 87 (50.5%) primary
endometrial cancers displayed positive CRKL immunoreactivity, which
was located in the nuclear and cytoplasmic compartment of the
carcinoma cells (Fig. 1B-D). The
association of CRKL overexpression with clinicopathological
characteristics is listed in Table I.
High CRKL immunostaining score in the primary endometrial carcinoma
was significantly correlated with advanced tumor grade (grade 2+3
vs. grade 1, P=0.0409). CRKL expression was relatively higher in
tumors with advanced T stage (T2-3, 66.6%) compared with tumors
with lower T stage (T1a, T1b and T1c, 49.3%), which did not reach
statistical significance. No significant correlation was observed
between CRKL level and other parameters such as International
Federation of Gynecology and Obstetrics stage (P=0.7001) or patient
age (P=0.7614).
 | Table I.Distribution of CRKL status in
endometrial carcinoma according to clinicopathological
characteristics. |
Table I.
Distribution of CRKL status in
endometrial carcinoma according to clinicopathological
characteristics.
| Characteristics | No. of patients |
CRKLweak/negative | CRKL positive | P-value |
|---|
| Age, years |
|
|
| 0.7614 |
|
<60 | 56 | 27 | 29 |
|
| ≥60 | 31 | 16 | 15 |
|
| Grade |
|
|
| 0.0409 |
| 1 | 37 | 23 | 14 |
|
| 2+3 | 50 | 20 | 30 |
|
| T stage |
|
|
| 0.7324 |
| T1a | 9 | 5 | 4 |
|
| T1b | 43 | 23 | 20 |
|
| T1c | 29 | 13 | 16 |
|
| T2-3 | 6 | 2 | 4 |
| FIGO stage |
|
|
| 0.7001 |
| I | 59 | 30 | 29 |
|
|
II+III | 28 | 13 | 15 |
|
CRKL promotes Ishikawa cell
proliferation and upregulates cell cycle proteins
To determine the biological roles of CRKL in
endometrial cancer, plasmid transfection was performed in the
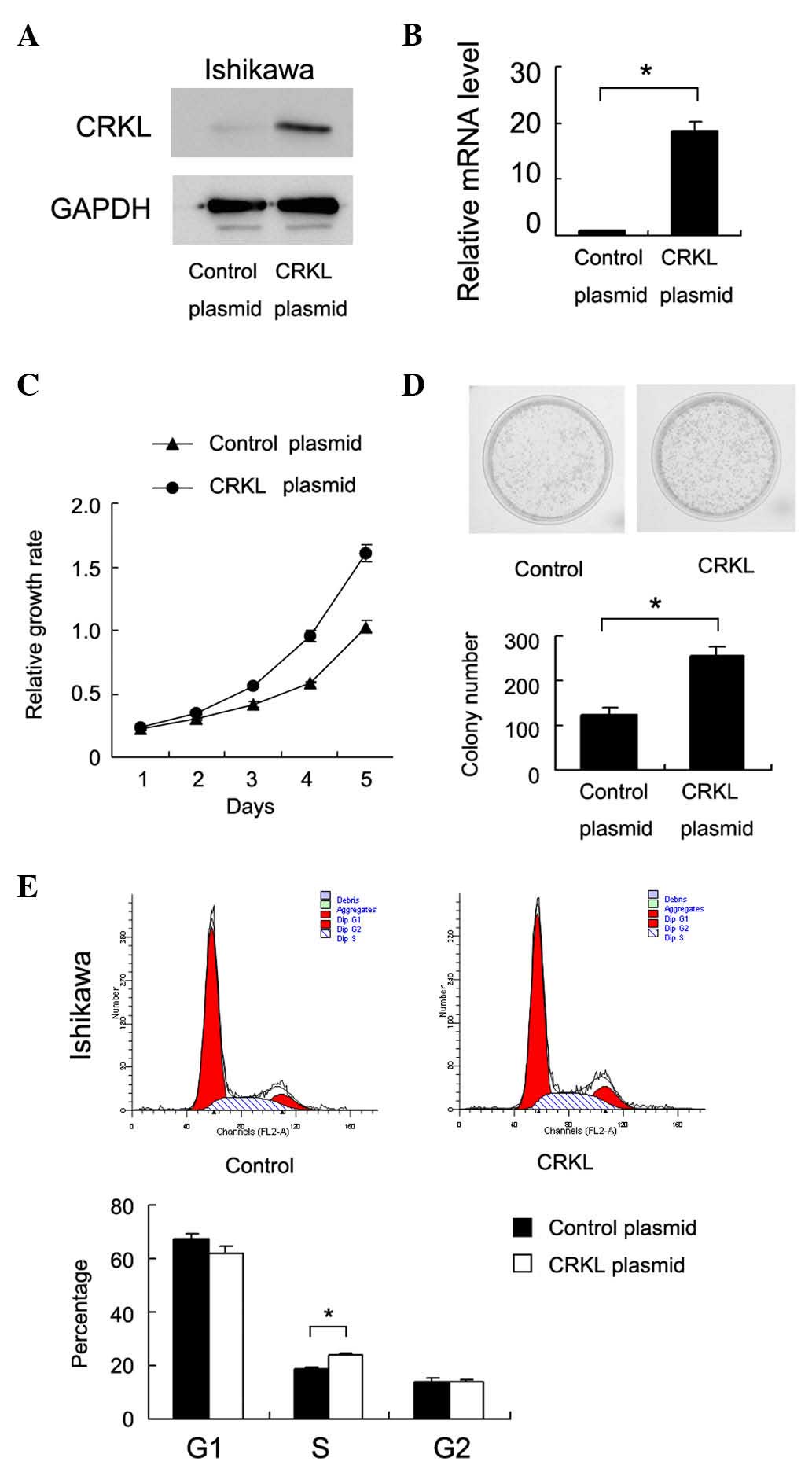
Ishikawa cell line. As shown in Fig. 2A
and B, CRKL plasmid transfection significantly upregulated CRKL
protein and messenger RNA expression. MTT assay revealed that CRKL
upregulation increased the cell proliferation rate (Fig. 2C). Colony formation assay was also
conducted, as shown in Fig. 2D, and
the results revealed that CRKL transfection significantly increased
the colony number of Ishikawa cells (83±9 colonies for empty
vector-transfected vs. 156±12 colonies for CRKL plasmid-transfected
cells, P<0.05). Cell cycle analysis was performed, and it was
observed that CRKL transfection increased the percentage of cells
in the S phase and decreased the percentage of cells in the G1
phase, thus suggesting that CRKL facilitated the G1-S cell cycle
transition (Fig. 2E). Accordingly,
CRKL transfection upregulated cyclin D1 and cyclin E expression
(Fig. 3A).
CRKL inhibits Ishikawa cell apoptosis
and upregulates Bcl-2 and survivin
In order to investigate the association between CRKL
and apoptosis in endometrial carcinoma cells, control cells and
CRKL-transfected cells were treated with cisplatin (1 µM, 24 h) and
subjected to cell apoptosis analysis. Compared with the control
group, the rate of cell apoptosis in CRKL-overexpressing Ishikawa
cells decreased significantly (empty vector vs. CRKL plasmid,
23.8±1.7 vs. 10.3±1.2%, respectively, P=0.009) (Fig. 3B). Accordingly, the levels of
caspase-3 and caspase-9 cleavage decreased upon CRKL upregulation.
The level of apoptosis-related proteins was also examined, and CRKL
transfection was observed to upregulate Bcl-2 and survivin
expression in the Ishikawa cell line. By contrast, the level of Bax
was downregulated following CRKL transfection (Fig. 3A).
Discussion
Endometrial carcinoma is one of the most common
gynaecological cancers worldwide (19,20).
Although previous studies have reported that multiple aberrantly
expressed genes in endometrial carcinoma could contribute to the
malignant behavior (15,21–24), novel
markers that are able to identify tumor progression and predict the
aggressive phenotype are still urgently required. Upregulation of
CRKL expression has been implicated in several human cancers,
including breast cancer, lung cancer and pancreatic cancer
(15,16,25,26), where
it leads to increased proliferation and invasion. However, its
biological roles and clinical significance in human endometrial
carcinoma remain unexplored. The present study examined CRKL
protein expression in 87 cases of endometrial carcinoma, and CRKL
overexpression was observed in 50.5% of cancer tissues. CRKL
overexpression positively correlated with advanced tumor grade,
thus suggesting its association with malignant phenotype. The
current data is in accordance with previous reports that confirmed
CRKL as an oncogene overexpressed in human endometrial
carcinomas.
There is a growing body of evidence suggesting that
CRKL is involved in carcinogenesis of solid tumors by regulating
their biological behavior, including proliferation, differentiation
and invasion (27–29). In order to assess the function of CRKL
in endometrial carcinoma progression, a CRKL-expression plasmid was
employed in the Ishikawa cell line. The results demonstrated that
transient transfection of the above CRKL plasmid caused an obvious
increase in the proliferation rate and colony formation ability of
Ishikawa cells. In addition, cell cycle analysis revealed that the
percentage of cells in the S phase was increased in
CRKL-transfected cells, thus suggesting that CRKL promotes cell
growth through the G1-S transition. The present study also examined
cell cycle-related factors, and observed that CRKL caused
upregulation of cyclin E and cyclin D1, which control cell cycle
progression through the restriction point at the G1-S phase
(26,30). Previous reports demonstrated that
cyclin D1 and cyclin E were overexpressed in endometrial carcinoma
and were important in cancer progression (31,32). The
current results were in accordance with their role in cell cycle
control.
The association of CRKL with apoptosis is not well
characterized. In the present study, CRKL overexpression led to
decreased cisplatin-induced apoptosis in Ishikawa cells. CRKL
transfection also caused decreased caspase-3 and caspase-9
cleavage, which suggests that CRKL may function as an important
modifier of the apoptosis process in endometrial carcinoma cells.
Regulation of cancer cell apoptosis involves the interaction of
multiple genes. In the current study, the change in the levels of
Bcl-2 and survivin in endometrial carcinoma cells upon CRKL
overexpression was examined, and it was observed that CRKL
overexpression increased the levels of Bcl-2 and survivin, both of
which have been reported to be involved in apoptosis inhibition in
numerous cancers, including endometrial carcinoma (33–36). The
present data strongly support the role of CRKL in regulating cell
apoptosis through Bcl-2 and survivin.
In conclusion, CRKL is overexpressed in human
endometrial carcinomas and correlates with advanced tumor grade.
CRKL promotes tumor proliferation through regulation of cyclin
proteins, and inhibits apoptosis through Bcl-2 and survivin
upregulation. CRKL may serve as a novel therapeutic target for
endometrial carcinoma.
Acknowledgements
This study was supported by the Shenyang Science and
Technology Project (grant no. F15-139-9-33).
References
|
1
|
Siegel R, Desantis C and Jemal A:
Colorectal cancer statistics, 2014. CA Cancer J Clin. 64:104–117.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sivridis E, Giatromanolaki A, Gatter KC,
Harris AL and Koukourakis MI: Tumor and Angiogenesis Research
Group: Association of hypoxia-inducible factors 1alpha and 2alpha
with activated angiogenic pathways and prognosis in patients with
endometrial carcinoma. Cancer. 95:1055–1063. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sakuragi N, Ohkouchi T, Hareyama H, Ikeda
K, Watari H, Fujimoto T, Kuwabara M, Yamamoto R, Sagawa T, Fujino T
and Fujimoto S: Bcl-2 expression and prognosis of patients with
endometrial carcinoma. Int J Cancer. 79:153–158. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wen SY, Zhang LN, Yang XM, Zhang YL, Ma L,
Ge QL, Jiang SH, Zhu XL, Xu W, Ding WJ, et al: LRG1 is an
independent prognostic factor for endometrial carcinoma. Tumour
Biol. 35:7125–7133. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Saarelainen SK, Staff S, Peltonen N,
Lehtimäki T, Isola J, Kujala PM, Vuento MH and Mäenpää JU:
Endoglin, VEGF and its receptors in predicting metastases in
endometrial carcinoma. Tumour Biol. 35:4651–4657. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Honkavuori-Toivola M, Talvensaari-Mattila
A, Soini Y, Turpeenniemi-Hujanen T and Santala M: Immunoreactivity
for TIMP-2 is associated with a favorable prognosis in endometrial
carcinoma. Tumour Biol. 33:935–941. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rhodes J, York RD, Tara D, Tajinda K and
Druker BJ: CrkL functions as a nuclear adaptor and transcriptional
activator in Bcr-Abl-expressing cells. Exp Hematol. 28:305–310.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Feller SM: Crk family adaptors-signalling
complex formation and biological roles. Oncogene. 20:6348–6371.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fidler IJ and Kripke ML: Genomic analysis
of primary tumors does not address the prevalence of metastatic
cells in the population. Nat Genet. 34:232003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hoeve J, Morris C, Heisterkamp N and
Groffen J: Isolation and chromosomal localization of CRKL, a human
crk-like gene. Oncogene. 8:2469–2474. 1993.PubMed/NCBI
|
|
11
|
ten Hoeve J, Kaartinen V, Fioretos T,
Haataja L, Voncken JW, Heisterkamp N and Groffen J: Cellular
interactions of CRKL, and SH2-SH3 adaptor protein. Cancer Res.
54:2563–2567. 1994.PubMed/NCBI
|
|
12
|
Beroukhim R, Mermel CH, Porter D, Wei G,
Raychaudhuri S, Donovan J, Barretina J, Boehm JS, Dobson J,
Urashima M, et al: The landscape of somatic copy-number alteration
across human cancers. Nature. 463:899–905. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Senechal K, Halpern J and Sawyers CL: The
CRKL adaptor protein transforms fibroblasts and functions in
transformation by the BCR-ABL oncogene. J Biol Chem.
271:23255–23261. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
van't Veer LJ, Dai H, van de Vijver MJ, He
YD, Hart AA, Mao M, Peterse HL, van der Kooy K, Marton MJ,
Witteveen AT, et al: Gene expression profiling predicts clinical
outcome of breast cancer. Nature. 415:530–536. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lin F, Chengyao X, Qingchang L, Qianze D,
Enhua W and Yan W: CRKL promotes lung cancer cell invasion through
ERK-MMP9 pathway. Mol Carcinog. (54 Suppl 1). E35–E44. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang Y, Dong QZ, Fu L, Stoecker M, Wang E
and Wang EH: Overexpression of CRKL correlates with poor prognosis
and cell proliferation in non-small cell lung cancer. Mol Carcinog.
52:890–899. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ali RH and Rouzbahman M: Endometrial
stromal tumours revisited: An update based on the 2014 WHO
classification. J Clin Pathol. 68:325–332. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cikos S, Bukovská A and Koppel J: Relative
quantification of mRNA: Comparison of methods currently used for
real-time PCR data analysis. BMC Mol Biol. 8:1132007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Buhtoiarova TN, Brenner CA and Singh M:
Endometrial carcinoma: Role of current and emerging biomarkers in
resolving persistent clinical dilemmas. Am J Clin Pathol. 145:8–21.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang L, Yan L, Cao M, Zhang H, Li C, Bai
Y, Yu P, Li M and Zhao X: SPAG9 promotes endometrial carcinoma cell
invasion through regulation of genes related to the
epithelial-mesenchymal transition. Eur J Gynaecol Oncol.
37:312–319. 2016.PubMed/NCBI
|
|
21
|
Lian X, Jiao Y, Yang Y, Wang Z, Xuan Q,
Liu H, Lu S, Wang Z, Liu Y, Li S, et al: CrkL regulates
SDF-1-induced breast cancer biology through balancing Erk1/2 and
PI3K/Akt pathways. Med Oncol. 32:4112015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lv S, Qin J, Yi R, Coreman M, Shi R, Kang
H and Yao C: CrkL efficiently mediates cell proliferation,
migration, and invasion induced by TGF-β pathway in glioblastoma. J
Mol Neurosci. 51:1046–1051. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nosaka Y, Arai A, Miyasaka N and Miura O:
CrkL mediates Ras-dependent activation of the Raf/ERK pathway
through the guanine nucleotide exchange factor C3G in hematopoietic
cells stimulated with erythropoietin or interleukin-3. J Biol Chem.
274:30154–30162. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Segovis CM, Schoon RA, Dick CJ, Nacusi LP,
Leibson PJ and Billadeau DD: PI3K links NKG2D signaling to a CrkL
pathway involved in natural killer cell adhesion, polarity, and
granule secretion. J Immunol. 182:6933–6942. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhao T, Miao Z, Wang Z, Xu Y, Wu J, Liu X,
You Y and Li J: Overexpression of CRKL correlates with malignant
cell proliferation in breast cancer. Tumour Biol. 34:2891–2897.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fu L, Dong Q, Xie C, Wang Y and Li Q: CRKL
protein overexpression enhances cell proliferation and invasion in
pancreatic cancer. Tumour Biol. 36:1015–1022. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Singer CF, Hudelist G, Lamm W, Mueller R,
Handl C, Kubista E and Czerwenka K: Active (p)CrkL is overexpressed
in human malignancies: Potential role as a surrogate parameter for
therapeutic tyrosine kinase inhibition. Oncol Rep. 15:353–359.
2006.PubMed/NCBI
|
|
28
|
Kim YH, Kwei KA, Girard L, Salari K, Kao
J, Pacyna-Gengelbach M, Wang P, Hernandez-Boussard T, Gazdar AF,
Petersen I, et al: Genomic and functional analysis identifies CRKL
as an oncogene amplified in lung cancer. Oncogene. 29:1421–1430.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fathers KE, Bell ES, Rajadurai CV, Cory S,
Zhao H, Mourskaia A, Zuo D, Madore J, Monast A, Mes-Masson AM, et
al: Crk adaptor proteins act as key signaling integrators for
breast tumorigenesis. Breast Cancer Res. 14:R742012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang J, Chen X, Li P, Su L, Yu B, Cai Q,
Li J, Yu Y, Liu B and Zhu Z: CRKL promotes cell proliferation in
gastric cancer and is negatively regulated by miR-126. Chem Biol
Interact. 206:230–238. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Quddus M Ruhul, Latkovich P, Castellani
WJ, Sung C James, Steinhoff MM, Briggs RC and Miranda RN:
Expression of cyclin D1 in normal, metaplastic, hyperplastic
endometrium and endometrioid carcinoma suggests a role in
endometrial carcinogenesis. Arch Pathol Lab Med. 126:459–463.
2002.PubMed/NCBI
|
|
32
|
Cassia R, Moreno-Bueno G,
Rodriguez-Perales S, Hardisson D, Cigudosa JC and Palacios J:
Cyclin E gene (CCNE) amplification and hCDC4 mutations in
endometrial carcinoma. J Pathol. 201:589–595. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
González-Rodilla I, Verna V, Muñoz AB,
Estévez J, Boix M and Schneider J: Expression of the
apoptosis-related genes Bcl-2 and p53 in clinical samples from
endometrial carcinoma patients. Anticancer Res. 31:4191–4193.
2011.PubMed/NCBI
|
|
34
|
Porichi O, Nikolaidou ME, Apostolaki A,
Tserkezoglou A, Arnogiannaki N, Kassanos D, Margaritis L and
Panotopoulou E: BCL-2, BAX and P53 expression profiles in
endometrial carcinoma as studied by real-time PCR and
immunohistochemistry. Anticancer Res. 29:3977–3982. 2009.PubMed/NCBI
|
|
35
|
Pallares J, Martinez-Guitarte JL, Dolcet
X, Llobet D, Rue M, Palacios J, Prat J and Matias-Guiu X: Survivin
expression in endometrial carcinoma: A tissue microarray study with
correlation with PTEN and STAT-3. Int J Gynecol Pathol. 24:247–253.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Takai N, Miyazaki T, Nishida M, Nasu K and
Miyakawa I: Survivin expression correlates with clinical stage,
histological grade, invasive behavior and survival rate in
endometrial carcinoma. Cancer letters. 184:105–116. 2002.
View Article : Google Scholar : PubMed/NCBI
|