Introduction
Nasopharyngeal carcinoma (NPC) is a distinct type of
cancer of the head and neck which is highly prevalent in Southern
China (1). Radiotherapy (RT) and
concurrent chemo-radiotherapy are the primary treatments for NPC in
the early and advanced stages, respectively (2). Although early-stage NPC is highly
radiocurable, metastasis to regional lymph nodes or distant organs
and local recurrence remain major issues for the treatment failure
of advanced-stage NPC. The molecular mechanisms underlying NPC
development and progression have are not yet completely
understood.
p300, a transcriptional co-activator of various
transcription factors, has been found to serve a central role in
the regulation of gene transcription through its histone
acetyltransferase activity. It has been shown to participate in
different cellular processes such as DNA damage repair, cell
growth, differentiation, apoptosis and migration (3). In a previous study by the authors of the
present study (4), it was identified
that p300 expression levels were higher in NPC tissues compared
with adjacent non-cancerous tissues, and p300 has been shown to be
overexpressed in a number of malignancies, such as breast cancer
(5), colorectal carcinomas (6), esophageal squamous cell carcinoma
(7) and hepatocellular carcinoma
(8). In addition, a previous study
confirmed that elevated p300 activity is correlated with poor
prognosis in NPC (4). These findings
suggest that p300 could be considered a rational therapeutic
strategy for NPC. Therefore, more extensive studies are required to
elucidate the molecular status of the p300 gene and its
potential oncogenic role in NPC.
Epithelial-mesenchymal transition (EMT) has been
demonstrated to be a fundamental process that could serve a vital
role in tumor progression (9). It is
characterized by cells losing their epithelial morphology and
acquiring mesenchymal markers. Emerging evidence has suggested that
EMT is involved in the metastatic behavior of NPC (10,11). In
addition, a previous study demonstrated that p300 may promote
cancer progression by inducing EMT in hepatocellular carcinoma
(12). However, whether or not p300
may promote cancer progression by inducing EMT in NPC has not been
reported.
In the present study, lentivirus-mediated small
interfering RNA (siRNA) techniques were applied to produce specific
and stable silencing of p300 in CNE-2 cells. In addition,
the study investigated the role of p300 in NPC metastasis and EMT
process.
Materials and methods
Cell culture
Human nasopharyngeal carcinoma cell lines CNE-1,
CNE-2, SUNE-5-8F, SUNE-6-10B and control cell line NP69 were grown
in Roswell Park Memorial Institute (RPMI)-1640 (Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA), supplemented with 10%
fetal bovine serum (FBS) (Gibco; Thermo Fisher Scientific, Inc.).
All cell lines were cultured in a 5% CO2 incubator at
37°C. When the cells reached the logarithmic growth phase,
succeeding experiments were performed.
Construction of lentivirus
vectors
To investigate the function of p300, the
vector LV-008 (Forevergen Biosciences, Guangzhou, China) with a U6
promoter was used to generate small hairpin (sh)RNA. The
p300 shRNA sequence is as follows: Sense,
5′-AACTGCACAAATGTCTAGTTCTTTTCAAGAGAAAGAACTAGACATTTGTGCTTTTTTC-3′
and antisense,
3′-TTGACTCTTCTCCCTCAGATATAAAGTTCTCTTTCTTGATCTGTAAACACGAAAAAAGAGCT-5′,
Negative Control (NC) were used as the control group: Sense,
5′-AACTTTCTCCGAACGTGTCACGTTTCAAGAGAACGTGACACGTTCGGAGAATTTTTTC-3′
and antisense,
3′-TTGAAAGAGGCTTGCACAGTGCAAAGTTCTCTTGCACTGTGCAAGCCTCTTAAAAAAGAGCT-5′.
Lentiviral vector DNA and package vectors were then transfected
into HEK-293T cells using Lipofectamine 2000 (Gibco; Thermo Fisher
Scientific, Inc.). Lentivirus supernatants were harvested at 48 and
72 h post-transfection. Lenti-sip300 (shp300) and src-siRNA (NC)
were then used to infect CNE-2 cells with 5–10 µg/ml polybrene.
After 48 h of infection, the cells were cultured in the medium with
2 µg/ml puromycin for 10–15 days to generate stable cell lines.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
determination
Total RNA was extracted from cultured cells and
fresh tissue with TRIzol (Invitrogen; Thermo Fisher Scientific,
Inc.) according to the manufacturer's instructions. RNA was reverse
transcribed by M-MLV Reverse Transcriptase (Promega Corporation,
Madison, WI, USA) according to the manufacturer's protocol. Primers
for P300 were as follows: Forward, 5′-CGTTGCCCTATCTCCGTCTC-3′ and
reverse, 5′-GGGAGCAATCGGGTAATTTTCC-3′. GAPDH was used as internal
control. GAPDH primers were as follows: Forward,
5′-GAGTCAACGGATTTGGTCGT-3′ and reverse, 5′-GACAAGCTTCCCGTTCTCAG-3′.
qPCR was performed on a MiniOpticon™ Real-Time PCR detection
instrument (Bio-Rad Laboratories, Inc., Hercules, CA, USA)
according to the manufacturer's instructions. All reactions were
performed in triplicate and the experiments were conducted three
times. Relative expression quantifications were performed using the
2−∆∆Cq method (13).
Western blot
Cells from all experimental groups were collected
using a cell scraper and proteins were extracted by cell lysis
buffer. Total protein was extracted from human NPC lines and liver
tissues using radioimmunoprecipitation assay buffer (Sigma-Aldrich;
Merck Millipore, Darmsdadt, Germany). Protein concentration was
assessed with a Bradford assay. Equal quantities of cell and tissue
lysates (30 µg protein) were separated by 10% SDS-PAGE, transferred
onto a polyvinylidene difluoride membrane (EMD Millipore,
Billerica, MA, USA) and blocked with 5% skimmed milk powder for 1 h
at room temperature. Membranes were then incubated with p300
antibody (catalog no. sc-584; 1:250 dilution; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) against target proteins at
4°C, followed by washing with TBS and Tween-20 (TBST) overnight.
Membranes were then incubated with the horseradish
peroxidase-labeled secondary antibody (1:1,000 dilution; YN-16;
Forevergen Biosciences) for 1 h at 37°C, and then washed with TBST.
The immunoreactive signals were detected with enhanced
chemiluminescence (Forevergen Biosciences). GAPDH was used as a
loading control.
In vitro cell invasion assay
Invasion assays were performed using transwell
chambers (BD Biosciences, Franklin Lakes, NJ, USA). Plates were
coated with Matrigel prior to cell seeding, cells were suspended in
RPMI-1640 supplemented with 0.1% FBS and added to the upper
chambers, and Dulbecco's modified Eagle's medium (Gibco; Thermo
Fisher Scientific, Inc.) with 10% FBS was added to the bottom
chambers. After overnight incubation, non-migratory cells on the
upper surface were removed. The cells that had passed through the
membrane were analyzed in 6 randomly selected microscopic fields.
All experiments were performed in triplicate.
Wound healing assay
Cells were seeded in six-well plates and incubated
for 24 h prior to the assay. For the wound healing assay,
monolayers were wounded by scraping with the tip of a sterile 200
µl pipette tip. Cell migration to the wounded surface was then
monitored by microscopy after 6, 24 and 48 h. Experiments were
performed a minimum of three times.
Immunofluorescence
Cells were plated at a density of 2×105
cells on cover glasses (Thermo Fisher Scientific, Inc.) for 24 h,
fixed using freshly prepared 4% paraformaldehyde and permeabilized
using 0.1% Triton X-100 in TBS. The expression of E-cadherin,
α-catenin, vimentin and N-cadherin were detected by incubation at
4°C with primary antibodies against E-cadherin (catalog no.
sc-7870; 1:1,000 dilution; Santa Cruz Biotechnology, Inc.),
α-catenin (catalog no. BD#610193; 1:1,000 dilution; BD Transduction
Laboratories; BD Biosciences), vimentin (catalog no. sc-6260;
1:1,000 dilution; Santa Cruz Biotechnology, Inc.) and N-cadherin
(catalog no. sc-53488; 1:1,000 dilution; Santa Cruz Biotechnology,
Inc.), and then incubated with Alexa Flour 488 Donkey anti-Rabbit
IgG(H+L) secondary antibody (catalog no. CA21206s; 1:500 dilution;
Invitrogen; Thermo Fisher Scientific, Inc.) at room temperature for
30 min. The coverslips were counterstained with DAPI and imaged
with an Axio Imager Z1 Microscope System (Carl Zeiss AG,
Oberkochen, Germany).
Immunoprecipitation (IP) assays
For each trial, cell extracts composed of
6.0×106 cells were prepared by solubilization in 400 µl
cell lysis buffer [20 mM Tris-HCl (pH 7.4), 150 mM NaCl, 1 mM EDTA,
1 mM EGTA, 1% Triton X-100, proteinase inhibitor cocktail] for 10
min at 4°C. After brief sonication, the lysates were cleared by
centrifugation at 15,000 × g for 15 min at 4°C, and the cell
extract was immunoprecipitated with 2 µg smad2/3 (Cell Signaling
Technology, Inc., Danvers, MA, USA) and incubated with 30 µl
protein A plus Gagarosehydrazide beads (Calbiochem; Merck
Millipore). overnight at 4°C. After washing three times with
agarose bead washing buffer, immunoprecipitated protein-antibody
complexes were subjected to western blotting, as aforementioned,
and then detected with Acetylated-Lysine antibody (catalog no.
9441; 1:1,000 dilution; Cell Signaling Technology, Inc.) and
Smad2/3 antibody (catalog no. 5678; 1:1,000 dilution; Cell
Signaling Technology, Inc.).
Statistical analysis
All the results are expressed as the mean ± standard
deviation and analyzed using a Student's t-test between two groups.
P<0.05 was considered to indicate a statistically significant
difference. Statistical analyses were performed using SPSS version
13.0 statistical software (SPSS, Inc., Chicago, IL, USA).
Results
Elevated expression of p300 in NPC
cell lines
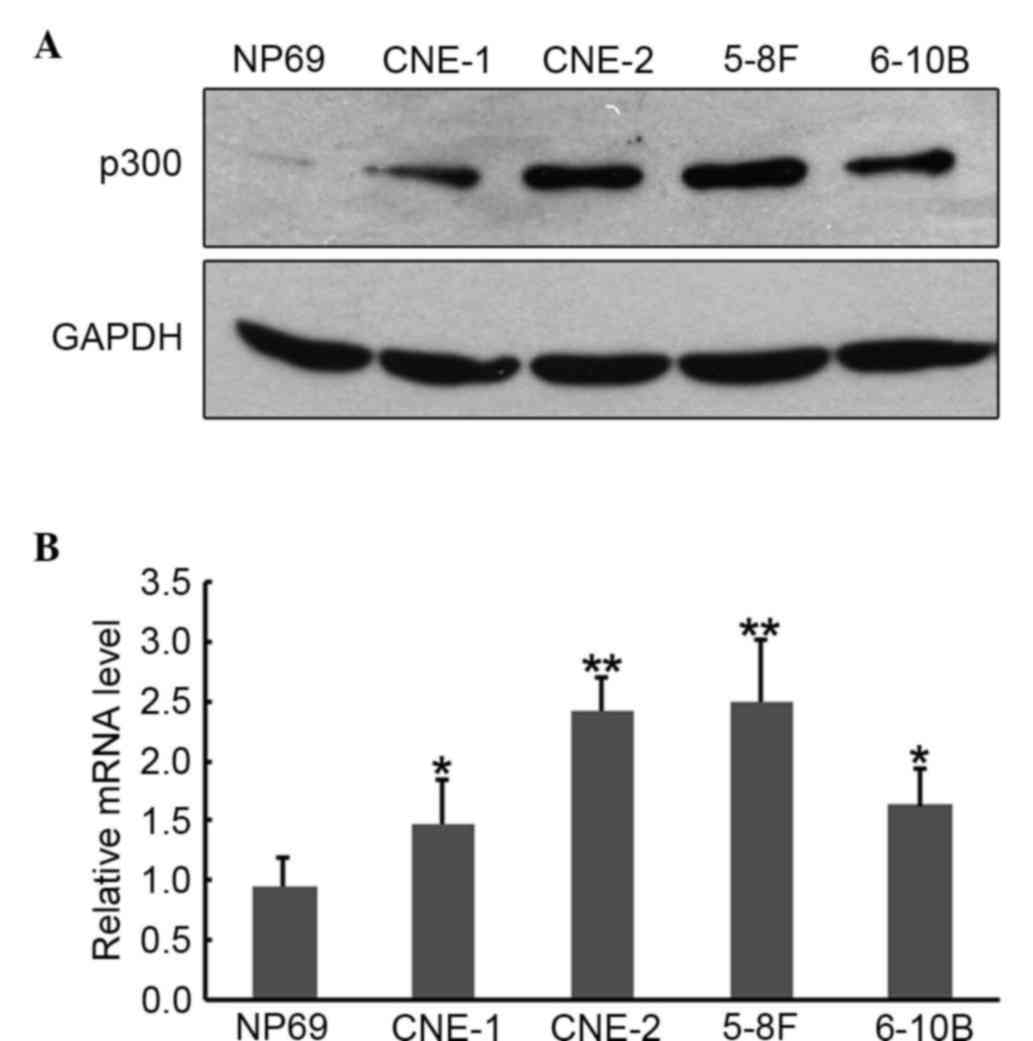
To examine the effects of p300 in functional
studies, it is important to select a cell culture system that
expresses the appropriate level of endogenous p300. p300 mRNA and
protein expression in various human NPC cell lines were confirmed
by RT-qPCR and western blot analysis. Western blotting (Fig. 1A) and RT-qPCR (Fig. 1B) results showed enhanced expression
of p300 protein and mRNA in all the four NPC cell lines as compared
with the immortalized non-tumorigenic cell line NP69. Based on this
expression pattern, CNE-2 cells were chosen for the following
loss-of-function studies.
Suppression of p300 mRNA and protein
expression of cells by lentivirus transduction
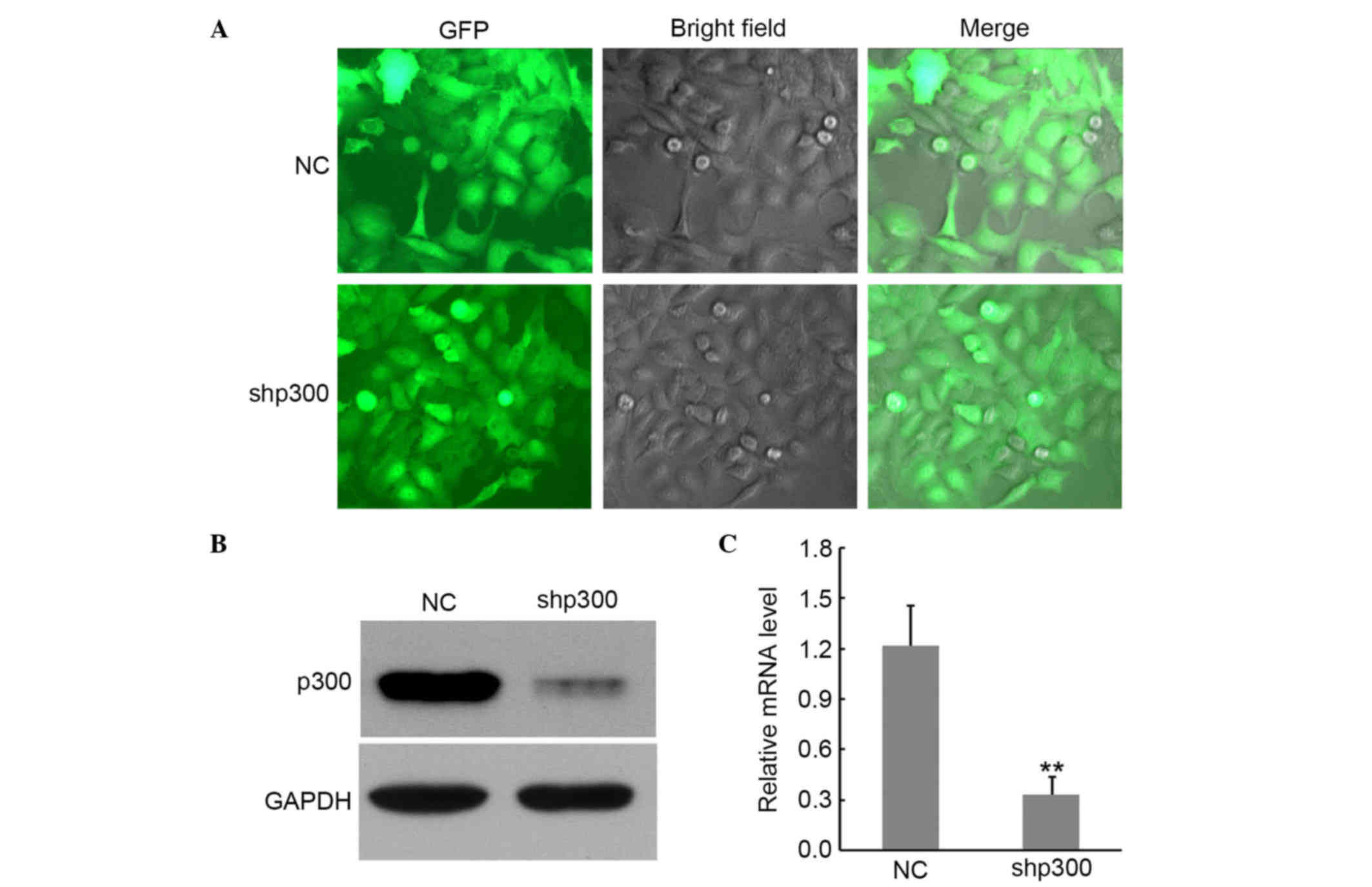
To further explore the particular function of p300
in NPC progression, p300 expression was silenced using RNA
interference. A lentivirus-delivered vector shp300 and NC was
constructed (Fig. 2A). RT-qPCR and
western blot were used to confirm the inhibition of p300. As shown
in Fig. 2B and C, compared with
untransfected cells, treatment with shp300 resulted in a
significant reduction of p300 in CNE-2 cells at both protein and
mRNA levels (P<0.01), while the p300 protein and mRNA expression
was almost unaffected in the NC group. These data suggested that
shp300 displays efficient mRNA degradation ability. Thus, shp300
cells were chosen for functional assays.
Suppression of p300 reduced the
migration and invasion ability of cells
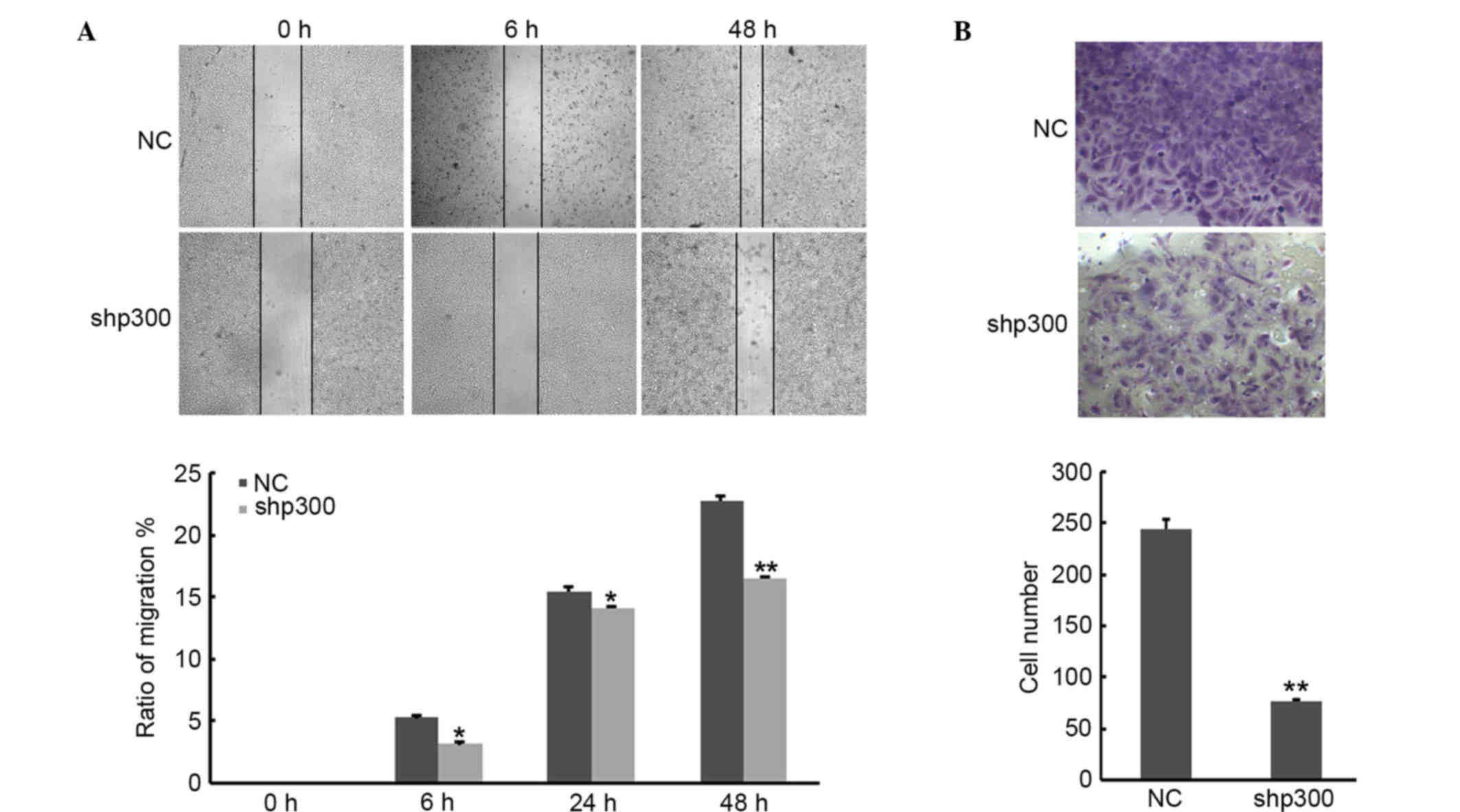
In order to investigate the potential influence of
p300 on cancer cell migration and invasion ability of NPC cells,
wound healing and invasion assays were performed. As shown in
Fig. 3A, data from the wound healing
assay revealed that depletion of p300 in cancer cells strongly
reduced cell migration. Similarly, the number of the cells that
migrated through the Matrigel significantly decreased in shp300
compared with NC group (P<0.01; Fig.
3B). Taken together, these data indicated that knockdown of
p300 decreased the migration and invasion abilities of CNE-2
cells.
p300 promoted EMT in cells
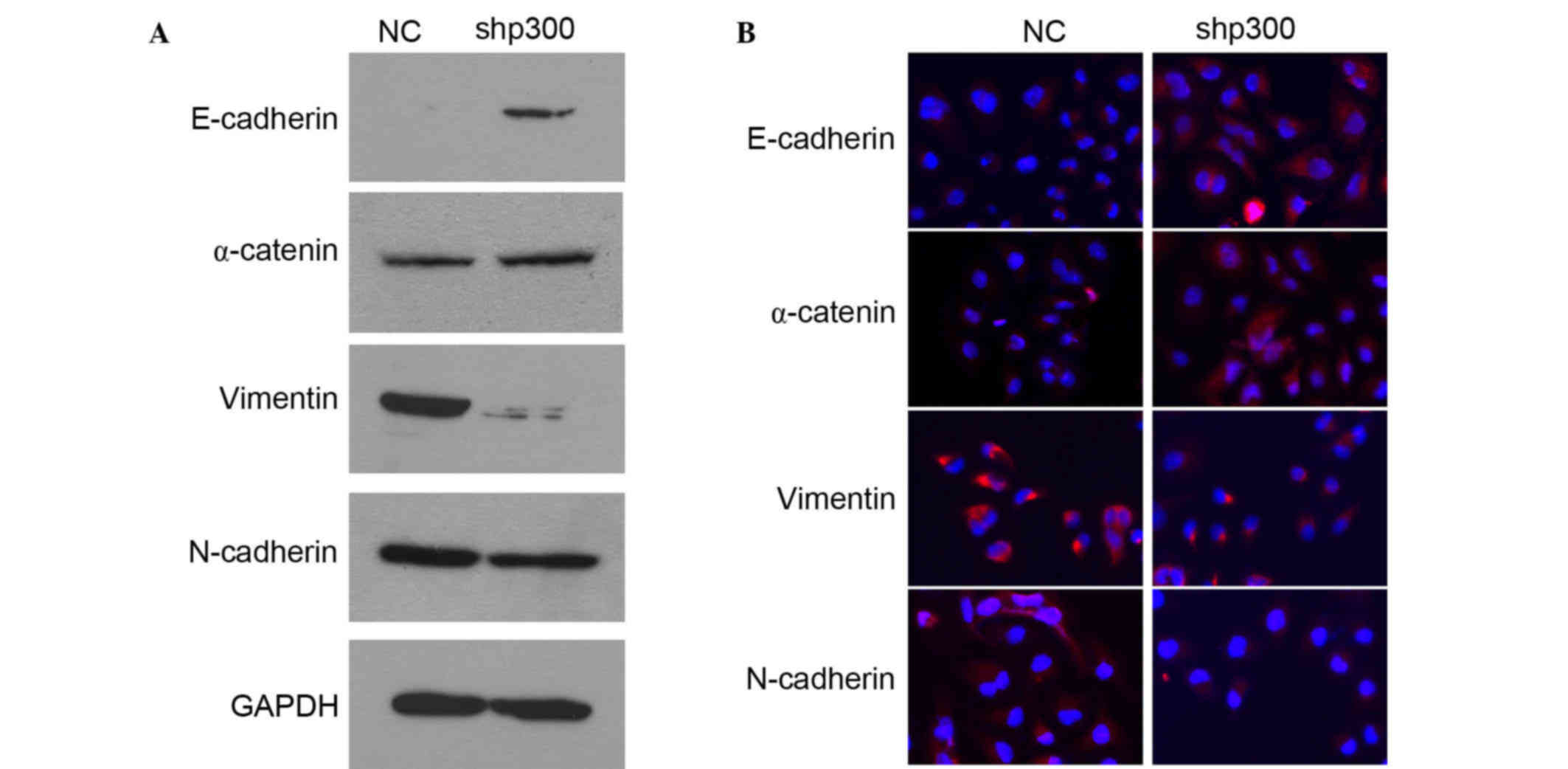
EMT is known to be important for epithelial cancer
metastasis (9). In order to study the
association between p300 and the EMT process, the protein levels of
several epithelial markers (E-cadherin, α-catenin) and mesenchymal
markers (vimentin, N-cadherin) were detected by western blot
analysis. As shown in Fig. 4A,
following knockdown of p300 in CNE-2 cells, the protein
levels of E-cadherin and α-catenin increased, whereas vimentin and
N-cadherin decreased. The EMT phenotype was confirmed by
immunofluoresence. As revealed in Fig.
4B, the epithelial markers E-cadherin and α-catenin
dramatically increased and the mesenchymal markers vimentin and
N-cadherin decreased in shp300 cells compared with the staining
observed in the control cells. All of these results suggest that
p300 is actively involved in maintaining EMT in NPC cells, and that
knockdown of p300 expression markedly increases the
expression levels of epithelial markers (E-cadherin, α-catenin) and
reduce the expression levels of mesenchymal markers (vimentin,
N-cadherin).
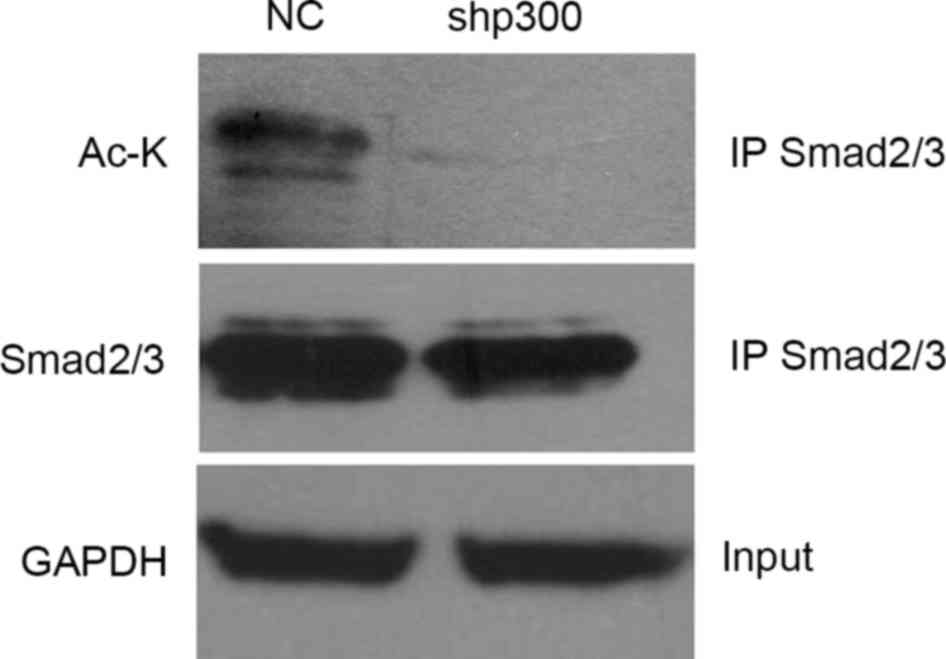
P300 promoted EMT through acetylation
of Smad2 and Smad3 in the TGF-β signaling pathway
To provide further insights into the signaling
mechanisms involved in p300-mediated EMT process, the protein
expression levels involved in the TGF-β signaling pathway were
measured and it was found that total smad2/3 expression levels
remained unchanged when p300 was decreased. An earlier study
suggested that both Smad2 and Smad3 directly interact with p300 in
the nucleus (14), therefore the
present study determined if p300 serves a role in the acetylation
of Smad2 and Smad3. These proteins were immunoprecipitated with
anti-Smad2 and anti-Smad3 antibodies from NC and shp300 cell
lysates, and their acetylation status were determined by
anti-acetylated-lysine antibodies. It was observed that knockdown
of the p300 gene decreased the acetylation of Smad2 and
Smad3 (Fig. 5).
Discussion
NPC is one of the most common cancers in southern
China and Southeast Asia (15,16).
Radiotherapy and chemotherapy are the most common treatments of
NPC. The 5-year survival rate is excellent for localized stages,
but there is no curative treatment for metastatic diseases. Thus it
is of paramount interest to identify the molecular mechanisms
affecting NPC invasion and metastasis, making it possible to tailor
individual treatments.
p300 has been shown to serve a variety of roles in a
series of biological processes, such as in cell proliferation,
senescence and apoptosis. It functions primarily as a mammalian
histone acetyltransferase that acetylates various nuclear proteins.
Previously, several studies have demonstrated that p300 was
evaluated as a prognostic factor in laryngeal squamous cell
carcinoma (17), breast cancer
(5), non-small cell lung cancers
(18), small cell lung cancer
(19) and esophageal cancers
(7).
A previous study demonstrated that the majority of
NPCs had a higher expression of p300 compared with in non-cancerous
nasopharyngeal mucosal tissues (4).
The present study reports, for the first time, that elevated p300
expression in NPC indicates a poor prognosis. In the current study,
the expression of p300 at the mRNA and protein levels in NPC cell
lines was confirmed by RT-qPCR and western blot analysis,
respectively. Consistent with our previous study (4), elevated expression of p300 was observed
in invasive NPC cells. These findings suggest that overexpression
of p300 may serve a key role in NPC progression. Therefore, the
functions of p300 need to be investigated further.
RNA interference-mediated gene silencing is widely
used as a powerful tool for the functional analysis of genes.
However, the use of RNA interference has been limited by the
transient duration of the silencing effect which cannot be passed
to cell progeny. To overcome these limitations, a lentiviral vector
mediating RNA interference was designed and constructed to obtain a
stable p300 knockdown effect. The results demonstrated that
lentivirus-delivered RNA interference could effectively reduce p300
expression.
Both cell migration and invasion are major features
that contribute to cancerous progression. A recent study provided
evidence that p300 is important for tumor cell growth and migration
(20). In the present study, it was
observed that knockdown of p300 significantly inhibited the
migration and invasiveness of CNE-2 cells. These findings are in
accordance with a previous report which suggested that inhibition
of p300 reduced migration and invasion in cultured LM3 cells
(21). However, the exact biological
mechanisms underlying p300-mediated migration and invasion
inhibition during NPC development are unknown.
EMT is considered to be a critical early event
involved in the invasion and metastasis of NPC. During EMT,
epithelial cells lose their characteristic cell-cell adhesion
structures and cell polarity, reduce levels of the epithelial cell
surface marker E-cadherin and increase levels of the mesenchymal
markers N-cadherin and vimentin. However, the role of p300 in this
process remains unclear. It has been reported that the knockdown of
p300 expression in hepatoma cells recovered E-cadherin expression,
inhibited the translocation of β-catenin into the nuclei and
contributes towards the promotion of cell proliferation during
tumor pathogenesis (12). Peña et
al (22) show that p300 levels
are important factors in the control of EMT-related molecules, such
as ZEB1 and SNAIL in human colon cancer. In the present study, the
expression levels of epithelial markers (E-cadherin, α-catenin)
were increased when p300 was downregulated, whereas expression of
mesenchymal markers (vimentin, N-cadherin) were decreased.
Previously, several studies have suggested that
TGF-β signaling serves a role in the control of the invasiveness of
NPC cells (23,24). In addition, TGF-β signaling is known
to serve a central role in the regulation of EMT (25). The direct downstream target of
activated TGF-β is the Smad family. Ko et al (14) identified that the acetylation of Smad2
and Smad3 by p300/CBP are essential for the regulation of
TGF-β-induced transcriptional activation of EMT markers in human
lung cancer cells. Furthermore, it was reported that acetylation of
Smad2/3 by p300/CBP is essential for the activation of the
transcription factor promoter complex in the TGF-β signaling
pathway (26). The data from the
present study demonstrated that p300 knockdown resulted in
downregulation of the acetylation of smad2/3. However, a change in
Smad2/3 expression was not observed. Taken together, the present
study shows that p300 serves an important role in regulating NPC
development and metastasis by inducing EMT via acetylation of Smad2
and Smad3 in the TGF-β signaling pathway. Therefore, p300 is
considered a novel target for the prevention of EMT. These finding
highlight the possibilities that p300 could be a potential target
for the therapeutic intervention of NPC.
Acknowledgements
The present study was supported by the Bureau of
Health of Guangzhou Municipality (grant no. 20141A011092) and the
Guangzhou Medical University Students Science and Technology
Innovation Project (grant no. 2013A002). The authors thank Dr
Jingyan Luo and Dr Bingquan Lai for their technical assistance.
References
|
1
|
Yu MC and Yuan JM: Epidemiology of
nasopharyngeal carcinoma. Semin Cancer Biol. 12:421–429. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang L, Zhao C, Ghimire B, Hong MH, Liu
Q, Zhang Y, Guo Y, Huang YJ and Guan ZZ: The role of concurrent
chemoradiotherapy in the treatment of locoregionally advanced
nasopharyngeal carcinoma among endemic population: A meta-analysis
of the phase III randomized trials. BMC Cancer. 10:5582010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Goodman RH and Smolik S: CBP/p300 in cell
growth, transformation, and development. Genes Dev. 14:1553–1577.
2000.PubMed/NCBI
|
|
4
|
Liao ZW, Zhou TC, Tan XJ, Song XL, Liu Y,
Shi XY, Huang WJ, Du LL, Tu BJ and Lin XD: High expression of p300
is linked to aggressive features and poor prognosis of
nasopharyngeal carcinoma. J Transl Med. 10:1102012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xiao XS, Cai MY, Chen JW, Guan XY, Kung
HF, Zeng YX and Xie D: High Expression of p300 in human breast
cancer correlates with tumor recurrence and predicts adverse
prognosis. Chin J Cancer Res. 23:201–207. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ishihama K, Yamakawa M, Semba S, Takeda H,
Kawata S, Kimura S and Kimura W: Expression of HDAC1 and CBP/p300
in human colorectal carcinomas. J Clin Pathol. 60:1205–1210. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li Y, Yang HX, Luo RZ, Zhang Y, Li M, Wang
X and Jia WH: High expression of p300 has an unfavorable impact on
survival in resectable esophageal squamous cell carcinoma. Ann
Thorac Surg. 91:1531–1538. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li M, Luo R, Chen JM, Cao Y, Lu JB, He JH,
Wu QL and Cai MY: High expression of transcriptional coactivator
p300 correlates with aggressive features and poor prognosis of
hepatocellular carcinoma. J Transl Med. 9:52011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Luo WR, Chen XY, Li SY, Wu AB and Yao KT:
Neoplastic spindle cells in nasopharyngeal carcinoma show features
of epithelial-mesenchymal transition. Histopathology. 61:113–122.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Song LB, Li J, Liao WT, Feng Y, Yu CP, Hu
LJ, Kong QL, Xu LH, Zhang X, Liu WL, et al: The polycomb group
protein Bmi-1 represses the tumor suppressor PTEN and induces
epithelial-mesenchymal transition in human nasopharyngeal
epithelial cells. J Clin Invest. 119:3626–3636. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yokomizo C, Yamaguchi K, Itoh Y, Nishimura
T, Umemura A, Minami M, Yasui K, Mitsuyoshi H, Fujii H, Tochiki N,
et al: High expression of p300 in HCC predicts shortened overall
survival in association with enhanced epithelial mesenchymal
transition of HCC cells. Cancer Lett. 310:140–147. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2−ΔΔCt method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ko H, So Y, Jeon H, Jeong MH, Choi HK, Ryu
SH, Lee SW, Yoon HG and Choi KC: TGF-β1-induced
epithelial-mesenchymal transition and acetylation of Smad2 and
Smad3 are negatively regulated by EGCG in human A549 lung cancer
cells. Cancer Lett. 335:205–213. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wei WI and Sham JS: Nasopharyngeal
carcinoma. Lancet. 365:2041–2054. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chang ET and Adami HO: The enigmatic
epidemiology of nasopharyngeal carcinoma. Cancer Epidemiol
Biomarkers Prev. 15:1765–1777. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen YF, Luo RZ, Li Y, Cui BK, Song M,
Yang AK and Chen WK: High expression levels of COX-2 and P300 are
associated with unfavorable survival in laryngeal squamous cell
carcinoma. Eur Arch Otorhinolaryngol. 270:1009–1017. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hou X, Li Y, Luo RZ, Fu JH, He JH, Zhang
LJ and Yang HX: High expression of the transcriptional co-activator
p300 predicts poor survival in resectable non-small cell lung
cancers. Eur J Surg Oncol. 38:523–530. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gao Y, Geng J, Hong X, Qi J, Teng Y, Yang
Y, Qu D and Chen G: Expression of p300 and CBP is associated with
poor prognosis in small cell lung cancer. Int J Clin Exp Pathol.
7:760–767. 2014.PubMed/NCBI
|
|
20
|
Zhou J, Zhan S, Tan W, Cheng R, Gong H and
Zhu Q: P300 binds to and acetylates MTA2 to promote colorectal
cancer cells growth. Biochem Biophys Res Commun. 444:387–390. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fermento ME, Gandini NA, Salomón DG,
Ferronato MJ, Vitale CA, Arévalo J, López Romero A, Nuñez M, Jung
M, Facchinetti MM and Curino AC: Inhibition of p300 suppresses
growth of breast cancer. Role of p300 subcellular localization. Exp
Mol Pathol. 97:411–424. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Peña C, García JM, García V, Silva J,
Domínguez G, Rodríguez R, Maximiano C, de Herreros A García, Muñoz
A and Bonilla F: The expression levels of the transcriptional
regulators p300 and CtBP modulate the correlations between SNAIL,
ZEB1, E-cadherin and vitamin D receptor in human colon carcinomas.
Int J Cancer. 119:2098–2104. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhao L, Lin L, Pan C, Shi M, Liao Y, Bin J
and Liao W: Flotillin-2 promotes nasopharyngeal carcinoma
metastasis and is necessary for the epithelial-mesenchymal
transition induced by transforming growth factor-β. Oncotarget.
6:9781–9793. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xiao J, Xiang Q, Xiao YC, Su ZJ, Huang ZF,
Zhang QH, Tan Y, Li XK and Huang YD: The effect of transforming
growth factor-beta1 on nasopharyngeal carcinoma cells: Insensitive
to cell growth but functional to TGF-beta/Smad pathway. J Exp Clin
Cancer Res. 29:352010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Morrison CD, Parvani JG and Schiemann WP:
The relevance of the TGF-β paradox to EMT-MET programs. Cancer
Lett. 341:30–40. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Inoue Y, Itoh Y, Abe K, Okamoto T, Daitoku
H, Fukamizu A, Onozaki K and Hayashi H: Smad3 is acetylated by
p300/CBP to regulate its transactivation activity. Oncogene.
26:500–508. 2007. View Article : Google Scholar : PubMed/NCBI
|