Introduction
High-grade gliomas (HGGs), such as glioblastoma
multiforme (GBM), are highly aggressive primary brain tumors and
locally invasive into surrounding normal brain tissues (1). For the multimodal treatment of GBM,
cytoreductive surgery is essential (4). The extent of tumor resection [starting
from 78% and increasing to nearly 100% of gadolinium-based
contrast-enhanced tumor volume on magnetic resonance imaging (MRI)]
improves the overall survival of patients with GBM (5). Subsequently, post-operative adjuvant
radiotherapy is required for managing invaded residual tumors in
HGGs (6). However, despite the
availability of multimodal treatment like surgery and
radiochemotherapy, the mean survival of patients with GBM remains
at only 12–14 months (2,3), and the recurrence rate of GBM is nearly
100% (1). Therefore, novel treatment
concepts for HGGs are necessary. Conventional MRI, including
contrast-enhanced T1-weighted imaging (CE-T1WI), is the gold
standard to evaluate tumor extension, post-operative residual
tumors and treatment responses after chemo-radiation for HGGs
(7). Contrast enhancement is able to
identify vital tumor tissue due to gadolinium-based contrast agent
(GBCA) leakage. This is a result of the abnormal permeability of
the blood-brain barrier (BBB) caused by an abundance of
dysfunctional tumor vessels in HGGs (8,9). However,
a highly infiltrative HGG tumor burden is not consistently
associated with abnormal BBB permeability (10). Previous pathological studies have
revealed that glioma cells can infiltrate the brain parenchyma far
beyond the contrast-enhanced lesions (9,11). Thus,
conventional radiological investigations using GBCA may
significantly underestimate the extent of HGG tumor growth,
particularly in cases of highly infiltrative HGGs (9). In addition, GBCA administration in
patients with renal dysfunction is limited in order to avoid the
development of nephrogenic systemic fibrosis (12). Novel candidate MRI contrast reagents
have not yet been identified as alternatives to GBCA.
5-aminolevulinic acid (5-ALA) is a natural
biochemical precursor of heme (13).
In cancer cells, 5-ALA is metabolized into protoporphyrin IX
(PpIX), which functions as a photosensitizer, following systemic
administration (14–16). Specifically, 5-ALA induces a high
accumulation of PpIX in glioma cells (17). Therefore, fluorescence-guided
resection using 5-ALA in HGG treatment has been useful in
determining tumor borders, making tumor resection easier compared
with conventional microsurgery (18).
5-ALA is water soluble, while PpIX is water insoluble (19). It has been speculated that converting
5-ALA to PpIX within glioma cells may increase the intracellular
free water content, and consequently induce an enhancement of the
T2 signal intensity. Therefore, in the present study, a prospective
assessment of the effect of 5-ALA-induced PpIX on MRI T2 signal
intensity in patients with HGGs was performed. In addition, the
potency of 5-ALA-induced PpIX as an enhancer of MRI T2 signal
intensity for HGGs is discussed.
Materials and methods
Study design
A prospective case study design was used, and
included eligible patients from September 2014 to February 2015 who
gave written informed consent. All patients underwent surgical
treatment at the Department of Neurosurgery, The Affiliated
Hospital of the University of Occupational and Environmental Health
(Kitakyushu, Japan). The study was approved by the institutional
review board of the University of Occupational and Environmental
Health (approval no. H26-075). The primary inclusion criteria were
cases of suspected HGG as determined by preoperative MRI, with
planned surgical resection, no contraindications for 5-ALA, and
informed patient consent. The exclusion criteria included recurrent
HGG, patient age of <20 years old and treatment history for
other brain tumors.
Study protocol
All MRIs were performed using a 3.0-T unit (Signa
Excite; GE Medical Systems, Milwaukee, Wisconsin) with a dedicated
8-channel phased-array coil (GE Medical Systems). All patients
underwent brain MRI scans both prior to and following 5-ALA
administration.
Patients underwent the institution's standard brain
MRI protocol for HGG, including an axial T2WI, a T1WI and a
CE-T1WI. The following imaging parameters were used for axial T2WI:
a TR/TE of 4,000/85; a flip angle of 90°; a bandwidth of 62.5 kHz;
a section thickness of 3 mm; a matrix of 512×224; a field of view
(FOV) of 18×18 cm; and an imaging time of 2 min 16 sec. For
CE-T1WI, the following imaging parameters were used following
administration of the contrast material. CE-T1WI (spin-echo) was
acquired with a section thickness of 3 mm; a FOV of 18 cm; a matrix
of 224×224; an imaging time of 2 min 40 sec; and 2 acquired
excitations. A three-dimensional fast spoiled gradient-echo (3D
fast SPGR) was acquired with the parameters of 10/4.1/700/7 min 20
sec [repetition time (ms)/echo time (ms)/inversion time/imaging
time], a flip angle of 10°, a 24 cm FOV, a 512×256 matrix, and 1.4
mm thick sections with a 2.5×2.5×8 mm resolution. The 3D fast SPGR
data were reconstructed in the sagittal and coronal planes. For all
patients, gadodiamide hydrate (Omniscan; Daiichi Pharmaceutical,
Tokyo, Japan) or gadopentetate dimeglumine (Magnevist; Bayer
Schering-Pharma, Berlin, Germany) was administered at a dose of 0.1
mmol/kg via intravenous bolus injection. Conventional MRI scans
were obtained from all patients at a maximum of 8 days prior to
surgery.
5-ALA was prepared by dissolving the compound in 50
ml of water (20 mg/kg), which was immediately administered orally
to each patient 3 h prior to anesthesia as previously described
(18). T2WIs were obtained ~2.5 h
post-5-ALA administration. Subsequently, patients immediately
underwent surgery, performed by two skilled neurosurgeons
(Department of Neurosurgery, University of Occupational and
Environmental Health). Patient registration in the neuronavigation
system was performed using automatic registration tools (BrainLab
AG, Feldkirchen, Germany) based on pre-operative CE-T1WIs. To avoid
photobleaching of 5-ALA-induced PpIX due to exposure to the
microscope's light, surgeons confirmed that the brain tumors
corresponded to CE lesions on the neuronavigation system, and
immediately evaluated the fluorescence of the 5-ALA-induced PpIX
within the tumors. The 5-ALA fluorescence was graded as ‘none’,
‘vague’, or ‘strong’ as previously described (20). ‘Strong’ fluorescence was characterized
as vivid red, while ‘vague’ fluorescence was defined as less vivid
pink. Intraoperatively, each patient was evaluated for their
fluorescence status (none, vague or strong) by two independent
observers. Fluorescent tumor specimens were obtained safely and
immediately snap-frozen in liquid nitrogen and stored at −80°C for
HPLC analysis. Simultaneously, tumor specimens were also obtained
for histopathology. No corticosteroids were administered to any
patients prior to obtaining tumor specimens.
HPLC analyses for 5-ALA-induced PpIX
in tumor specimens
Accumulation of 5-ALA-induced PpIX in HGGs was
confirmed using HPLC analyses. Using a previously described HPLC
analysis method with porphyrin metabolites (21), tumor specimens (1 mm in diameter) were
treated with 200 µl of 0.1 M NaOH and homogenized on ice with a
PowerMaster II (Array Solutions, Sunnyvale, TX, USA). Tumor
specimens consisted of at least 2 samples from each patient.
Aliquots (10 µl) of NaOH-treated samples were transferred to a
protein concentration assay (Quick Start™ Bradford Dye Reagent,
Bio-Rad Laboratories, Inc., Hercules, CA, USA), while the remaining
50 µl was denatured by the addition of N,N-dimethylformamide:
Isopropanol (100:1, v/v) solution added at 3X the sample volume
(150 µl). Following overnight storage in the dark, the prepared
samples were subjected to HPLC analysis as previously described
(13,22) with the following modifications.
Briefly, the porphyrins were separated using the Prominence HPLC
system (Shimadzu, Kyoto, Japan), which was equipped with a
reversed-phase C18 column, SG300, 5 µm, 4.6×250 mm; (CAPCELL PAK,
Shiseido, Tokyo, Japan) and maintained at 40°C. The elution
solvents were solvent A (1 M ammonium acetate, including 12.5%
acetonitrile, pH 5.2) and solvent B (50 mM ammonium acetate,
including 80% acetonitrile, pH 5.2). The elution was first
performed with solvent A for 5 min and subsequently with a linear
gradient of solvent B (0–100%) for 25 min, followed by an elution
with solvent B for 10 min. The elution flow was maintained at a
constant rate by using a fluorospectrometer (excitation at 404 nm,
detection at 624 nm). The porphyrin concentrations in the samples
were estimated using calibration curves obtained with standard
porphyrins.
Image analysis
It was hypothesized that 5-ALA-induced PpIX would
induce changes in the T2 signal intensity in HGGs as 5-ALA is water
soluble, while PpIX is water insoluble (19). Therefore, an experienced
neuroradiologist was blinded to the clinical information (the
intraoperative findings of the 5-ALA-induced PpIX fluorescence and
pathological diagnosis). The neuroradiologist reviewed the T2WI
pre- and post-5-ALA administration in each patient. According to
the CE lesion on the CE-T1WI (which was defined as a tumor)
obtained prior to 5-ALA administration, an axial slice with a
maximum amount of tumor was defined as the standard slice. Three
slices, which included the standard slice and adjacent slices above
and below the standard slice, were used for the axial T2WI
evaluations. Similar to previous studies (23–25), the
regions of interest (ROI) for the signal intensity analyses were
drawn directly on the T2WI. Briefly, all images, including the T2WI
and the CE-T1WI prior to and following 5-ALA administration, were
displayed on a diagnostic monitor (Flexscan L365; Eizo Nanao
Corporation, Ishikawa, Japan) simultaneously. All ROIs were an
arbitrarily chosen, uniform shape and size (elliptical, 50
mm2). The ROIs were identified within tumors on the
T2WI, and corresponded to the CE lesion on the CE-T1WI in 4 spots
in each slice. Therefore, a total of 12 ROIs were assessed in each
patient pre- and post-5-ALA administration. The ROI placement
avoided areas of necrosis, hemorrhage, calcification, and engorged
vessels, in accordance with other sequences of MRI and computed
tomography (CT) (23,25). ROIs were also placed within the normal
white matter of the contralateral brain region (the contralateral
cerebellar peduncle in the cerebellar glioma). To avoid scaling
problems on each MRI scan image, signal intensity ratios were
calculated for all images by using the following formula: Signal
intensity ratio = signal intensity of tumor / signal intensity of
normal white matter (23).
Histopathology
A pathologist from the University of Occupational
and Environmental Health performed the subsequent histopathology.
Tumor specimens for histological assessment were obtained during
surgery according to the imaging results on the neuronavigation
system and the 5-ALA fluorescence, and were immediately fixed in
10% neutral buffered formalin for 24 h at room temperature. Then,
tumor specimens embedded in paraffin were cut systematically at 4
µm thickness using a sliding microtome (Leica SM2010R; Leica
Microsystems, Wetzler, Germany) for staining with hematoxylin and
eosin, followed by immunohistochemistry. All immunohistochemical
stainings were carried out using Dako Envision kit (Dako, Glostrup,
Denmark) according to the manufacturer's protocol, and using the
following commercially available antibodies: Anti-glial fibrillary
acidic protein (dilution, 1:500; #Z0344; Dako), Anti-Oligo2
(dilution, 1:20; #18953; Immuno-Biological Laboratories Co., Ltd.,
Fujioka, Japan), anti-S100 (dilution, 1:300; #Z0311; Dako),
anti-IDH1 R132H (dilution, 1:20; #DIA-H09; Dianova GmbH, Hamburg,
Germany), anti-p53 (dilution, 1:50; #M7001; Dako) and anti-Ki-67
(dilution, 1:150; #M7240; Dako). The slides were incubated with the
primary antibodies for 30 min at room temperature. HGGs were
classified neuropathologically according to the World Health
Organization classification of tumors of the central nervous system
(26).
Statistical analysis
Statistical analyses were performed using StatView
5.0 (SAS Institute Inc., Cary, North Carolina). T2 signal intensity
was compared prior to and following 5-ALA administration using a
paired t-test Accumulation of 5-ALA-induced PpIX was analyzed with
an unpaired t-test. The data are presented as the mean ± standard
error. P<0.01 was considered to indicate a statistically
significant difference.
Results
Patient characteristics
Surgery was performed on 9 patients with suspected
HGGs, which were identified from preoperative MRIs from September
2014 to February 2015. Of these, 5 patients (recurrent cases, n=4;
child case, n=1) were excluded according to the exclusion criteria.
Therefore, 4 patients (3 male, 1 female; mean age, 60±18.1 years)
were included in this study. Patient demographics are summarized in
Table I. A total of 3 patients were
pathologically diagnosed with glioblastoma multiforme (GBM) and 1
with anaplastic oligodendroglioma (AO). The tumors were located in
the right frontal lobe in 2 GBM cases, the right cerebellar
hemisphere in 1 GBM case and the right cingulate gyrus in the AO
case. The CE-T1WI exhibited a heterogeneous and irregularly thick
margin in patients with GBM, and focally enhanced heterogeneity in
the patient with AO. The mean duration of the MRI examination (from
initial MRI to surgery and post-5-ALA administration MRI) was 4
days.
 | Table I.Clinical characteristics of
high-grade gliomas. |
Table I.
Clinical characteristics of
high-grade gliomas.
| Case | Age, years | Gender | Pathological
diagnosis | Tumor location | MRI findings
(CE-T1WI) | Duration from
initial MRI to surgery | PpIX accumulation
intraoperative findings |
|---|
| 1 | 61 | M | GBM | Right frontal
lobe | Irregular, enhanced
thick margin | 5 days | Strong |
| 2 | 63 | F | GBM | Right frontal
lobe | Wholly enhanced,
heterogeneous | 1 days | Strong |
| 3 | 36 | M | AO | Right cingulate
gyrus | Focally enhanced,
heterogeneous | 8 days | None |
| 4 | 80 | M | GBM | Right cerebellar
hemisphere | Irregular, enhanced
thick margin | 2 days | Strong |
Intraoperative observations and
5-ALA-induced PpIX accumulation
The intraoperative findings for the 5-ALA
fluorescence were ‘strong’ in all 3 GBM cases and ‘none’ in the AO
case. The quantitative HPLC analysis demonstrated that the amount
of 5-ALA-induced PpIX in tumors of ‘strong’/GBM group was
113.1±32.6 pmol/mg of protein. The 5-ALA-induced PpIX was not
detected in the tumor of the ‘none’/AO group (<0.1 pmol/mg). The
former group tended to produce higher 5-ALA-induced PpIX values
than the latter, although no statistically significant differences
were identified (P=0.2927).
Evaluation of T2 signal intensity
prior to and following 5-ALA administration
In total, 48 within-tumor ROIs were obtained from
the T2WI prior to and following 5-ALA administration. In the
‘strong’ group, the relative value of the T2 signal intensity
within the tumors post-5-ALA administration was significantly
higher than the signal intensity value prior to 5-ALA
administration (1.537±0.021 and 1.577±0.023, respectively;
P=0.0055). By contrast, the value of the T2 signal intensity in the
‘none’ group did not differ significantly between tumors obtained
prior to or following 5-ALA administration (1.551±0.039 and
1.572±0.040 respectively; P=0.1281) (Table II).
 | Table II.Changes in T2 signal intensity before
and after 5-ALA administration. |
Table II.
Changes in T2 signal intensity before
and after 5-ALA administration.
|
|
| Relative signal
intensity value (T2) |
|---|
|
|
|
|
|---|
| PpIX
fluorescence | 5-ALA | Mean ± SE | P-value |
|---|
| Strong (n=36) | Pre | 1.537±0.021 | 0.0055 |
|
| Post | 1.577±0.023 |
|
| None (n=12) | Pre | 1.551±0.039 | 0.1281 |
|
| Post | 1.572±0.040 |
|
Representative cases
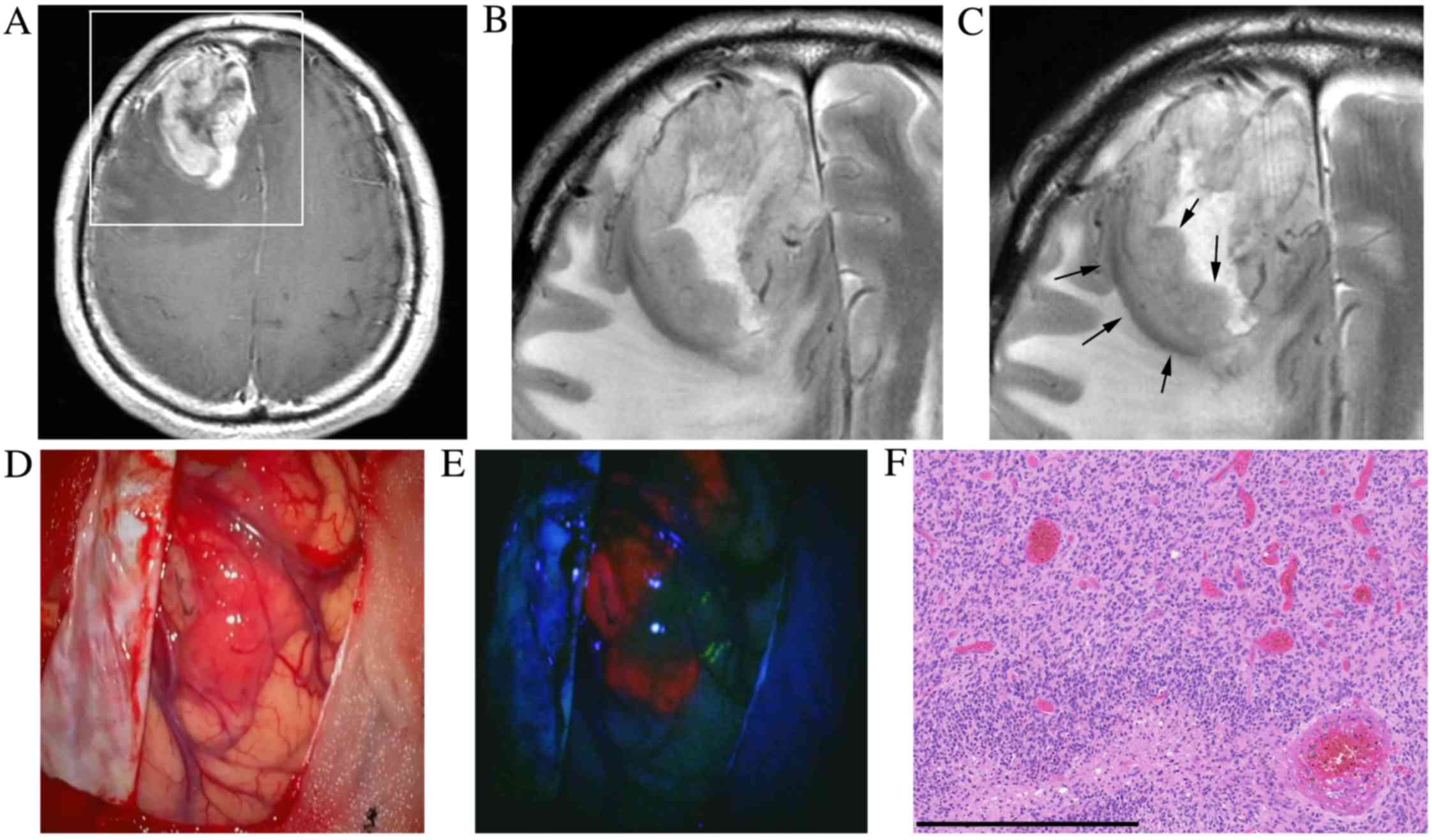
A 61-year-old healthy male presented with left
hemiparesis and cerebral cortical dysfunction (case 1). MRI
revealed an irregular, enhanced mass with peritumoral brain edema
in the right frontal lobe (Fig. 1A and
B). The patient underwent surgery 5 days later. The T2WI
post-5-ALA administration and prior to surgery exhibited a slightly
higher signal within the tumor compared with the T2WI prior to
5-ALA administration (initial MRI) (Fig.
1C). Intraoperative findings demonstrated a ‘strong’ 5-ALA
fluorescence within the tumor (Fig. 1D
and E). Histopathological examination identified
hypercellularity, marked nuclear atypia and prominent vascular
proliferation consistent with GBM (Fig.
1F).
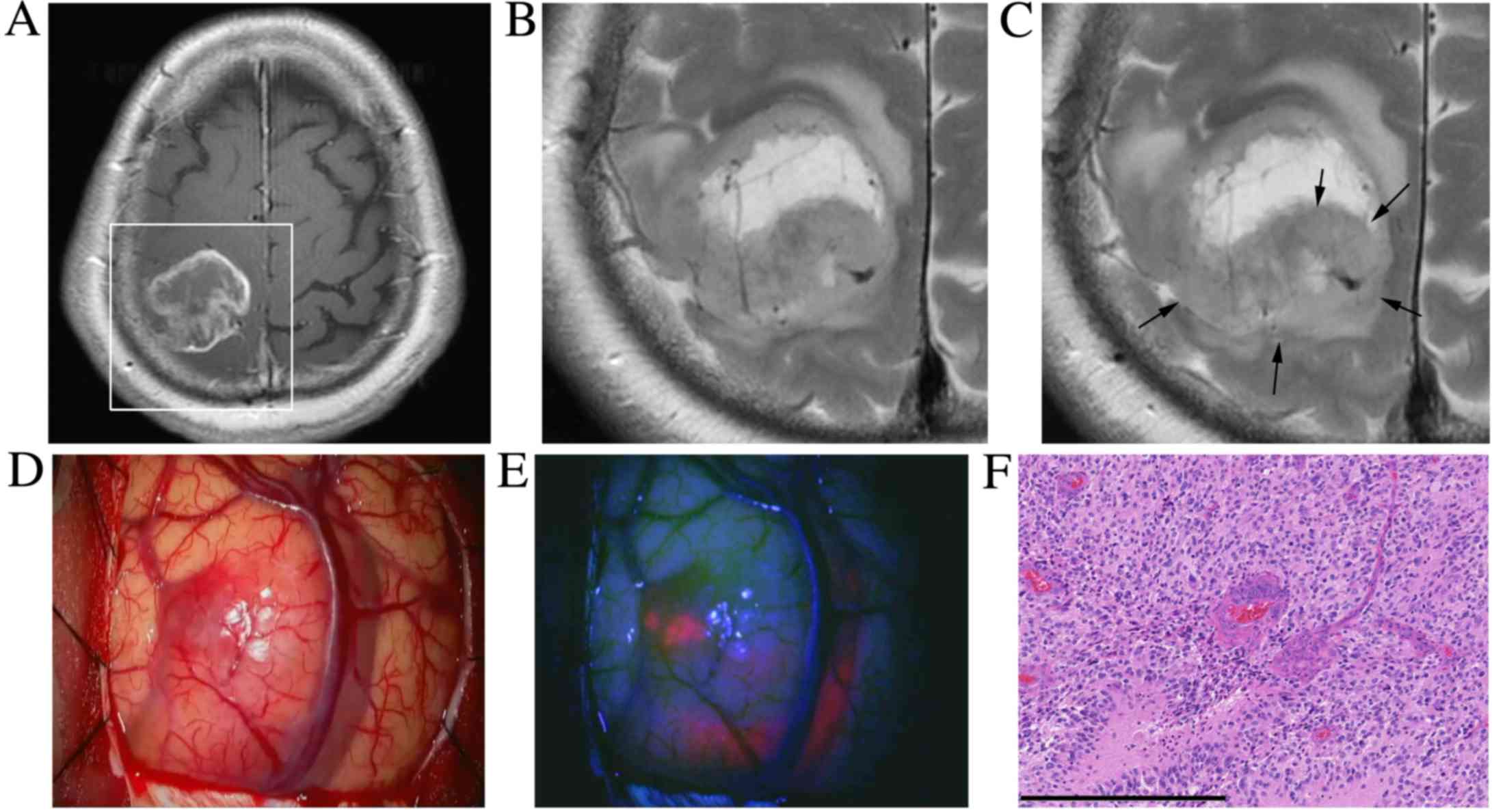
A 63-year-old woman presented with left hemiparesis
(case 2). An MRI scan revealed a heterogeneously enhanced mass in
the right frontal lobe with peritumoral brain edema (Fig. 2A and B). The patient underwent surgery
the following day. Prior to surgery, the T2WI following 5-ALA
administration exhibited a slightly increased T2 signal within the
tumor compared with the T2WI prior to 5-ALA administration
(Fig. 2C). Intraoperative findings
demonstrated a ‘strong’ 5-ALA fluorescence within the tumor
(Fig. 2D and E). Histopathological
examination confirmed the diagnosis of GBM (Fig. 2F).
Discussion
The present study demonstrated that 5-ALA-induced
PpIX enhanced the T2 signal intensity in HGGs. In particular, GBM
with a high accumulation of PpIX had an increased T2 signal
intensity within the tumors compared with AO, which had a low
accumulation of PpIX. To the best of our knowledge, the present
study is the first to assess the effects of 5-ALA-induced PpIX on
MRI signaling, in particular in T2WI in clinical HGG cases.
Several studies have demonstrated that pre-treatment
CE-T1WI tumor volume, early CE-T1WI tumor volume changes, the
treatment response criteria and residual CE-T1WI volumes in the
early follow up MRI scans may serve as predictors for both
progression free survival and overall survival in HGGs (27–30).
Bevacizumab is a well-known vascular endothelial growth factor
inhibitor, which functions as a powerful corticosteroid to decrease
the permeability of the BBB in HGGs (31). Bevacizumab is able to induce an
initially high radiographic response rate, with a profound
reduction in GBCA enhancement in MRI scans obtained as soon as 24 h
after the first bevacizumab dose in HGG cases (27). However, this rapid decrease in the
degree of contrast enhancement may not represent the true changes
in the cellular burden or tumor biology (32). Therefore, certain studies have
attempted to predict the bevacizumab response in HGGs using
subtraction maps of CE-T1WI, or modified relative cerebral blood
volume imaging based on GBCA administration (32,33).
Metabolic imaging using positron emission tomography (PET) amino
acid radiolabeled tracers may overcome certain disadvantages of
MRI, as the uptake of an amino acid tracer occurs primarily
independently of the regional tumor perfusion and BBB permeability.
Therefore, this technique may more accurately estimate the tumor
size and extension of the metabolically active tumor (34–37).
However, the spatial resolution of PET images is extremely low
compared with MRI. In addition, the general use of the PET system
is also low compared with MRI due to equipment location and the
handling of radiolabeled reagents. Therefore, metabolic imaging
with high spatial resolution, such as MRI, is required for HGG
management. In the present study, it was demonstrated that high
accumulation of 5-ALA-induced PpIX within tumor cells consequently
lead to T2 signal intensity enhancement within HGGs; conventional
MRI could observe these phenomena. These results suggest that it is
possible to use 5-ALA clinically in the metabolic imaging of HGGs
using MRI.
A number of previous studies have reported that the
normal BBB is impermeable to 5-ALA (38,39), while
others have reported that 5-ALA is able to cross the normal BBB to
a certain degree at high blood concentrations (40–42). Thus,
in the case of HGGs, the BBB breakdown within tumors is considered
to serve a permissive role in the uptake and processing of
5-ALA-induced PpIX (43). However,
5-ALA does not perform as well as GBCA in HGG cases. A previous
study demonstrated that 5-ALA-induced PpIX fluorescence had a
higher diagnostic accuracy compared with GBCA in a murine model of
infiltrative human glioma (44).
Moreover, another prospective study reported that 5-ALA-induced
PpIX was superior to GBCA-enhanced intraoperative MRI with regards
to its sensitivity and specificity in patients with HGG (8). In addition, 5-ALA-induced PpIX
fluorescence was observed in recurrent GBM without contrast
enhancement of GBCA following bevacizumab treatment (45). Considering its increased vascular
permeability, increased cellular metabolism and a modified tumor
microenvironment, 5-ALA-induced PpIX may depict dispersed
infiltrative glioma cells more accurately compared with GBCA
(46).
The mechanism underlying the enhancement of the T2
signal intensity by 5-ALA-induced PpIX remains unclear. Only one
study has attempted to depict experimental gliomas using 5-ALA with
MRI in vivo (47). This study
demonstrated the complete excretion of 5-ALA-induced PpIX and an
increase in intracellular iron 24 h after 5-ALA administration, and
subsequently, a decrease of T2* signal intensity, which is used to
depict paramagnetic deoxyhemoglobin, methemoglobin or hemosiderin
on MRI (48), in brain tumors
(37). Histological evaluation
revealed multifocal iron deposits within the tumor adjacent to
normal brain tissue in mice 24 h after 5-ALA treatment (37). 5-ALA does not only accumulate as PpIX
in cancer. Recent studies have reported that 5-ALA induced the
restoration of oxidative phosphorylation, the suppression of
glycolysis, the disruption of the Warburg effect in cancer cells,
and the induction of tumor cytotoxic macrophages to the surface of
subcutaneous experimental gliomas following 5-ALA administration
(21,49). These phenomena may affect iron
metabolism in glioma cells 24 h after 5-ALA administration, and
consequently the T2* signal intensity may decrease. In the present
study, the T2 signal intensity in HGGs was increased ~3 h after
5-ALA administration. A high accumulation of 5-ALA-induced PpIX in
the tumors was also quantified using HPLC analysis during this
period. Therefore, it may be speculated that 5-ALA-induced PpIX is
important in the enhancement of T2 signal intensity within tumors.
Possible mechanisms underlying PpIX-induced enhancement of T2
signal intensity are as follows. Firstly, relative increase of
intracellular content by water insoluble, 5-ALA-induced PpIX may
affect enhancement of T2 signal in HGG. Secondly, PpIX is well
known for its diamagnetism (50).
Thus, accumulated PpIX within the tumor may induce an increase in
T2 signal intensity, as well as oxyhemoglobin (51). The present study examined the T2WI of
patients following 5-ALA administration only due to the
psychophysiological stress that occurs prior to surgery. Therefore,
future studies should consider other MRI sequences including T1WI,
diffusion imaging, T2*, and intraoperative MRI under general
anesthesia.
The current study has several limitations. Firstly,
ROIs in T2WIs were obtained from only 4 patients. Thus, future
studies should consider the use of a larger sample size. Secondly,
the duration of the MRI examination prior to and following
administration of 5-ALA was 1 to 8 days. Therefore, the possibility
that there was an increase of intratumoral edema that developed
during these periods, and affected the enhancement of the T2 signal
intensity cannot be excluded. Thus, the duration of the MRI
examination should be shortened.
In conclusion, previous studies have focused on the
enhancement of the T1 signal intensity using metal-based contrast
reagents, including GBCA, in HGG MRI. The present study supports
the possibility of using 5-ALA-induced PpIX as a contrast reagent
for the enhancement of the T2 signal intensity in conventional MRI
for HGGs. At present, the enhancement effect of 5-ALA-induced PpIX
is not sufficient for clinical application. However, both the 5-ALA
reagent and conventional MRI systems have already been widely used
for clinical neurosurgery globally. Therefore, the application of
5-ALA as an MRI contrast reagent is possible, whilst the
development of a specific MRI sequence for 5-ALA-induced PpIX is
essential.
References
|
1
|
Shapiro WR, Green SB, Burger PC, Mahaley
MS Jr, Selker RG, VanGilder JC, Robertson JT, Ransohoff J, Mealey J
Jr and Strike TA: Randomized trial of three chemotherapy regimens
and two radiotherapy regimens and two radiotherapy regimens in
postoperative treatment of malignant glioma. Brain Tumor
Cooperative Group Trial 8001. J Neurosurg. 71:1–9. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Stupp R, Mason WP, van den Bent MJ, Weller
M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn
U, et al: Radiotherapy plus concomitant and adjuvant temozolomide
for glioblastoma. N Engl J Med. 352:987–996. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Westphal M, Ram Z, Riddle V, Hilt D and
Bortey E: Executive Committee of the Gliadel Study Group: Gliadel
wafer in initial surgery for malignant glioma: Long-term follow-up
of a multicenter controlled trial. Acta Neurochir (Wien).
148:269–275. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lacroix M, AbiSaid D, Fourney DR, Gokaslan
ZL, Shi W, DeMonte F, Lang FF, McCutcheon IE, Hassenbusch SJ,
Holland E, et al: A multivariate analysis of 416 patients with
glioblastoma multiforme: Prognosis, extent of resection and
survival. J Neurosurg. 95:190–198. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sanai N, Polley MY, McDermott MW, Parsa AT
and Berger MS: An extent of resection threshold for newly diagnosed
glioblastomas. J Neurosurg. 115:3–8. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Laperriere N, Zuraw L and Cairncross G:
Cancer Care Ontario Practice Guidelines Initiative Neuro-Oncology
Disease Site Group: Radiotherapy for newly diagnosed malignant
glioma in adults: A systematic review. Radiother Oncol. 64:259–273.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wen PY, Macdonald DR, Reardon DA,
Cloughesy TF, Sorensen AG, Galanis E, Degroot J, Wick W, Gilbert
MR, Lassman AB, et al: Updated response assessment criteria for
high-grade gliomas: Response assessment in neuro-oncology working
group. J Clin Oncol. 28:1963–1972. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Coburger J, Engelke J, Scheuerle A, Thal
DR, Hlavac M, Wirtz CR and König R: Tumor detection with
5-aminolevulinic acid fluorescence and Gd-DTPA-enhanced
intraoperative MRI at the border of contrast-enhancing lesions: A
prospective study based on histopathological assessment. Neurosurg
Focus. 36:E32014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Claes A, Idema AJ and Wesseling P: Diffuse
glioma growth: A guerilla war. Acta Neuropathol. 114:443–458. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hutterer M, Hattingen E, Palm C,
Proescholdt MA and Hau P: Current standards and new concepts in MRI
and PET response assessment of antiangiogenic therapies in
high-grade glioma patients. Neuro Oncol. 17:784–800. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Earnest F IV, Kelly PJ, Scheithauer BW,
Kall BA, Cascino TL, Ehman RL, Forbes GS and Axley PL: Cerebral
astrocytomas: Histopathologic correlation of MR and CT contrast
enhancement with stereotactic biopsy. Radiology. 166:823–827. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang Y, Alkasab TK, Narin O, Nazarian RM,
Kaewlai R, Kay J and Abujudeh HH: Incidence of nephrogenic systemic
fibrosis after adoption of restrictive gadolinium-based contrast
agent guidelines. Radiology. 260:105–111. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ishizuka M, Hagiya Y, Mizokami Y, Honda K,
Tabata K, Kamachi T, Takahashi K, Abe F, Tanaka T, Nakajima M, et
al: Porphyrins in urine after administration of 5-aminolevulinic
acid as a potential tumor marker. Photodiagnosis Photodyn Ther.
8:328–331. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yamamoto J, Yamamoto S, Hirano T, Li S,
Koide M, Kohno E, Okada M, Inenaga C, Tokuyama T, Yokota N, et al:
Monitoring of singlet oxygen is useful for predicting the
photodynamic effects in the treatment for experimental glioma. Clin
Cancer Res. 12:7132–7139. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ishizuka M, Abe F, Sano Y, Takahashi K,
Inoue K, Nakajima M, Kohda T, Komatsu N, Ogura S and Tanaka T:
Novel development of 5-aminolevurinic acid (ALA) in cancer
diagnoses and therapy. Int Immunopharmacol. 11:358–365. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yamamoto J, Ogura S, Tanaka T, Kitagawa T,
Nakano Y, Saito T, Takahashi M, Akiba D and Nishizawa S:
Radiosensitizing effect of 5-aminolevulinic acid-induced
protoporphyrin IX in glioma cells in vitro. Oncol Rep.
27:1748–1752. 2012.PubMed/NCBI
|
|
17
|
Stummer W, Stocker S, Wagner S, Stepp H,
Fritsch C, Goetz C, Goetz AE, Kiefmann R and Reulen HJ:
Intraoperative detection of malignant gliomas by 5-aminolevulinic
acid-induced porphyrin fluorescence. Neurosurgery. 42:518–526.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Stummer W, Pichlmeier U, Meinel T,
Wiestler OD, Zanella F and Reulen HJ: ALA-Glioma Study Group:
Fluorescence-guided surgery with 5-aminolevulinic acid for
resection of malignant glioma: A randomised controlled multicentre
phase III trial. Lancet Oncol. 7:392–401. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Junga CI, Yang JI, Park CH, Lee JB and
Park HR: Formation of the Metal Complexes between Protoporphyrin IX
and Divalent Metal Cations in the EnvironmentMolecular
Environmental Soil Science at the Interfaces in the Earth's
Critical Zone. Xu J and Huang PM: Springer Berlin Heidelberg;
Berlin: pp. 97–99. 2010, View Article : Google Scholar
|
|
20
|
Stummer W, Novotny A, Stepp H, Goetz C,
Bise K and Reulen HJ: Fluorescence-guided resection of glioblastoma
multiforme by using 5-aminolevulinic acid-induced porphyrins: A
prospective study in 52 consecutive patients. J Neurosurg.
93:1003–1013. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yamamoto J, Ogura S, Shimajiri S, Nakano
Y, Akiba D, Kitagawa T, Ueta K, Tanaka T and Nishizawa S:
5-aminolevulinic acid-induced protoporphyrin IX with multi-dose
ionizing irradiation enhances host antitumor response and strongly
inhibits tumor growth in experimental glioma in vivo. Mol Med Rep.
11:1813–1819. 2015.PubMed/NCBI
|
|
22
|
Mlkvy P, Messmann H, Pauer M, Stewart JC,
Millson CE, MacRobert AJ and Bown SG: Distribution and photodynamic
effects of meso-tetrahydroxyphenylchlorin (mTHPC) in the pancreas
and adjacent tissues in the Syrian golden hamster. Br J Cancer.
73:1473–1479. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yamamoto J, Kakeda S, Shimajiri S,
Takahashi M, Watanabe K, Kai Y, Moriya J, Korogi Y and Nishizawa S:
Tumor consistency of pituitary macroadenomas: Predictive analysis
on the basis of imaging features with contrast-enhanced 3D FIESTA
at 3T. AJNR Am J Neuroradiol. 35:297–303. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pierallini A, Caramia F, Falcone C,
Tinelli E, Paonessa A, Ciddio AB, Fiorelli M, Bianco F, Natalizi S,
Ferrante L and Bozzao L: Pituitary macroadenomas: Preoperative
evaluation of consistency with diffusion-weighted MR
imaging-initial experience. Radiology. 239:223–231. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Watanabe K, Kakeda S, Yamamoto J, Ide S,
Ohnari N, Nishizawa S and Korogi Y: Prediction of hard meningiomas:
Quantitative evaluation based on the magnetic resonance signal
intensity. Acta Radiol. 57:333–340. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Louis DN, Ohgaki H, Wiestler OD, Cavenee
WK, Burger PC, Jouvet A, Scheithauer BW and Kleihues P: The 2007
WHO classification of tumours of the central nervous system. Acta
Neuropathol. 114:97–109. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kreisl TN, Kim L, Moore K, Duic P, Royce
C, Stroud I, Garren N, Mackey M, Butman JA, Camphausen K, et al:
Phase II trial of single-agent bevacizumab followed by bevacizumab
plus irinotecan at tumor progression in recurrent glioblastoma. J
Clin Oncol. 27:740–745. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hasselbalch B, Lassen U, Hansen S,
Holmberg M, Sørensen M, Kosteljanetz M, Broholm H, Stockhausen MT
and Poulsen HS: Cetuximab, bevacizumab, and irinotecan for patients
with primary glioblastoma and progression after radiation therapy
and temozolomide: A phase II trial. Neuro Oncol. 12:508–516.
2010.PubMed/NCBI
|
|
29
|
Prados M, Cloughesy T, Samant M, Fang L,
Wen PY, Mikkelsen T, Schiff D, Abrey LE, Yung WK, Paleologos N, et
al: Response as a predictor of survival in patients with recurrent
glioblastoma treated with bevacizumab. Neuro Oncol. 13:143–151.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Huang RY, Rahman R, Hamdan A, Kane C, Chen
C, Norden AD, Reardon DA, Mukundun S and Wen PY: Recurrent
glioblastoma: Volumetric assessment and stratification of patient
survival with early posttreatment magnetic resonance imaging in
patients treated with bevacizumab. Cancer. 119:3479–3488. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Henson JW, Ulmer S and Harris GJ: Brain
tumor imaging in clinical trials. AJNR Am J Neuroradiol.
29:419–424. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Schmainda KM, Prah M, Connelly J, Rand SD,
Hoffman RG, Mueller W and Malkin MG: Dynamic-susceptibility
contrast agent MRI measures of relative cerebral blood volume
predict response to bevacizumab in recurrent high-grade glioma.
Neuro Oncol. 16:880–888. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ellingson BM, Kim HJ, Woodworth DC, Pope
WB, Cloughesy JN, Harris RJ, Lai A, Nghiemphu PL and Cloughesy TF:
Recurrent glioblastoma treated with bevacizumab: Contrast-enhanced
T1-weighted subtraction maps improve tumor delineation and aid
prediction of survival in a multicenter clinical trial. Radiology.
271:200–210. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Dunet V, Rossier C, Buck A, Stupp R and
Prior JO: Performance of 18F-fluoro-ethyl-tyrosine (18F-FET) PET
for the differential diagnosis of primary brain tumor: A systematic
review and Metaanalysis. J Nucl Med. 53:207–214. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Nihashi T, Dahabreh IJ and Terasawa T: PET
in the clinical management of glioma: Evidence map. AJR Am J
Roentgenol. 200:W654–W660. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hutterer M, Nowosielski M, Putzer D,
Jansen NL, Seiz M, Schocke M, McCoy M, Göbel G, la Fougère C,
Virgolini IJ, et al: [18F]-fluoro-ethyl-L-tyrosine PET: A valuable
diagnostic tool in neuro-oncology, but not all that glitters is
glioma. Neuro Oncol. 15:341–351. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Galldiks N, Rapp M, Stoffels G, Fink GR,
Shah NJ, Coenen HH, Sabel M and Langen KJ: Response assessment of
bevacizumab in patients with recurrent malignant glioma using
[18F]Fluoroethyl-L-tyrosine PET in comparison to MRI. Eur J Nucl
Med Mol Imaging. 40:22–33. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Garcia SC, Moretti MB, Garay MV and Batlle
A: Delta-aminolevulinic acid transport through blood-brain barrier.
Gen Pharmacol. 31:579–582. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Terr L and Weiner LP: An autoradiographic
study of delta-aminolevulinic acid uptake by mouse brain. Exp
Neurol. 79:564–568. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
McGillion FB, Thompson GG, Moore MR and
Goldberg A: The passage of delta-aminolaevulinic acid across the
blood-brain barrier of the rat: Effect of ethanol. Biochem
Pharmacol. 23:472–474. 1974. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ennis SR, Novotny A, Xiang J, Shakui P,
Masada T, Stummer W, Smith DE and Keep RF: Transport of
5-aminolevulinic acid between blood and brain. Brain Res.
959:226–234. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Gibson SL, Havens JJ, Foster TH and Hilf
R: Time-dependent intracellular accumulation of
delta-aminolevulinic acid, induction of porphyrin synthesis and
subsequent phototoxicity. Photochem Photobiol. 65:416–421. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Stummer W, Reulen HJ, Novotny A, Stepp H
and Tonn JC: Fluorescence-guided resections of malignant gliomas-an
overview. Acta Neurochir Suppl. 88:9–12. 2003.PubMed/NCBI
|
|
44
|
Samkoe KS, GibbsStrauss SL, Yang HH,
Hekmatyar S Khan, Hoopes P Jack, O'Hara JA, Kauppinen RA and Pogue
BW: Protoporphyrin IX fluorescence contrast in invasive
glioblastomas is linearly correlated with Gd enhanced magnetic
resonance image contrast but has higher diagnostic accuracy. J
Biomed Opt. 16:0960082011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wachter D, Kallenberg K, Wrede A,
SchulzSchaeffer W, Behm T and Rohde V: Fluorescence-guided
operation in recurrent glioblastoma multiforme treated with
bevacizumab-fluorescence of the noncontrast enhancing tumor tissue?
J Neurol Surg A Cent Eur Neurosurg. 73:401–406. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Collaud S, Juzeniene A, Moan J and Lange
N: On the selectivity of 5-aminolevulinic acid-induced
protoporphyrin IX formation. Curr Med Chem Anticancer Agents.
4:301–316. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Cho HR, Kim DH, Kim D, Doble P, Bishop D,
Hare D, Park CK, Moon WK, Han MH and Choi SH: Malignant glioma: MR
imaging by using 5-aminolevulinic acid in an animal model.
Radiology. 272:720–730. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Chavhan GB, Babyn PS, Thomas B, Shroff MM
and Haacke EM: Principles, techniques, and applications of
T2*-based MR imaging and its special applications. Radiographics.
29:1433–1449. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Sugiyama Y, Hagiya Y, Nakajima M, Ishizuka
M, Tanaka T and Ogura S: The heme precursor 5-aminolevulinic acid
disrupts the Warburg effect in tumor cells and induces
caspase-dependent apoptosis. Oncol Rep. 31:1282–1286.
2014.PubMed/NCBI
|
|
50
|
Desideri A, Caccuri AM, Polizio F, Bastoni
R and Federici G: Electron paramagnetic resonance identification of
a highly reactive thiol group in the proximity of the catalytic
site of human placenta glutathione transferase. J Biol Chem.
266:2063–2066. 1991.PubMed/NCBI
|
|
51
|
Hiwatashi A, Kinoshita T, Moritani T, Wang
HZ, Shrier DA, Numaguchi Y, Ekholm SE and Westesson PL:
Hypointensity on diffusion-weighted MRI of the brain related to T2
shortening and susceptibility effects. AJR Am J Roentgenol.
181:1705–1709. 2003. View Article : Google Scholar : PubMed/NCBI
|