Introduction
Chemotherapy and radiotherapy are common treatment
approaches for breast cancer (1).
Although the technology underlying chemotherapy and radiotherapy
has been developed, resistance to chemotherapy and radiotherapy
remains an issue (2). An important
characteristic of breast cancer is the presence of hypoxia
(3). For example, the median partial
pressure of oxygen (PO2) level measured in breast cancer
was 10 mmHg, while the median PO2 level was 65 mmHg in
normal breast tissue (4). The hypoxic
microenvironment in tumors is frequently caused by imbalance of
oxygen demand and supply, and the malfunctioning of the blood
vessels feeding tumors (5). This
hypoxic condition makes tumor cells initiate various adaptation
processes (6). In addition, various
clinical trials have demonstrated that the presence of hypoxia is a
major cause of treatment failure (7–11). The
hypoxia-tolerant cancer cells are resistant to chemotherapy and
radiotherapy (6).
Resistance to chemotherapy and radiotherapy may be
overcome by a variety of measures demonstrated by experiments.
Increasing oxygen pressure within the tumor [for example,
administration of red blood cells (12) and hyperbaric oxygenation (13)], and administration of
chemoradiotherapy sensitization agents [for example,
nitroimidazoles, including nimorazole (14)] are among the approaches. However, the
effectiveness of such measures remains controversial, and no clear
benefits of these measures have been demonstrated (13). In particular, when increasing the
oxygen pressure within the tumor, the oxygen content needs to be
controlled within a small range or it cannot sensitize the tumor to
radiotherapy (15). Furthermore, in a
previous study, the radiotherapy sensitizer tirapazamine did not
improve the treatment outcome and increased the incidence of
adverse events (16).
Carbon nanotubes (CNTs) have been exploited in
several biomedical applications (17,18). CNTs
have been proposed to exhibit the potential to cross the cell
membrane (19) and effectively
transport molecules into the cytoplasm (20). Both single-walled (SW) CNTs and
multi-walled nanotubes are being considered as effective
nanocarriers for drug transportation (21).
Considering the characteristics of CNTs, the present
study speculated that SWCNTs may serve as a nanocarrier for
delivery of O2, with the aim of alleviating the hypoxic
conditions of cancer lesions and increasing sensitivity to
chemotherapy and radiotherapy in tumor cells.
Despite the application of SWCNTs as O2
delivery vehicles, tombarthite, a mineral containing 17 chemical
elements, was used to increase the dispersion of SWCNTs in order to
reduce the toxicity (22,23). Folic acid (FA) was also used, in an
attempt to make the material selectively accumulate in cancer
lesions.
In the present study, the synthesis of the novel
oxygen-carrying tombarthite-modified FA-conjugated chitosan
(R-O2-FA-CHI)-SWCNT nanocarrier for targeted delivery of
O2 was described, and the chemoradiotherapy sensitizing
properties of this novel material were investigated in
vitro. The potential underlying mechanisms involved were also
assessed.
Materials and methods
Purification of the SWCNTs and
preparation of R-O2-SWCNTs
Purification of the SWCNTs
The purification of the SWCNTs with purity >95%,
a length of 0.5–2 µm and a diameter of 10–20 nm (Nanjing XianFeng
Nano Material Technology Co., Ltd., Nanjing, China) was performed
by the School of Mechanical and Power Engineering, Shanghai
Jiaotong University (Shanghai, China). The SWCNTs (100 mg) were
added to a mixture of 98% H2SO4 and 78%
HNO3 (v/v, 3:1, 50 ml) in a conical flask and sonicated
at room temperature for 2 h. The SWCNTs were washed with ultrapure
water and dried. Subsequently, 300 ml deionized water was added to
the conical flask, causing the SWCNT to sink and three layers to
form. The upper layer was a clear liquid and was removed after 6
min. The residual turbid solution (middle layer) was filtered
through a microporous membrane (0.45 µm; Beijing Xinwei Technology
Group Co., Ltd., Beijing, China), and was then washed with
ultrapure water to neutral pH. The material was dried at 60°C in a
baking oven.
Preparation of R-O2-SWCNTs
The purified SWCNTs were immersed in tombarthite
modifier liquid (m/v, 2 mg/ml) containing 1.5% of the tombarthite
element lanthanum, 96% ethyl alcohol, 0.2% EDTA, 0.8%
NH4Cl, 0.5% HNO3 and 1% carbamide, for 2 h,
followed by sonication for 1–3 h. Subsequently, the product was
filtered through a microporous filtration membrane (0.45 µm) and
dried.
Production of R-O2-SWCNTs
The R-O2-SWCNTs were placed in the sample
chamber of a vacuum heating system and heated to 300–400°C and
vacuumized for 5 h, to the vacuum degree of 10−3 Pa. A
total of 9 MPa pure oxygen was pumped into the sample chamber
following cooling to room temperature, while maintaining the vacuum
status. The R-O2-SWCNTs were obtained when the pressure
in the sample chamber remained stable for 2–3 h.
Preparation of
R-O2-FA-CHI-SWCNTs
Conjugation of CHI
The R-O2-SWCNTs (20 mg) were sonicated in
CHI (Shanghai YuanYe Biotechnology Co., Ltd., Shanghai, China)
solution (40 mg in 0.05 mol/l acetic acid) for 20 min and
subsequently stirred at room temperature for 16 h. The modified
SWCNTs were collected and filtered through a 10 KDa molecular
weight cut-off membrane (Shanghai Lvniao Biocompany, Shanghai,
China) to remove excess chitosan, and subsequently washed with
ultrapure water several times.
Conjugation of FA
FA (Shanghai YuanYe Biotechnology Co., Ltd.) and
N,N-(3-dimethylaminopropyl)-N'-ethyl carbodiimide hydrochloride
(EDC; Shanghai YuanYe Biotechnology Co., Ltd.) were added to a
solution of R-O2-CHI-SWCNTs in PBS at pH 7.4, with final
concentrations of 1.5 mg/ml FA, 1.2 mg/ml EDC and 1 mg/ml
CHI-SWCNTs. The mixture was allowed to react overnight at room
temperature in the dark. The solution was subsequently dialyzed
three times to ensure complete excess removal of unconjugated FA
and EDC, and subsequently dried at 30°C. In this way,
R-O2-FA-CHI-SWCNTs were obtained. Ultrapure water was
used as a solvent in the experiment.
Characterization of
R-O2-FA-CHI-SWCNT
X-ray photoelectron spectroscopy (XPS) to
characterize the newly synthesized material was performed on a PHI
Quantera II Scanning XPS Microprobe with an aluminium anode
(Physical Electronics, Inc., Chanhassen, MN, USA). Low resolution
survey scans (pass energy, 280 eV; time per step, 100 ms) were
performed to determine the elemental composition of the CNTs, and
high resolution element scans (pass energy, 26 eV; time per step,
500 ms) were conducted to the obtain bonding information of each
element. The UV-visible absorption spectral measurements were
performed on the Lambda 17 UV-vis 8500 spectrometer (PerkinElmer,
Inc., Waltham, MA, USA) with a 1-cm pathlength quartz cuvette.
Stock solution (10 mg/ml) of each sample was prepared in water. The
spectra were recorded at 20°C.
Cell lines and cell culture
Human breast cancer cell lines MDA-MB-231 and
ZR-75-1 were obtained from the Shanghai Institutes for Biological
Sciences, Chinese Academy of Sciences (Shanghai, China). Cells were
cultured in an environment of 5% CO2 at 37°C in
RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc., Waltham,
MA, USA) or high glucose Dulbecco's modified Eagle's medium (Gibco;
Thermo Fisher Scientific, Inc.) supplemented with 10% fetal bovine
serum (FBS; Gibco; Thermo Fisher Scientific, Inc.) and 1% 100 U
penicillin (Gibco; Thermo Fisher Scientific, Inc.) and 100 U
streptomycin (Gibco; Thermo Fisher Scientific, Inc.). Cells were
incubated under normoxic (21% O2 and 5% CO2)
or hypoxic (1% O2 and 5% CO2) conditions.
When each cell type had grown to 80% confluence, the cells were
washed three times with PBS (10 mM sodium phosphate buffer, pH 7.2;
GE Healthcare Life Sciences, Logan, UT, USA), and incubated at 37°C
with 0.25% trypsin-EDTA until they dissociated from the flask,
centrifuged at 177 × g for 5 min at room temperature and
resuspended in fresh media. The resuspended pellet was passaged at
a ratio of 1:3 into a new flask.
Treatment with chemotherapy drugs and irradiation
procedure
A total of five frequently-used chemotherapy drugs
in clinical practice of treating breast cancer: 5-fluorouracil,
epirubicin, pirarubicin, paclitaxel, docetaxel and carboplatin,
were used at the given concentrations offered by the pharmaceutical
department of XinHua Hospital (Shanghai, China; 3 mg/ml
5-fluorouracil; 0.5 mg/ml epirubicin; 0.08 mg/ml pirarubicin; 0.46
mg/ml paclitaxel; and 1.0 mg/ml carboplatin). Irradiation was
performed at room temperature with single doses of X-rays ranging
from 2 to 8 Gy, using a linear accelerator with 6 MeV photons/100
cm focus-surface distance, with a dose rate of 2.0 Gy/min.
Cell viability assay
MDA-MB-231 and ZR-75-1 cells were seeded into
96-well culture plates (Corning Incorporated, Corning, NY, USA) at
5,000 cells/well. After culturing overnight, the cells were washed
with FBS-free RPMI-1640. MDA-MB-231 and ZR-75-1 cells were cultured
with ordinary medium or FA-free medium and divided into the
following groups: Blank control group,
R-O2-FA-CHI-SWCNTs-treated group, chemotherapy-group and
R-O2-FA-CHI-SWCNTs-chemotherapy group under hypoxic
conditions. The control cells were incubated with ultrapure water
instead of drug. The treatment group cells were incubated with
R-O2-FA-CHI-SWCNTs, chemotherapy drugs or
R-O2-FA-CHI-SWCNTs plus chemotherapy drugs for 48 h at
37°C.
Subsequently, the cells were washed three times with
PBS and FBS-free RPMI-1640 (100 µl) was used to substitute the
culture medium. A total of 10 µl water-soluble tetrazolium salts-1
(WST-1) reagent (Roche Diagnostics, Indianapolis, IN, USA) was
added to each well and incubated for an additional 2.5 h at 37°C.
The plates were read at 450 nM using a microplate reader (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). Each experiment was
performed independently at least three times.
Colony forming assay
The survival and proliferation potential of cells
treated with R-O2-FA-CHI-SWCNTs and/or ionizing
radiation was assessed by colony forming assay. Initially,
exponentially growing cells in 6-well plates were irradiated (0, 2,
4, 6 or 8 Gy) following incubation with or without
R-O2-FA-CHI-SWCNTs under hypoxic conditions for 48 h at
37°C. Following irradiation, the cells were washed with PBS twice
and trypsinized, suspended in complete medium, counted, diluted
serially to appropriate densities and re-plated in new 6-well
culture plates, allowing the formation of macroscopic colonies.
Following incubation at 37°C for 14–21 days, cells were fixed with
methanol, and stained with Giemsa. Colonies containing >50 cells
were counted. The plating efficiency (PE) and surviving fraction
(SF) were calculated as follows: PE (%) = (colony number /
inoculating cell number) × 100; SF (%) = PE (tested group) / PE
(0-Gy group) × 100. The cell-survival curve was plotted with
GraphPad Prism version 5.0 software (GraphPad Software, Inc., La
Jolla, CA, USA), using the linear-quadratic formula SF = exp[-(αD +
βD2)], where α and β describe survival curve
characteristics that classify cellular response to radiation, and D
indicates the dose of radiation. The sensitization enhancement
ratio (SER) was calculated as follows: SER = SF2 (tested group) /
SF2 (0 Gy group).
Cell apoptosis assay
MDA-MB-231 and ZR-75-1 cells were seeded into 6-well
dishes (Corning Incorporated) overnight. For the chemotherapy
experiment, cells were treated with chemotherapy drugs at the given
concentration, R-O2-FA-CHI-SWCNTs or chemotherapy drugs
and R-O2-FA-CHI-SWCNTs for 48 h under hypoxic conditions
at 37°C. For the radiotherapy experiment, cells were incubated in
hypoxic conditions with or without R-O2-FA-CHI-SWCNTs
for 48 h at 37°C, and treated with radiotherapy (4 Gy), followed by
incubation for another 24 h at 37°C.
Cells were collected and double-stained for cell
apoptosis and death detection. The apoptotic cells were stained by
Annexin V, while the necrotic cells were stained with propidium
iodide (PI). The Annexin V and PI staining was carried out by using
an Apoptosis Detection kit (BD Biosciences, Franklin Lakes, NJ,
USA). The cells were suspended in binding buffer with a cell
concentration of ~106 cells/ml after they were harvested
and washed with cold PBS buffer. Subsequently, 5 µl Annexin V and 1
µl PI were added into a 100 µl suspension of cells, and stained for
15 min at room temperature. Following staining, 400 µl binding
buffer was added into the above 100 µl cell suspension. Finally,
the stained cells were analyzed by fluorescence-activated cell
sorting (BD LSR II; BD Biosciences).
Protein extraction and western blot analysis
Total protein extraction was performed as described
below. Cells were washed twice with ice-cold PBS. Total cell lysate
was prepared using radioimmunoprecipitation assay buffer (Beyotime
Institute of Biotechnology, Haimen, China) supplemented with
phenylmethylsulfonyl fluoride (Beyotime Institute of Biotechnology)
(v/v, 100:1). Subsequently, lysate was centrifuged at 177 ×
g for 10 min at 4°C to collect the supernatant. Protein
lysate concentrations were determined by the bicinchoninic acid
protein assay kit (Beyotime Institute of Biotechnology).
Western blotting reagents (Beyotime Institute of
Biotechnology) were used as received. To quantify protein levels,
equal amounts of protein (40 µg) were subjected to 10 or 15%
SDS-PAGE. Separated proteins were transferred to a polyvinylidene
difluoride membrane, which was then exposed to 5% non-fat dried
milk in TBS containing 0.1% Tween 20 (0.1% TBST) for 1 h at room
temperature, and incubated overnight at 4°C with antibodies against
B-cell lymphoma 2 (Bcl-2; 1:500; E17; Abcam, Cambridge, UK),
hypoxia-inducible factor 1-α (HIF-1α; 1:2,000; EP1215Y; Abcam),
survivin (1:5,000; EP2880Y; Abcam), Ku80 (1:1,000; EPR3468; Abcam),
P-glycoprotein (P-gp; 1:1,000; EPR10363; Abcam), multidrug
resistance-associated protein 1 (MRP-1; 1:500; MRPm5; Abcam), RAD51
(1:10,000; EPR4030 (3); Abcam) and
β-actin (1:1,000; AC-74; Beyotime Institute of Biotechnology). The
membranes were subsequently washed with 0.1% TBST prior to
incubation with horseradish peroxidase-conjugated goat anti-rabbit
(1:1,000; A0208; Beyotime Institute of Biotechnology) or -mouse
(1:1,000, A0216; Beyotime Institute of Biotechnology) secondary
antibodies. Immune complexes were detected with chemiluminescence
reagents and measured using ECL Plus (Bio-Rad Laboratories, Inc.,
Hercules, CA). Band intensities were quantified using ImageJ
software, version 2.1.4.7 (National Institutes of Health, Bethesda,
MD, USA).
Statistical analysis
Statistical analysis was performed using SPSS
version 20 (IBM SPSS, Armonk, NY, USA). Data are presented as the
mean ± standard deviation of the results from three or four
independent experiments. Statistical comparisons were performed
using Student's t-test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Synthesis of
R-O2-FA-CHI-SWCNTs
The starting materials were oxygen
R-O2-SWCNTs, which were synthesized by the School of
Mechanical and Power Engineering, Shanghai Jiaotong University.
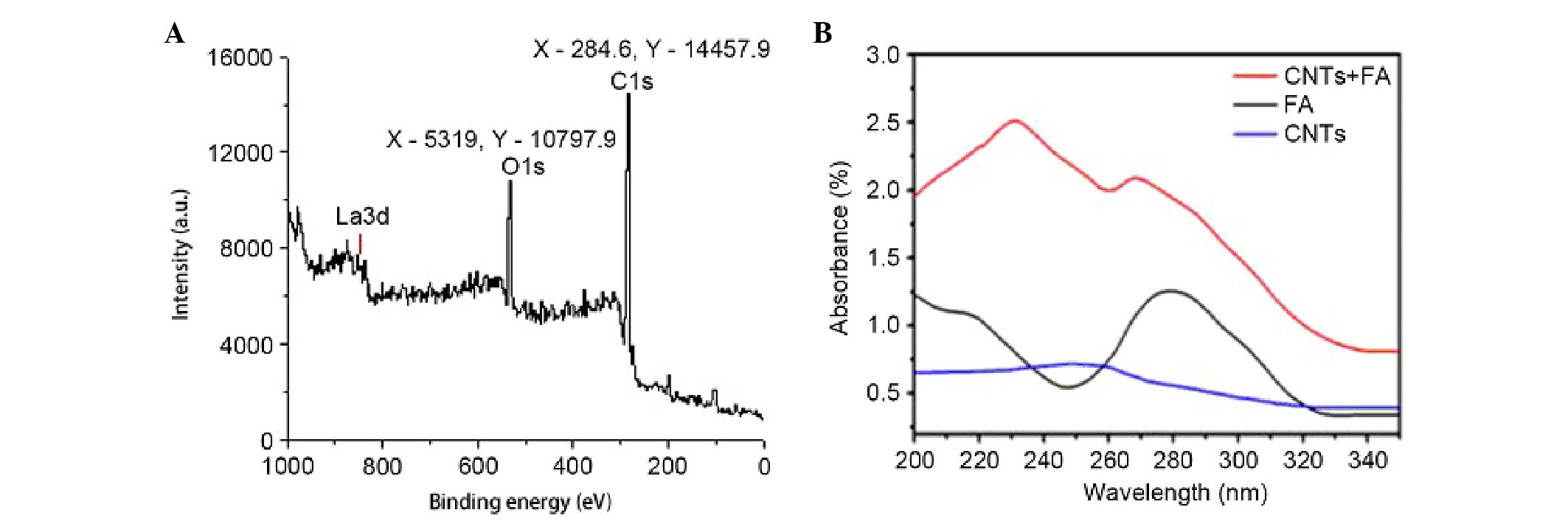
Fig. 1A shows the results of XPS
analysis which confirmed the conjugation with O2 and R.
Subsequently, R-O2-SWCNTs were functionalized with CHI
and FA. The final product was denoted as
R-O2-FA-CHI-SWCNT. As shown in Fig. 1B, the conjugation with FA was
confirmed by examining the ultraviolet visible (UV-vis) absorption
spectra. The absorbance peaks at 280 nm in UV-vis spectra
corresponded to the characteristic peaks of FA.
Cytotoxicity of
R-O2-FA-CHI-SWCNTs
The toxicity of SWCNTs was has been widely discussed
and disputed in published articles (24–26). In
the present study, the effect of R-O2-FA-CHI-SWCNTs on
the proliferation of MDA-MB-231 and ZR-75-1 cells was determined by
WST-1 assay. Fig. 2 shows the results
of WST-1 assays on the proliferation ratio of MDA-MB-231 and
ZR-75-1 cells following treatment with
R-O2-FA-CHI-SWCNTs at concentrations from 5–500 µg/ml
for 48 h. No significant inhibition of cell proliferation was
observed when the concentration of R-O2-FA-CHI-SWCNTs
was <100 µg/ml, indicating no clear cellular toxicity of the
novel functionalized CNTs below a certain dose. However, the cell
viability was significantly inhibited by
R-O2-FA-CHI-SWCNTs when the concentration of the
material was >150 µg/ml (P<0.05). The toxic effect of the
synthesized material at various doses indicated a concentration
threshold of viability inhibition at 100–150 µg/ml. Accordingly,
100 µg/ml concentration of R-O2-FA-CHI-SWCNTs was used
for further experiments to identify the chemotherapy and
radiotherapy sensitizing effect.
R-O2-FA-CHI-SWCNTs increase
the sensitivity of cells to chemotherapy
In the present study, the anticancer effects of
chemotherapy or chemotherapy and R-O2-FA-CHI-SWCNTs
combined treatment were determined by the WST-1 assay on two human
breast carcinoma cell lines: MDA-MB-231 (which harbor FA receptor)
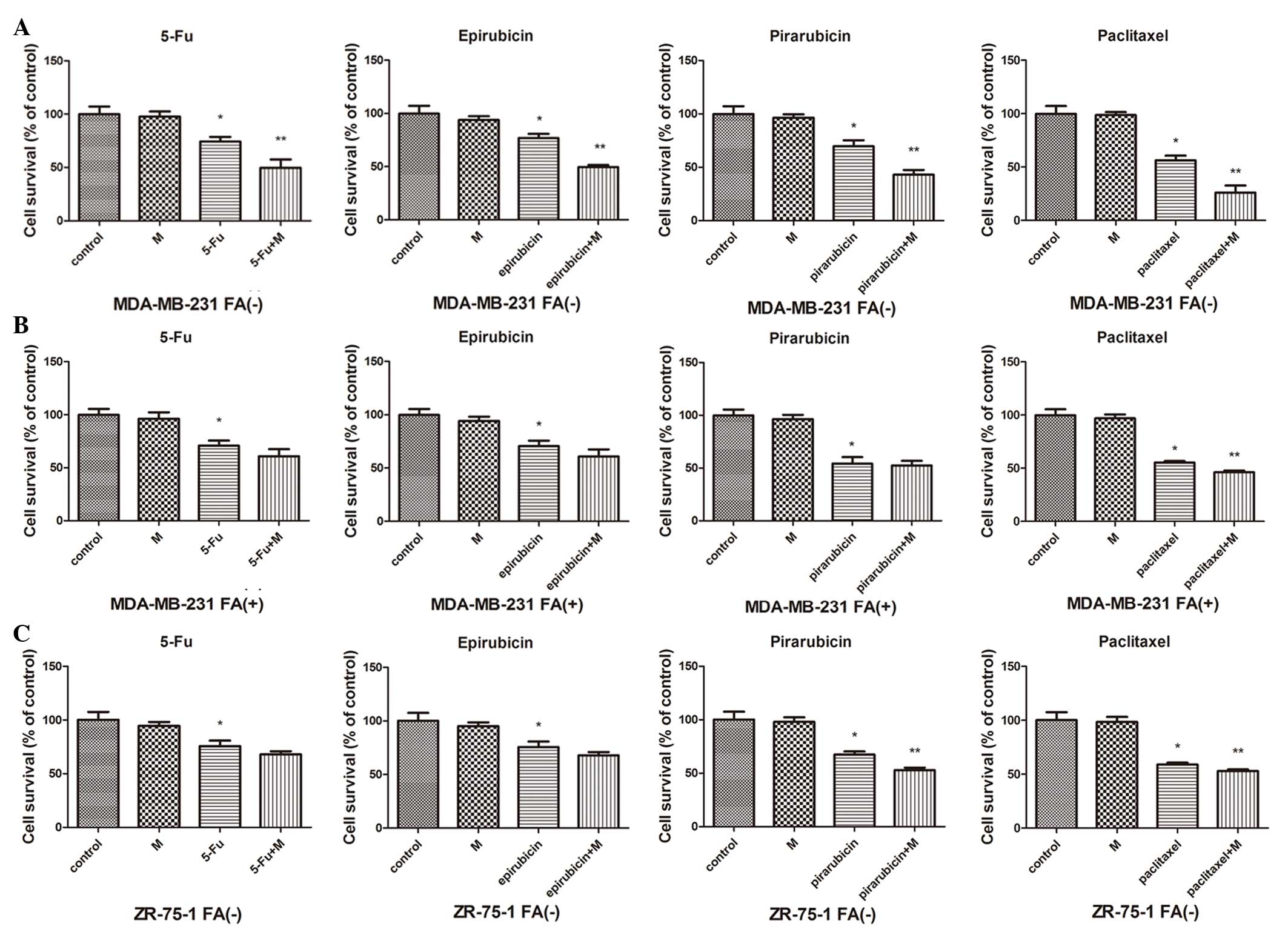
and ZR-75-1 (which are FA receptor negative). As shown in Fig. 3, treatment with
R-O2-FA-CHI-SWCNTs and chemotherapy reduced the survival
of MDA-MB-231 cells in FA-free medium significantly compared to
chemotherapy alone cells (P<0.05). In MDA-MB-231 cells in medium
containing FA, treatment with R-O2-FA-CHI-SWCNTs and
paclitaxel caused a reduction of cell survival significantly
compared to treatment with paclitaxel alone (P<0.05), and caused
a modest but not significant reduction in the other chemotherapy
drug groups. In ZR-75-1 cells, a significant reduction in cell
survival caused by adding R-O2-FA-CHI-SWCNTs was
observed in the pirarubicin and paclitaxel groups (P<0.05);
however, the effect of R-O2-FA-CHI-SWCNTs was modest but
not significant in the remaining two chemotherapy drug groups.
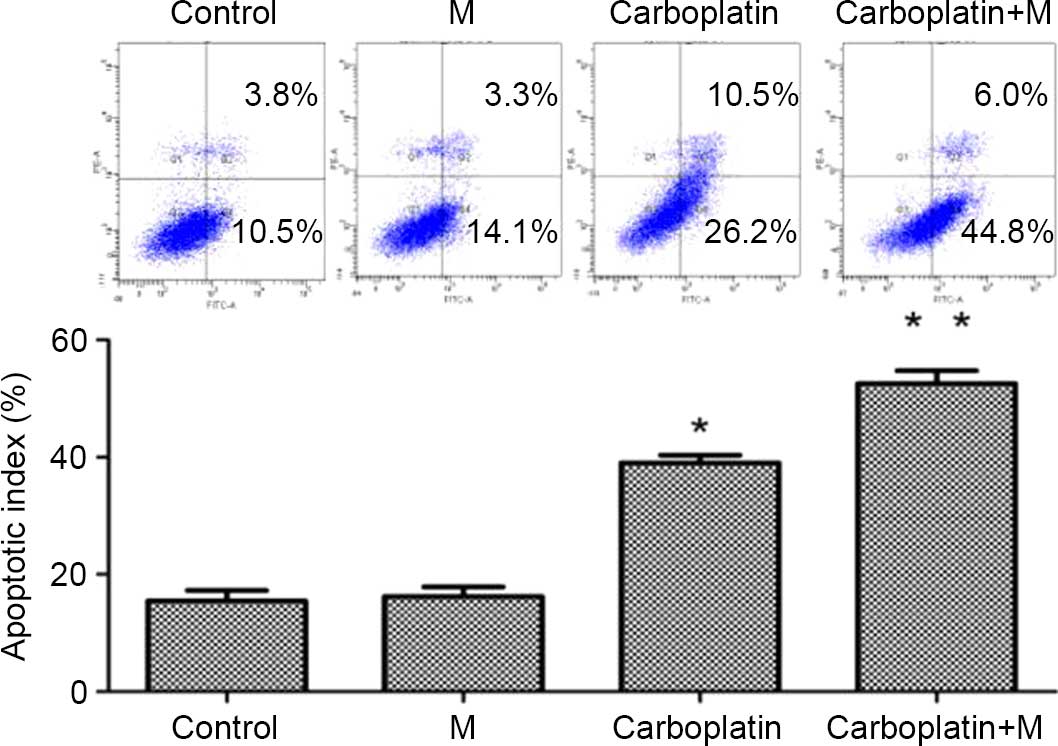
An Annexin V-fluorescein isothiocyanate (FITC)/PI
experiment was performed to further investigate the effect of
sensitization from R-O2-FA-CHI-SWCNTs in hypoxic
conditions. As shown in Fig. 4, the
apoptotic rate was 39.0±1.31% when treated alone with carboplatin,
and that increased to 52.5±2.21% when treated with carboplatin and
R-O2-FA-CHI-SWCNTs in combination. As
R-O2-FA-CHI-SWCNT treatment alone had no effect on
apoptosis in MDA-MB-231 cells, it appears that
R-O2-FA-CHI-SWCNTs sensitizes the breast cancer cell
lines to chemotherapy.
R-O2-FA-CHI-SWCNTs increase
the sensitivity of cells to radiotherapy
To investigate whether R-O2-FA-CHI-SWCNTs
modulated the response of MDA-MB-231 and ZR-75-1 cells to
radiation, 100 µg/ml R-O2-FA-CHI-SWCNTs was added to
cells 30 min prior to irradiation. As shown in Fig. 5, pre-incubation with
R-O2-FA-CHI-SWCNTs reduced clonogenic survival in
comparison to irradiated controls. As shown in Table I, the SER values for MDA-MB-231 cells
in FA-free medium was 1.11; the SER values for MDA-MB-231 cells in
medium containing FA was 1.02, and the SER values for ZR-75-1 cells
was 1.03.
 | Table I.Radiation response variables of
MDA-MB-231 and ZR-75-1 cells following combined treatment with
M. |
Table I.
Radiation response variables of
MDA-MB-231 and ZR-75-1 cells following combined treatment with
M.
| Cell line
treatment | α | β | α/β | SER |
|---|
| MDA-MB-231 FA
(−) |
|
|
|
|
| IR | 0.03823 | 0.01486 | 2.57 |
|
|
IR+M | 0.08692 | 0.02028 | 4.29 | 1.11 |
| MDA-MB-231 FA
(+) |
|
|
|
|
| IR | 0.03234 | 0.01372 | 2.36 |
|
|
IR+M | 0.04842 | 0.01857 | 2.61 | 1.02 |
| ZR-75-1 |
|
|
|
|
| IR | 0.02944 | 0.01281 | 2.30 |
|
|
IR+M | 0.04587 | 0.01587 | 2.89 | 1.03 |
To further assess the effect of
R-O2-FA-CHI-SWCNTs on the radiotherapy sensitivity of
MDA-MB-231 cell lines, an Annexin V-FITC/PI experiment was
performed. As shown in Fig. 6, the
apoptotic rate of MDA-MB-23 cells was 43.1±4.36% at an irradiation
dose of 4 Gy. R-O2-FA-CHI-SWCNTs increased the apoptotic
rate of irradiated (4 Gy) MDA-MB-231 cells to 53.4±2.41%
(P<0.05).
Effect of
R-O2-FA-CHI-SWCNTs on proteins associated with
apoptosis, and chemotherapy and radiotherapy sensitivity in
MDA-MB-231 cells
To investigate the proteins involved in the
sensitizing effect of R-O2-FA-CHI-SWCNTs administrated
in combination with epirubicin or radiotherapy (4 Gy) on MDA-MB-231
cells, the expression of certain proteins were determined by
western blotting.
As illustrated in Fig.
7, treatment with R-O2-FA-CHI-SWCNTs in combination
with epirubicin or radiotherapy resulted in a decrease in Bcl-2 and
survivin levels, which may be partially responsible for the
apoptotic tendency, and a decrease of HIF-1α levels, which
indicated the amelioration of hypoxic status. The levels of P-gp
and MRP-1, which are associated with chemoresistance, and the
expression of RAD51 and Ku80, which are associated with
radioresistance, was downregulated.
 | Figure 7.(A) Epirubicin group, western blot
analysis was performed against Bcl-2, survivin, P-gp, MRP-1 and
HIF-1α. β-actin was used as a loading control. (B) Radiotherapy
group, western blot analysis was performed against Bcl-2, survivin,
RAD51, Ku80 and HIF-1α. β-actin was used as a loading control. M,
tombarthite-modified-folic acid-chitosan-single-walled carbon
nanotubes; IR, irradiation; Bcl-2, B-cell lymphoma 2; P-gp,
P-glycoprotein; MRP-1, multidrug resistance-associated protein 1;
Hif-1α, Hypoxia-inducible factor 1-α. |
Discussion
Breast cancer poses a major challenge to the health
of women worldwide. The modalities of treatment include surgery,
chemotherapy, and hormonal and radiation therapies (1). A significant cause of breast cancer
treatment failure is the cancer cells becoming resistant to drugs
or radiation, leading to progression of the tumor into an invasive
and metastatic phenotype (27). The
inability of chemotherapy or radiotherapy to totally eradicate
tumors may be due to the presence of hypoxic cells (28–30).
Therefore, oxygen is extremely important for effective anticancer
chemotherapy and radiotherapy.
The utilization of nanomedicine in oncology has
drawn attention in recent years, particularly in the field of
breast cancer research (31–33). Efforts have been made to apply
nanomedicine in cancer treatment to improve the efficiency of
anticancer regimens (17,18,34,35). In
the present study, SWCNTs were used as the carrier of oxygen, which
was expected to increase the sensitivity of cancer cells to
chemotherapy and radiotherapy.
FA receptor (FR) is a
glycosylphosphatidylinositol-linked membrane glycoprotein and is
overexpressed on the surfaces of numerous cancer cells, including
breast cancer cells; it is almost absent in the majority of normal
tissues (36–38). FA is internalized into the cytoplasm
via FR-mediated endocytosis (37–39).
Functionalization of R-O2-SWCNTs with FA may target this
receptor specifically. In the present study, a novel oxygen carrier
based on single-walled carbon nanotubes was successfully
synthesized, which was subsequently shown to have characteristics
of targeted and good dispersibility (40).
The WST-1 assay and cell colony formation assay in
the present experiment revealed that administration of
R-O2-FA-CHI-SWCNTs plus chemotherapy or radiotherapy
yielded increased inhibition of cell proliferation compared with
chemotherapy or radiotherapy alone, and the inhibition was more
marked and the sensitivity enhancement rate was the highest in
MDA-MB-231 cells in FA-free medium. As ZR-75-1 cells are
FR-negative, and MDA-MB-231 cells are FR-positive, FA in the medium
binds with FR, so the FR was partly blocked and could not bind with
the synthesized material. Therefore, it may be assumed that
R-O2-FA-CHI-SWCNTs release O2, which spreads
to the cancer cells through free diffusion. The enhanced inhibition
of cell proliferation in MDA-MB-231 cells in FA-free medium
suggests that R-O2-FA-CHI-SWCNTs are able to supply
oxygen to cancer cells through the free diffusion of oxygen, as
well as the binding of FA to FR.
Flow cytometry in the present study confirmed that
R-O2-FA-CHI-SWCNTs plus chemotherapy or radiotherapy
induced an increased rate of cell apoptosis compared to
chemotherapy or radiotherapy alone.
Bcl-2 is an anti-apoptotic protein, and has a
significant role in regulating cell apoptosis (41,42). Bcl-2
interacts indirectly with Bcl-2-like protein 4 and prevents caspase
activation, including caspase-9 (43,44).
Overexpression of Bcl-2 is a significant pathway of resistance in
treating cancer with chemotherapy and radiotherapy (45). Survivin is an inhibitor of apoptosis
protein, and is poorly expressed in normal breast tissue and
overexpressed in neoplastic breast tissue (46). Therefore, downregulation of Bcl-2 and
survivin may be a potential strategy for breast cancer therapy. In
the present study, under hypoxic conditions, the addition of
R-O2-FA-CHI-SWCNTs may have inhibited Bcl-2 and
survivin, which demonstrates its effect of increasing sensitivity
to chemoradiotherapy.
Under hypoxic conditions, breast cancer cells must
adapt to exist in the microenvironment, and the expression of
HIF-1α is elevated (47,48). In the present experiment, the
administration of R-O2-FA-CHI-SWCNTs downregulated the
expression of HIF-1α, which indicated that the hypoxic conditions
and chemoradiotherapy resistance were alleviated. Furthermore, the
decrease of MRP-1 and P-gp expression caused by adding
R-O2-FA-CHI-SWCNTs may potentially reduce tumor
resistance to chemotherapy.
In radiotherapy, DNA damage is created by direct
ionization from radiation, or is induced by interaction with oxygen
centered radicals that are formed by the ionization of water
surrounding DNA (49–51). Unrepaired DNA double-strand breaks
(DSBs) may lead to fatal changes, including chromosomal aberrations
(52). In the absence of molecular
oxygen, the damage is more repairable, as oxygen is able to react
with the broken ends of DNA, making them less easily repaired by a
cell (53,54). There are two major signaling pathways
to repair potentially lethal DNA DSBs: Homologous recombination
(HR) and nonhomologous DNA end joining (NHEJ) (55). RAD51 is a central player in the
HR-mediated repair of DSBs (56), and
Ku80 is one of the important factors that can function to mediate
NHEJ (56). Based on the results of
the present study, which identified decreased levels of RAD51 and
Ku80 proteins, it may be hypothesized that radiosensitivity is
increased with R-O2-FA-CHI-SWCNTs administration, and
the radiosensitizing effect may involve impairment of DSBs
repair.
In conclusion, the present study successfully
synthesized a novel functionalized SWCNT, which was denoted as
R-O2-FA-CHI-SWCNTs, and the results of the present study
suggest that administration of R-O2-FA-CHI-SWCNTs
sensitizes human breast cancer cells to chemotherapy and
radiotherapy by alleviating hypoxic conditions, leading to
increased rates of proliferation inhibition and apoptosis of breast
cancer cells. Apoptosis associated proteins (Bcl-2 and survivin),
proteins indicating chemosensitivity (MRP-1 and P-gp), hypoxia
associated protein HIF-1α and proteins involved in DSB repair in
radiotherapy (RAD51 and Ku80) were all downregulated. Animal
experiments are required in order to confirm the results of the
present study. Further experiments in different cell lines are
required to investigate if the novel synthesized
R-O2-FA-CHI-SWCNTs are an effective chemoradiotherapy
sensitizer.
Acknowledgments
The present study was funded by the National Natural
Science Foundation of China (grant no., 81102016). The authors
thank Dr Yingbin Liu, the head of the Department of General Surgery
and Laboratory of General Surgery in Xinhua Hospital Affiliated to
Shanghai Jiao Tong University, School of Medicine and the Research
Institute of Biliary Tract Disease, affiliated to Shanghai Jiaotong
University, School of Medicine (Shanghai, China) for laboratory
technical support.
References
|
1
|
National Comprehensive Cancer Network
(NCNN), . Breat cancerNCCN Clinical Practice Guidelines in
Oncology. 3rd. NCNN; Fort Washington, PA, USA: 2013, PubMed/NCBI
|
|
2
|
Ward C, Langdon SP, Mullen P, Harris AL,
Harrison DJ, Supuran CT and Kunkler IH: New strategies for
targeting the hypoxic tumour microenvironment in breast cancer.
Cancer Treat Rev. 39:171–179. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rademakers SE, Span PN, Kaanders JH, Sweep
FC, van der Kogel AJ and Bussink J: Molecular aspects of tumour
hypoxia. Mol Oncol. 2:41–53. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Vaupel P, Mayer A and Höckel M: Tumor
hypoxia and malignant progression. Methods Enzymol. 381:335–354.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rundqvist H and Johnson RS: Tumour
oxygenation: Implications for breast cancer prognosis. J Intern
Med. 274:105–112. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Trédan O, Galmarini CM, Patel K and
Tannock IF: Drug resistance and the solid tumor microenvironment. J
Natl Cancer Inst. 99:1441–1454. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Vaupel P and Mayer A: Hypoxia in cancer:
Significance and impact on clinical outcome. Cancer Metastasis Rev.
26:225–239. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nordsmark M, Overgaard M and Overgaard J:
Pretreatment oxygenation predicts radiation response in advanced
squamous cell carcinoma of the head and neck. Radiother Oncol.
41:31–39. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Denny WA: The role of hypoxia-activated
prodrugs in cancer therapy. Lancet Oncol. 1:25–29. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Guise CP, Mowday AM, Ashoorzadeh A, Yuan
R, Lin WH, Wu DH, Smaill JB, Patterson AV and Ding K: Bioreductive
prodrugs as cancer therapeutics: targeting tumor hypoxia. Chin J
Cancer. 33:80–86. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liapis V, Labrinidis A, Zinonos I, Hay S,
Ponomarev V, Panagopoulos V, DeNichilo M, Ingman W, Atkins GJ,
Findlay DM, et al: Hypoxia-activated pro-drug TH-302 exhibits
potent tumor suppressive activity and cooperates with chemotherapy
against osteosarcoma. Cancer Lett. 357:160–169. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kimura H, Braun RD, Ong ET, Hsu R, Secomb
TW, Papahadjopoulos D, Hong K and Dewhirst MW: Fluctuation in red
cell flux in tumor microvessels can lead to transient hypoxia and
reoxygenation in tumor parenchyma. Cancer Res. 56:5522–5528.
1996.PubMed/NCBI
|
|
13
|
Bennett MH, Feldmeier J, Smee R and
Milross C: Hyperbaric oxygenation for tumor sensitization to
radiotherapy. Cochrane Database Syst Rev. 18:CD0050072012.
|
|
14
|
Overgaard J, Eriksen JG, Nordsmark M,
Alsner J and Horsman MR: Danish Head and Neck Cancer Study Group:
Plasma osteopontin, hypoxia, and response to the hypoxia sensitiser
nimorazole in radiotherapy of head and neck cancer: Results from
the DAHANCA 5 randomised double-blind placebo-controlled trial.
Lancet Oncol. 6:757–764. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xuhui Ma, Zhenjing Miao and Hongyun Wang:
Some problems with hyperbaric oxygenation in treating cancers.
Linchuang Junyi Zazhi. 38:862–864. 2010.
|
|
16
|
Williamson SK, Crowley JJ, Lara PN Jr,
McCoy J, Lau DH, Tucker RW, Mills GM and Gandara DR: Southwest
Oncology Group Trial S0003: Phase III trial of paclitaxel plus
carboplatin with or without tirapazamine in advanced non-small-cell
lung cancer: Southwest Oncology Group Trial S0003. J Clin Oncol.
23:9097–9104. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jevamohan P, Hasumura T, Nagaoka Y,
Yoshida Y, Maekawa T and Kumar DS: Accelerated killing of cancer
cells using a multifunctional single-walled carbon nanotube-based
system for targeted drug delivery in combination with photothermal
therapy. Int J Nanomedicine. 8:2653–2667. 2013.PubMed/NCBI
|
|
18
|
Das M, Datir SR, Singh RP and Jain S:
Augmented anticancer activity of a targeted, intracellularly
activatable, theranostic nanomedicine based on fluorescent and
radiolabeled, methotrexate-folic Acid-multiwalled carbon nanotube
conjugate. Mol Pharm. 10:2543–2557. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lin Y, Taylor S, Li H, Fernando KA Shiral,
Qu L, Wang W, Gu L, Zhou B and Sun Y: Advances toward
bioapplications of carbon nanotubes. J Mater Chem. 14:527–541.
2004. View Article : Google Scholar
|
|
20
|
Bianco A, Kostarelos K and Prato M:
Applications of carbon nanotubes in drug delivery. Curr Opin Chem
Biol. 9:674–679. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Prato M, Kostarelos K and Bianco A:
Functionalized carbon nanotubes in drug design and discovery. Acc
Chem Res. 41:60–68. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xu J, Zhu MH, Liu JJ and Zhao Z: The
state-of-art of researches on rare earth surface engineering and
its tribological applications. China Surface Engineering. 1:20–23.
2001.
|
|
23
|
Sun ZY and Cheng XH: Friction/wear
behaviors of rare earth treated carbon nanotubes/amino silane
self-assembled composite film on silicon substrate. Tribology.
31:156–160. 2011.
|
|
24
|
Ravichandran P, Baluchamy S, Gopikrishnan
R, Biradar S, Ramesh V, Goornavar V, Thomas R, Wilson BL, Jeffers
R, Hall JC and Ramesh GT: Pulmonary biocompatibility assessment of
inhaled single-wall and multiwall carbon nanotubes in BALB/c mice.
J Biol Chem. 286:29725–29733. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lam CW, James JT, McCluskey R, Arepalli S
and Hunter RL: A review of carbon nanotube toxicity and assessment
of potential occupational and environmental health risks. Crit Rev
Toxicol. 36:189–217. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang Y, Lin Q, Wu K, Zhu M, Lu Y, Chen J,
Huang S, Cheng X and Weng Z: Experimental study of bio-security of
functionalized single-walled and multi-walled carbon nanotubes.
Nano Biomed Eng. 3:249–255. 2011. View Article : Google Scholar
|
|
27
|
Eccles SA, Aboaqye EO, Ali S, Anderson AS,
Armes J, Berditchevski F, Blaydes JP, Brennan K, Brown NJ, Bryant
HE, et al: Critical research gaps and translational priorities for
the successful prevention and treatment of breast cancer. Breast
Cancer Res. 15:R922013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gray LH, Conger AD, Ebert M, Hornsey S and
Scott OC: The concentration of oxygen dissolved in tissues at the
time of irradiation as a factor in radiotherapy. Br J Radiol.
26:638–648. 1953. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bush RS, Jenkin RD, Allt WE, Beale FA,
Bean H, Dembo AJ and Pringle JF: Definitive evidence for hypoxic
cells influence in the cure in cancer therapy. Br J Cancer Suppl.
3:302–306. 1978.PubMed/NCBI
|
|
30
|
Brown JM: Evidence for acutely hypoxic
cells in mouse tumor, and a possible mechanism of reoxygenation. Br
J Radiol. 52:650–656. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tan A, Yildirimer L, Rajadas J, De La Peña
H, Pastorin G and Seifalian A: Quantum dots and carbon nanotubes in
oncology: A review on emerging theranostic applications in
nanomedicine. Nanomedicine (Lond). 6:1101–1114. 2016. View Article : Google Scholar
|
|
32
|
Bregoli L, Movia D, Gavigan-Imedio JD,
Lysaght J, Reynolds J and Prina-Mello A: Nanomedicine applied to
translational oncology: A future perspective on cancer treatment.
Nanomedicine. 12:81–103. 2015.PubMed/NCBI
|
|
33
|
Herreros E, Morales S, Cortés C, Cabaña M,
Peñaloza JP, Jara L, Geraldo D, Otero C and Fernández-Ramires R:
Advances in nanomedicine towards clinical application in oncology
and immunology. Curr Pharm Biotechnol. 15:864–879. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Joshi P, Chakraborti S, Ramirez-Vick JE,
Ansari ZA, Shanker V, Chakrabarti P and Singh SP: The anticancer
activity of chloroquine-gold nanoparticles against MCF-7 breast
cancer cells. Colloids Surf B Biointerfaces. 95:195–200. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang H, Li F, Du C, Wang H, Mahato RI and
Huang Y: Doxorubicin and lapatinib combination nanomedicine for
treating resistant breast cancer. Mol Pharm. 11:2600–2611. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Leamon CP and Reddy JA: Folate-targeted
chemotherapy. Drug Deliv Rev. 56:1127–1141. 2004. View Article : Google Scholar
|
|
37
|
Sudimack J and Lee RJ: Targeted drug
delivery via the folate receptor. Adv Drug Deliv Rev. 41:147–162.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Low PS and Kularatne SA: Folate-targeted
therapeutic and imaging agents for cancer. Curr Opin Chem Biol.
13:256–262. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang S and Low PS: Folate-mediated
targeting antineoplastic drugs, imaging agents and nucleic acids to
cancer cells. J Control Release. 53:39–48. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Cheng X: A preparation method of rare
earth modified-folic acid-chitosan-SWCNTs. China Patent
103,007,285. Filed July 20, 2012; issued. November 24–2012
|
|
41
|
Korsmeyer SJ, Shutter JR, Veis DJ, Merry
DE and Oltvai ZN: Bcl-2/Bax: A rheostat that regulates an
anti-oxidant pathway and cell death. Semin Cancer Biol.
4:327–25332. 1993.PubMed/NCBI
|
|
42
|
Lindsay J, Esposti MD and Gilmore AP:
Bcl-2 proteins and mitochondria-specificity in membrane targeting
for death. Biochim Biophys Acta. 1813:532–539. 2001. View Article : Google Scholar
|
|
43
|
Chinnaiyan AM, Orth K, O'Rourke K, Duan H,
Poirier GG and Dixit VM: Molecular ordering of the cell death
pathway. Bcl-2 and Bcl-xL function upstream of the CED-3-like
apoptotic proteases. J Biol Chem. 271:4573–4576. 1996.PubMed/NCBI
|
|
44
|
Kitanaka C, Namiki T, Noguchi K, Mochizuki
T, Kagaya S, Chi S, Hayashi A, Asai A, Tsujimoto Y and Kuchino Y:
Caspase-dependent apoptosis of COS-7 cells induced by Bax
overexpression: Differential effects of Bcl-2 and Bcl-xL on
Bax-induced caspase activation and apoptosis. Oncogene.
15:1763–1772. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Shiu LY, Chang LC, Liang CH, Huang YS,
Sheu HM and Kuo KW: Solamargine induces apoptosis and sensitizes
breast cancer cells to cisplatin. Food Chem Toxicol. 45:2155–2164.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
de Moraes G Nestal, Vasconcelos FC, Delbue
D, Mognol GP, Sternberg C, Viola JP and Maia RC: Doxorubicin
induces cell death in breast cancer cells regardless of survivin
and XIAP expression levels. Eur J Cell Biol. 92:247–256. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Lee JW, Bae SH, Jeong JW, Kim SH and Kim
KW: Hypoxia-inducible factor (HIF-1) alpha: Its protein stability
and biological functions. Exp Mol Med. 36:1–12. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Choi KS, Bae MK, Jeong JW, Moon HE and Kim
KW: Hypoxia-induced angiogenesis during carcinogenesis. J Biochem
Mol Biol. 36:120–127. 2003.PubMed/NCBI
|
|
49
|
Powell S and McMillan TJ: DNA damage and
repair following treatment with ionizing radiation. Radiother
Oncol. 19:95–108. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Santivasi WL and Xia F: Ionizing
radiation-induced DNA damage, response, and repair. Antioxid Redox
Signal. 21:251–259. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Bryant PE: Repair and chromosomal damage.
Radiother Oncol. 72:251–256. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Kavanagh JN, Redmond KM, Schettino G and
Prise KM: DNA double strand break repair: A radiation perspective.
Antioxid Redox Signal. 18:2458–2472. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Deschner EE and Gray LH: Influence of
oxygen tension on x-ray-induced chromosomal damage in Ehrlich
ascites tumor cells irradiated in vitro and in vivo. Radiat Res.
11:115–146. 1959. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Gray LH, Conger AD, Ebert M, Hornsey S and
Scott OC: The concentration of oxygen dissolved in tissue at the
time of irradiation as a factor in radiotherapy. Br J Radiol.
26:638–648. 1953. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Choudhury A, Cuddihy A and Bristow RG:
Radiation and new molecular agents part 1: Targeting ATM-ATR
checkpoints, DNA repair, and the proteasome. Semin Radiat Oncol.
16:51–58. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Lieber MR: The mechanism of human
nonhomologous DNA end joining. J Biol Chem,. 283:1–5. 2008.
View Article : Google Scholar
|