Introduction
Cervical cancer is the second most common type of
cancer among women worldwide, with a mortality rate of ~300,000
annually (1). Metastasis is
responsible for the vast burden of cancer-associated morbidity and
mortality. Overexpression of epithelial growth factor receptor
(EGF-R) is detected in 70–90% of all cervical cancer cases
(2–4).
EGF-R activation induces the epithelial-mesenchymal transition
(EMT), which is accompanied by the overexpression of mesenchymal
markers. In contrast, EGF-R inhibition, by a tyrosine kinase
inhibitor or antibody, provokes EMT, which is accompanied by the
upregulation of epithelial-marker proteins, such as E-cadherin
(E-cad) and Zonula occludens-1 (5).
The EMT is a multi-step process that includes dysfunctional
cell-cell adhesive interactions, loss of cell-cell junctions and
reorganization of the cytoskeleton, which is associated with cell
proliferation, metastasis and immune escape.
Annexin A2 (ANXA2), a calcium-dependent phospholipid
binding protein, is abundantly present in various cancer cells, and
has multiple roles in regulating cellular function, particularly
tumor differentiation, clinical outcomes and metastatic potential
(6,7).
Previous studies have implicated ANXA2 in various biological
functions, including mitogenic signal transduction, fibrinolysis,
immune response, proliferation, carcinogenesis and tumor
progression (7–11). ANXA2 has been demonstrated to be a
co-receptor for both plasminogen and tissue-type plasminogen
activator, which cleaves inactive plasminogen to yield the active
serine proteinase, plasmin (12,13).
Subsequent studies elucidated that the conversion of plasminogen to
plasmin is induced by ANXA2 promoting metastasis, which leads to
the activation of metalloproteinases, degradation of extracellular
matrix components, and promotion of neoangiogenesis (14–17).
However, the underlying mechanisms of ANXA2 and EMT remain
obscure.
The present study utilized wound healing assays,
western blotting, flow cytometry and MTT assays to demonstrate that
ANXA2 is a key regulatory factor of EGF-induced EMT in CaSki
cervical cancer cells.
Materials and methods
Cell lines
CaSki, HeLa and SiHa cells were purchased from the
Cell Bank of China (Wuhan, China) and were cultured under standard
conditions in RPMI-1640 medium (Shanghai Biosun Sci&Tech Co.,
Ltd., Shanghai, China) supplemented with 10% fetal bovine serum
(Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA), 100
µg/ml streptomycin and 100 units/ml penicillin. The cell lines were
maintained in a humidified incubator containing 5% CO2
at 37°C. The cells were passaged twice a week at an initial density
of 1×106 cells/ml. CasKi cells were cultured with
various concentrations (0, 5, 10, 50 and 100 ng/ml) of EGF (Santa
Cruz Biotechnology Inc., Dallas, TX, USA).
Plasmid construction and
stable/transient transfection
The human genomic fragment of ANXA2, amplified by
nested-polymerase chain reaction (PCR) and ~1,076 bp, was cloned
into the pcDNA3.1 (+) vector. Primers for nested-PCR of ANXA2 were
as follows: Sense 5′-CAGCATTTGGGGACGCTCTCAGC-3′ and anti-sense
5′-ATTTCTGGACGCTCAGGCCGTGT-3′; sense
5′-TCCTCGAGCATTTGGGGACGCTCTCAGCTCTC-3′ and anti-sense
5′-GCGGATCCCTTCAGTCATCTCCACCACACAGG-3′. To generate a cell line
that stably expresses ANXA2, CaSki cells were transfected with
pcDNA3.1-ANXA2 using Lipofectamine 2000 reagent (Invitrogen; Thermo
Fisher Scientific, Inc.). Following selection with G418, a single
clone that overexpressed ANXA2 was identified by western blotting.
To knockdown ANXA2, the ANXA2 siRNA reagent (sc-270151; Santa Cruz
Biotechnology Inc.) was used for transfection.
Wound healing assay
Cells were plated at 2×105/l cells per
well in a 6-well plate and grown overnight under standard
conditions. A straight-line scratch was made on a confluent
monolayer of cells using a sterile 1-ml disposable serological
pipette. To remove debris and to smooth the edge of the scratch,
the cells were washed with 1 ml PBS. Images of cell proliferation
were captured using a Nikon Eclipse TS100 microscope (Nikon Corp.,
Tokyo, Japan) at 0, 24 and 48 h after the scratch was made.
Western blot
Total protein was extracted from cells using cell
lysis buffer (0.5% NP-40, 0.5% SDS, 1.5 Mm Tris-HC, pH 7.4 and 15
mM NaCl). Protein samples (20 µg/lane) were separated by 5% (spacer
gel) and then 10% (separation gel) SDS-PAGE, and transferred to
polyvinylidene difluoride membranes. Following blocking with 5%
skimmed milk, the membranes were incubated overnight with primary
antibodies against E-cadherin (sc-7870; 1:1,000; Santa Cruz
Biotechnology, Inc.), N-cad (BA0637; 1:500; Boster Biological
Technology, Ltd., Wuhan, China), ANXA2 (A2485; 1:3,000;
Sigma-Aldrich; Merck Millipore, Darmstadt, Germany) and β-actin
(sc-7210; Santa Cruz Biotechnology, Inc.). The membranes were
treated with goat-anti rabbit horseradish peroxidase-conjugated
secondary antibody (A32732; 1:1,000; Invitrogen; Thermo Fisher
Scientific, Inc.) for 1 h at 37°C. Target protein bands were
determined using the enhanced chemiluminescence (ECL) reagents
provided in the ECL+PLUS kit (GE Healthcare Bio-Sciences,
Pittsburgh, PA, USA). Protein bands were quantified using Quantity
One 4.62 software (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
β-actin was used as an internal control.
Flow cytometry
Flow cytometric analysis was used to determine the
distribution of cells in the cell cycle sub-phases. Cells were
harvested in the logarithmic growth phase and fixed overnight with
80% ethanol. Cells were washed with cold PBS and stained with
propidium iodide (0.05 mg/ml) and RNase A (0.5 mg/ml) and were
analyzed using a flow cytometer.
MTT assay
Cells with different ANXA2 expression levels were
seeded at a density of 1×105 cells into 96-well culture
plates and grown to 40% confluence. The medium in each well was
removed, and 20 µl MTT solution (5 mg/ml) was added to each well.
Following incubation at 37°C for 4 h, the medium was removed and
150 µl DMSO was added to each well. The optical density at 570 nm
was recorded. The cell growth rate was calculated as follows:
Cell growth rate = ANh / A0h × 100% (N~24, 48, 72,
96, 120).
Statistical analysis
All data are presented as the mean ± standard
deviation. Statistical analysis between the groups was assessed
using Student's two-tailed t-test and analysis of variance.
P<0.05 was considered to indicate a statistically significant
difference.
Results
EGF induces EMT and promotes cell
viability
Different tumor cell lines exhibit different
metastasis properties, which are associated with the expression
levels of E-cad (18). Initially,
E-cad expression levels were tested in different cervical cancer
cell lines, including HeLa, SiHa and CaSki. E-cad expression levels
in CaSki cells were markedly increased, as compared with SiHa and
HeLa cells (Fig. 1A). Due to this,
CaSki cells were used for the epithelial model and were cultured
under standard conditions with or without EGF (100 ng/ml) for 24 h.
Morphological changes were assessed. After 48 h of EGF (100 ng/ml)
treatment, CaSki cells became more fusiform and the connections
between the cells decreased (Fig.
1B). Moreover, the E-cad and N-cad expression levels were
analyzed by western blotting, which suggesed that EGF treatment was
able to upregulate N-cad expression levels and downregulate E-cad
expression levels (Fig. 1C). EGF
treatment disrupted the cell cycle distribution by decreasing the
G0/G1 phase (normal, 70.9%; EGF treatment, 56.9%) and increasing
the G2/M (normal, 7.6%; EGF treatment, 13.3%) and S phases (normal,
21.5%; EGF treatment, 29.7%) under EGF treatment (Fig. 1D). These data indicate that EGF may
induce EMT and promote cell viability by interfering with the cell
cycle. Similar results have been published previously (19).
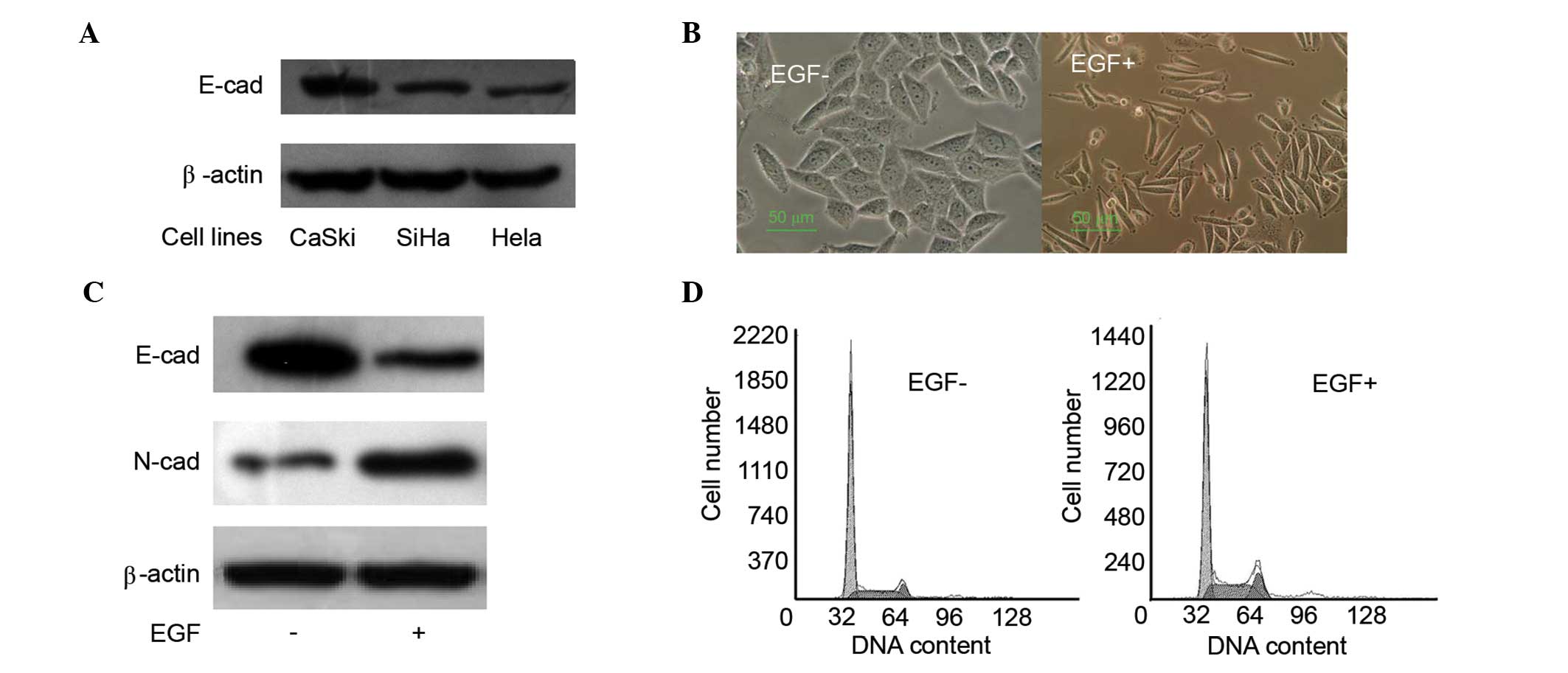 | Figure 1.EGF induces EMT and promotes cell
proliferation. (A) E-cad expression levels were determined by
western blot analysis, and the expression levels in CaSki were much
higher than that of SiHa and HeLa cells. (B) Morphological changes
were detected following 24 h EGF (100 ng/ml) treatment. CaSki cells
became more fusiform after EGF treatment. (C) E-cad and N-cad
expression levels were tested by western blot analysis. EGF
treatment induced downregulation of E-cad and upregulation of N-cad
in CaSki cells. (D) Cell cycle distribution was tested by flow
cytometry. Cell cycle distribution was disrupted by decreasing the
G0/G1 phase (normal, 70.9%; EGF, 56.9%) and increasing the G2/M
(normal, 7.6%; EGF, 13.3%) and S phases (normal, 21.5%; EGF, 29.7%)
with EGF treatment. EGF, epidermal growth factor; EMT,
epithelial-mesenchymal transition; E-cad, E-cadherin; N-cad,
N-cadherin. |
EGF treatment upregulates ANXA2
Previous studies have illustrated that ANXA2 is
involved in the EMT process (20). To
investigate the association between ANXA2 and EGF treatment, total
protein was collected from different CaSki cells that were treated
with different EGF concentrations (0, 5, 10, 50 and 100 ng/ml;
Fig. 2A) or with different treatment
durations (0, 12, 24, 36 and 48 h; Fig.
2B). Expression levels of ANXA2 after 24 h of 100 ng/ml EGF
treatment were significantly higher than that of cells without EGF
treatment or after 24 h at a lower concentration (P<0.01).
Furthermore, ANXA2 expression levels were higher when treated with
EGF (100 ng/ml) for a longer duration. These results suggested that
EGF promotes ANXA2 expression, and that EGF expression levels in
cells are increased by prolonged exposure or exposure to an
increased concentration (Fig. 2).
Roles of ANXA2 in EMT, growth and
migration
Although ANXA2 was significantly upregulated in the
EGF-induced EMT process, it is possible that this is an unrelated
phenomenon or a compensatory event. To clarify the role of ANXA2 in
the EMT process, CaSki cell lines that had ANXA2 overexpression or
knockdown were established by transfecting pcDNA 3.1-ANXA2 or ANXA2
siRNAs, which was evaluated by western blot analysis (Fig. 3A). Using these cell lines as a model,
it was demonstrated that ANXA2 downregulated E-cad expression,
which was similar to the effect noted after 24 h of EGF (100 ng/ml)
treatment. The downregulation of E-cad induced by EGF was partially
reversed by ANXA2 siRNA transfection. N-cad, which is a mesenchymal
marker protein and is upregulated with EGF treatment, was
upregulated by ANXA2 overexpression. Furthermore, the upregulation
of N-cad induced by EGF was partially reversed by ANXA2 siRNA
transfection (Fig. 3B). With
overexpression of ANXA2, the cell morphology became more fusiform,
and the morphological changes induced by 24-h treatment with EGF
(50 ng/ml) were partially reversed by ANXA2 siRNA transfection
(Fig. 3C).
 | Figure 3.Roles of ANXA2 in the EMT process. To
clarify the role of ANXA2 in the EMT process, CaSki cell lines with
overexpressed or knocked down ANXA2 were created by transfecting
pcDNA 3.1-ANXA2 or ANXA2 siRNAs. (A) Overexpression or knockdown of
ANXA2 was verified by western blot analysis. 1 and 2 were
transfected with ANXA2 siRNA, 3 was normal CaSki cells, 5 was
transfected with pcDNA 3.1, and 4 and 6 were transfected with
pcDNA3.1-ANXA2. (B) E-cad and N-cad expression levels were tested
by western blot analysis; 1 was normal CaSki cells, 2 was knockdown
of ANXA2 in CaSki cells with 24 h of EGF (100 ng/ml) treatment, 3
was forced expression of ANXA2 in CaSki cells, 4 was normal CaSki
cells with EGF treatment. Forced ANXA2 expression downregulated
E-cad expression levels and upregulated N-cad expression levels,
which was similar to 24 h of EGF (100 ng/ml) treatment. However,
the upregulation of N-cad and downregulation of E-cad induced by
EGF was partially reversed by knockdown of ANXA2. Densitometric
analysis of three independent western blots. Values are presented
as the means of three trials with the standard deviation indicated
by error bars. **P<0.01 vs. the control. (C) Morphological
changes with ANXA2 transfection. Morphology was more fusiform after
ANXA2 overexpression, and the morphological changes induced by 24 h
of EGF (50 ng/ml) treatment were partially reversed by ANXA2 siRNA
transfection. (D) Cell viability was analyzed by MTT assay, which
showed that forced ANXA2 expression promoted cell growth, which was
similar to EGF treatment. P<0.01 vs. the normal cells. However,
knockdown of ANXA2 inhibited cell growth. P<0.05 vs. the normal
cells. EGF, epidermal growth factor; EMT, epithelial-mesenchymal
transition; E-cad, E-cadherin; N-cad, N-cadherin; ANXA2, Annexin
A2. |
Moreover, ANXA2 promoted cell growth and interfered
with the cell cycle by decreasing the cell ratio in G0/G1 phase
(normal, 70.9%; ANXA2 upregulation, 53.7%), but it increased the
cell ratio in S phase (normal, 21.5%; ANXA2 upregulation, 29.6%)
and G2/M phase (normal, 7.6%; ANXA2 upregulation, 16.8%), which was
similar to EGF treatment. However, knockdown of ANXA2 had the
opposite effect compared with normal CaSki cells, which involved an
increased cell ratio in the G0/G1 phase (ANXA2 downregulation,
79.1%) and a decreased cell ratio in S phase (ANXA2 downregulation,
11.1%) and G2/M phase (ANXA2 downregulation, 9.8%; Figs. 3D and 4).
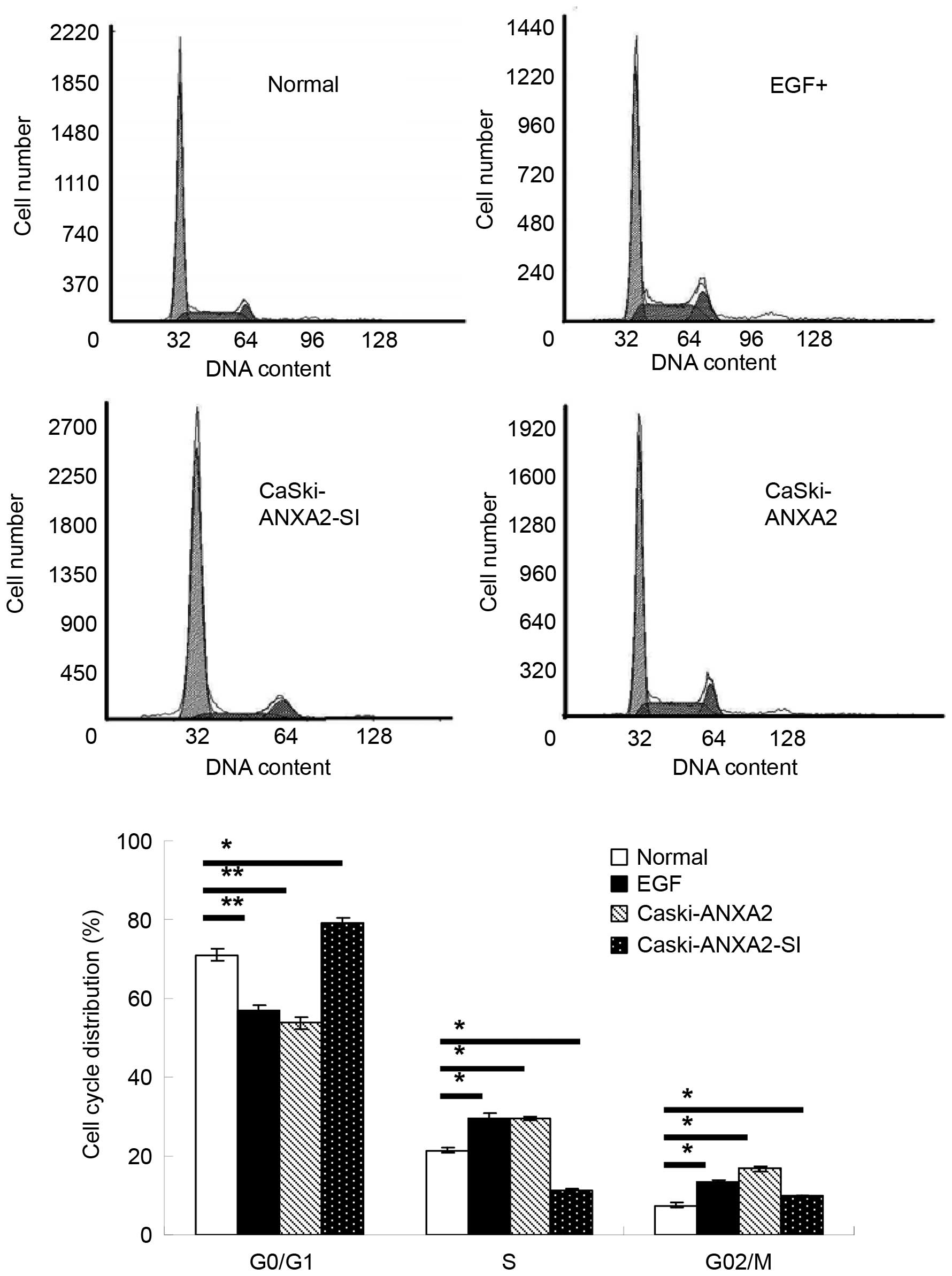 | Figure 4.Effects of ANXA2 on cell cycle
distribution. Flow cytometry was used to test the effects of
overexpression and knockdown of ANXA2 on the cell cycle. There was
a decreased cell ratio in G0/G1 phase (normal, 70.9%; ANXA2
upregulation, 53.7%) and an increased cell ratio in S phase
(normal, 21.5%; ANXA2 upregulation, 29.6%) and G2/M phase (norma,
7.6%; ANXA2 upregulation, 16.8%) when ANXA2 expression was forced.
With ANXA2 knockdown, the cell ratio in G0/G1 phase increased
(ANXA2 upregulation, 79.1%), and decreased in S phase (ANXA2
upregulation, 11.1%) and G2/M phase (ANXA2 downregulation, 9.8%),
which was evaluated using flow cytometry. Treatment with EGF (100
ng/ml) for 24 h was used as a positive control, and normal CaSki
cells were used as a normal control. Values are presented as the
means of three trials with the standard deviation indicated by
error bars. *P<0.05 and **P<0.01 vs. the control cells. EGF,
epidermal growth factor; EMT, epithelial-mesenchymal transition;
ANXA2, Annexin A2. |
The results of the present study suggested that
overexpression of ANXA2 may promote EMT, which was upregulated in
the EGF-induced EMT process (Fig. 3).
To further investigate the role of ANXA2 in migration, a wound
healing assay was evaluated in normal, EGF-treated,
ANXA2-upregulated and ANXA2-downregulated CaSki cell lines. These
results suggest that upregulated ANXA2 or EGF may promote CaSki
cell migration compared with normal cells, but silencing of ANXA2
had the opposite effect (Fig. 5).
 | Figure 5.Wound healing assay. CaSki cells that
were treated with EGF or overexpression or knockdown of ANXA2 were
plated at 2×105/l cells per well in a 6-well plate and
cultured overnight under standard conditions. A straight-line
scratch was made on a confluent monolayer of cells using a sterile,
1-ml, disposable serological pipette. Images of cell proliferation
were captured and the close field was calculated using a Nikon
Eclipse TS100 microscope at 0, 24 and 48 h after the scratch.
Sample 1 was normal CaSki cells, 2 was CaSki cells with EGF
treatment, 3 was CaSki cell with ANXA2 overexpression and 4 was
CaSki cells with downregulated ANXA2. The relative close field
ratio of ANXA2 overexpressed cells was much higher than that of the
normal and ANXA2 knockdown cells at 48 h after scratching. EGF
treatment was used as a positive control. Values are presented as
the means of three trials with the standard deviation indicated by
error bars. *P<0.05 and **P<0.01. EGF, epidermal growth
factor; EMT, epithelial-mesenchymal transition; E-cad, E-cadherin;
N-cad, N-cadherin; ANXA2, Annexin A2. |
Discussion
EMT is a dynamic process that can be regulated and
reversed by various factors, including miRNA (21). Alterations in cell morphology and
function during the EMT process are accompanied by changes in
protein expression profiles, including the loss of epithelial
markers and the de novo expression of mesenchymal markers.
Previous studies have revealed that EMT can be triggered by the
interplay of extracellular signals, including extracellular matrix
components and soluble growth factors, such as transforming growth
factor-β and fibroblast growth factor families, EGF, insulin-like
growth factor and scatter factor/hepatocyte growth factor in cancer
progression (22,23).
The present study explored the possible mechanism of
EGF-induced EMT, in addition to how ANXA2 has a crucial role in
CaSki progression. The overexpression of EGF-R is an independent
predictor for poor prognosis in cervical cancer (24). Moreover, EGF-R overexpression is
associated with a poor response to chemoradiation (25). Initially, it was suggested that the
CaSki cell line has higher E-cad expression levels than other
cervical cancer cell lines, such as SiHa and HeLa cells. As a
result, CaSki cells may be epithelial-like cells in cervical
cancer, and thus were used as a model cell line in the present
study. The present results demonstrated that EGF-induced EMT is
accompanied by high levels of ANXA2 expression. With EGF treatment,
these cells became more spindle-shaped and had
mesenchymal-associated molecular profiles, such as de novo
expression of N-cadherin and increased migration activity. Grewal
and Enrich (26) suggested that ANXA2
is downstream of the EGF-R signal pathway. To clarify the
regulatory role of ANXA2 in EGF treatment, the role of ANXA2 in the
viability and migration of human CaSki cervical cancer cells was
investigated. Stable expression of ANXA2 significantly promotes
cell growth by interfering with the cell cycle in vitro.
However, silencing of ANXA2 inhibited the cell growth and
distribution of the S phase less than in normal cells.
Overexpression of ANXA2 was able to promote cell migration
activity, whereas depletion of ANXA2 inhibited migration. It has
also been suggested that ANXA2 maintains constitutive activation of
EGF-R downstream signaling intermediates, contributing to cell
proliferation, migration and viability (27), which is considered a potential factor
that regulates cell growth, invasion and chemo-resistance (20). ANXA2 may facilitate cell proliferation
by regulating p53 via c-Jun N-terminal kinase/c-Jun in HCC since
disruption of the p53/miRNA-34 axis causes abnormal apoptosis and
progression (28).
The present results also indicated that the
overexpression of ANXA2 induces E-cad downregulation and N-cad
upregulation with structural alterations, which is similar to EGF
treatment alone. However, silencing ANXA2 reversed the
downregulation of E-cad and upregulation of N-cad that was induced
by EGF treatment. In mesenchymal-like cells, downregulation of
E-cadherin or upregulation of N-cad is characterized as the major
hallmark responsible for the loss of cell-cell contacts in EMT
events (21). E-cadherin, which is
present in mature adherens junctions, is a pivotal molecule that
maintains epithelial cell polarity. E-cadherin binds to β-catenin
and forms a protein complex that links to the actin cytoskeleton.
E-cadherin has anti-proliferation, anti-invasion and
anti-metastasis functions, and loss of E-cadherin contributes to
metastatic dissemination in numerous cancer types (5). The mechanisms of E-cadherin loss in
malignant cancers include genetic mutation, epigenetic silencing,
transcription repression and proteolytic processes (4). Another research group has also
demonstrated that upregulation of ANXA2 is accompanied by the EMT
process in endometrial cells, and forced expression of ANXA2 may
mediate phenotypic mesenchymal-like cellular changes with
structural and functional alteration in a β-catenin/TCF
signal-associated manner (29). This
could be reversed by inhibition of ANXA2 expression, and another
study suggested that ANXA2 is closely associated with tumor
progression in HeLa cells (30).
It has previously been suggested that ANXA2
depletion delays EGF-R endocytic trafficking via cofilin activation
and enhances EGF-R signaling and metastasis formation (27). However, this data also suggested that
this inhibition coincides with enhanced EGF-induced cell migration
and downstream signaling via JNK and Akt, which may explain why
ANXA2 knockdown increases lung metastasis formation in mice
(31).
The findings of the present study demonstrated that
in CaSki cells, ANXA2 acts as an important regulatory factor in
EGF-induced EMT. ANXA2 promoted EGF-induced EMT, cell viability and
migration activity in CaSki cells in vitro. This suggests
that depletion of ANXA2 may structurally and functionally reverse
EGF-induced EMT.
Acknowledgements
We would like to thank Professor Zhaoqi Liu and Dr
Changbai Liu at the Institute of Molecular Biology of Three Gorges
University (Yichang, China) for their technical advice and
assistance. This work was supported by grants from the Projects of
Natural Science Foundation of China (grant nos. 81603345 and
81374024) and Projects of Hubei Science foundation (grant no.
2013CFA079). We thank the staff of the Institute of Molecular
Biology of Three Gorges University.
References
|
1
|
Mathur SP, Mathur RS and Young RC:
Cervical epidermal growth factor-receptor (EGF-R) and serum
insulin-like growth factor II (IGF-II) levels are potential markers
for cervical cancer. Am J Reprod Immunol. 44:222–230. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kim GE, Kim YB, Cho NH, Chung HC, Pyo HR,
Lee JD, Park TK, Koom WS, Chun M and Suh CO: Synchronous
coexpression of epidermal growth factor receptor and
cyclooxygenase-2 in carcinomas of the uterine cervix: A potential
predictor of poor survival. Clin Cancer Res. 10:1366–1374. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Oh MJ, Choi JH, Kim IH, Lee YH, Huh JY,
Park YK, Lee KW, Chough SY, Joo KS, Ku BS and Saw HS: Detection of
epidermal growth factor receptor in the serum of patients with
cervical carcinoma. Clin Cancer Res. 6:4760–4763. 2000.PubMed/NCBI
|
|
4
|
Lee MY, Chou CY, Tang MJ and Shen MR:
Epithelial-mesenchymal transition in cervical cancer: Correlation
with tumor progression, epidermal growth factor receptor
overexpression, and snail up-regulation. Clin Cancer Res.
14:4743–4750. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yoshida K, Yoshida S, Choisunirachon N,
Saito T, Matsumoto K, Saeki K, Mochizuki M, Nishimura R, Sasaki N
and Nakagawa T: The relationship between clinicopathological
features and expression of epithelial and mesenchymal markers in
spontaneous canine mammary gland tumors. J Vet Med Sci.
76:1321–1327. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gerke V and Moss SE: Annexins: From
structure to function. Physiol Rev. 82:331–371. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Flood EC and Hajjar KA: The annexin A2
system and vascular homeostasis. Vascul Pharmacol. 54:59–67. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Brichory FM, Misek DE, Yim AM, Krause MC,
Giordano TJ, Beer DG and Hanash SM: An immune response manifested
by the common occurrence of annexins I and II autoantibodies and
high circulating levels of IL-6 in lung cancer. Proc Natl Acad Sci
USA. 98:9824–9829. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Keutzer JC and Hirschhorn RR: The
growth-regulated gene 1B6 is identified as the heavy chain of
calpactin I. Exp Cell Res. 188:153–159. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sharma MR, Koltowski L, Ownbey RT,
Tuszynski GP and Sharma MC: Angiogenesis-associated protein annexin
II in breast cancer: Selective expression in invasive breast cancer
and contribution to tumor invasion and progression. Exp Mol Pathol.
81:146–156. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sharma MR, Rothman V, Tuszynski GP and
Sharma MC: Antibody-directed targeting of angiostatin's receptor
annexin II inhibits Lewis lung carcinoma tumor growth via blocking
of plasminogen activation: Possible biochemical mechanism of
angiostatin's action. Exp Mol Pathol. 81:136–145. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hwang J, Hodis HN, Hsiai TK, Asatryan L
and Sevanian A: Role of annexin II in estrogen-induced macrophage
matrix metalloproteinase-9 activity: The modulating effect of
statins. Atherosclerosis. 189:76–82. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Brownstein C, Deora AB, Jacovina AT,
Weintraub R, Gertler M, Khan KM, Falcone DJ and Hajjar KA: Annexin
II mediates plasminogen-dependent matrix invasion by human
monocytes: Enhanced expression by macrophages. Blood. 103:317–324.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhao P, Zhang W, Tang J, Ma XK, Dai JY, Li
Y, Jiang JL, Zhang SH and Chen ZN: Annexin II promotes invasion and
migration of human hepatocellular carcinoma cells in vitro via its
interaction with HAb18G/CD147. Cancer Sci. 101:387–395. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sharma M, Ownbey RT and Sharma MC: Breast
cancer cell surface annexin II induces cell migration and
neoangiogenesis via tPA dependent plasmin generation. Exp Mol
Pathol. 88:278–286. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ohno Y, Izumi M, Kawamura T, Nishimura T,
Mukai K and Tachibana M: Annexin II represents metastatic potential
in clear-cell renal cell carcinoma. Br J Cancer. 101:287–294. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lokman NA, Ween MP, Oehler MK and
Ricciardelli C: The role of annexin A2 in tumorigenesis and cancer
progression. Cancer Microenviron. 4:199–208. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Serrano MJ, Ortega FG, Alvarez-Cubero MJ,
Nadal R, Sanchez-Rovira P, Salido M, Rodríguez M, García-Puche JL,
Delgado-Rodriguez M, Solé F, et al: EMT and EGFR in CTCs
cytokeratin negative non-metastatic breast cancer. Oncotarget.
5:7486–7497. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bhat FA, Sharmila G, Balakrishnan S,
Arunkumar R, Elumalai P, Suganya S, Singh P Raja, Srinivasan N and
Arunakaran J: Quercetin reverses EGF-induced epithelial to
mesenchymal transition and invasiveness in prostate cancer (PC-3)
cell line via EGFR/PI3K/Akt pathway. J Nutr Biochem. 25:1132–1139.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zheng L, Foley K, Huang L, Leubner A, Mo
G, Olino K, Edil BH, Mizuma M, Sharma R, Le DT, et al: Tyrosine 23
phosphorylation-dependent cell-surface localization of annexin A2
is required for invasion and metastases of pancreatic cancer. PLoS
One. 6:e193902011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lei C, Wang Y, Huang Y, Yu H, Huang Y, Wu
L and Huang L: Up-regulated miR155 reverses the
epithelial-mesenchymal transition induced by EGF and increases
chemo-sensitivity to cisplatin in human Caski cervical cancer
cells. PLoS One. 7:e523102012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Huber MA, Kraut N and Beug H: Molecular
requirements for epithelial-mesenchymal transition during tumor
progression. Curr Opin Cell Biol. 17:548–558. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mimeault M and Batra SK: Interplay of
distinct growth factors during epithelial mesenchymal transition of
cancer progenitor cells and molecular targeting as novel cancer
therapies. Ann Oncol. 18:1605–1619. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kersemaekers AM, Fleuren GJ, Kenter GG,
Van den Broek LJ, Uljee SM, Hermans J and Van de Vijver MJ:
Oncogene alterations in carcinomas of the uterine cervix:
Overexpression of the epidermal growth factor receptor is
associated with poor prognosis. Clin Cancer Res. 5:577–586.
1999.PubMed/NCBI
|
|
25
|
Noordhuis MG, Eijsink JJ, Ten Hoor KA,
Roossink F, Hollema H, Arts HJ, Pras E, Maduro JH, Reyners AK, de
Bock GH, et al: Expression of epidermal growth factor receptor
(EGFR) and activated EGFR predict poor response to (chemo)
radiation and survival in cervical cancer. Clin Cancer Res.
15:7389–7397. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Grewal T and Enrich C: Annexins-modulators
of EGF receptor signalling and trafficking. Cell Signal.
21:847–858. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
de Graauw M, Cao L, Winkel L, van
Miltenburg MH, le Dévedéc SE, Klop M, Yan K, Pont C, Rogkoti VM,
Tijsma A, et al: Annexin A2 depletion delays EGFR endocytic
trafficking via cofilin activation and enhances EGFR signaling and
metastasis formation. Oncogene. 33:2610–2619. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cha YH, Kim NH, Park C, Lee I, Kim HS and
Yook JI: MiRNA-34 intrinsically links p53 tumor suppressor and Wnt
signaling. Cell Cycle. 11:1273–1281. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhou S, Yi T, Liu R, Bian C, Qi X, He X,
Wang K, Li J, Zhao X, Huang C and Wei Y: Proteomics identification
of annexin A2 as a key mediator in the metastasis and
proangiogenesis of endometrial cells in human adenomyosis. Mol Cell
Proteomics. 11:M1122012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yim EK, Tong SY, Ho EM, Bae JH, Um SJ and
Park JS: Anticancer effects on TACC3 by treatment of paclitaxel in
HPV-18 positive cervical carcinoma cells. Oncol Rep. 21:549–557.
2009.PubMed/NCBI
|
|
31
|
Andey T, Marepally S, Patel A, Jackson T,
Sarkar S, O'Connell M, Reddy RC, Chellappan S, Singh P and Singh M:
Cationic lipid guided short-hairpin RNA interference of annexin A2
attenuates tumor growth and metastasis in a mouse lung cancer stem
cell model. J Control Release. 184:67–78. 2014. View Article : Google Scholar : PubMed/NCBI
|



















