Introduction
Hepatocellular carcinoma (HCC) is the second leading
cause of cancer-associated mortality worldwide (1). HCC represents the most common
histological type of liver cancer, accounting for 70–85% of cases
(2). Its treatment may include local
ablation, surgical resection, trans-catheter arterial
chemoembolization and the administration of chemotherapeutic agents
(3,4).
However, at the time of their diagnosis, HCC tumors may be too
large or may have expanded into nearby major blood vessels or
metastasized, rendering the majority of HCC patients unsuitable for
treatment by surgical resection (5).
In addition, chemotherapy provides only a modest improvement to the
overall survival time of patients due to the lack of specificity of
the agent as well as the intolerable side effects that are often
induced (6). Thus, novel anticancer
therapeutic agents for use in the treatment of HCC are urgently
required. In this regard, polysaccharides may be promising
candidates. Numerous studies have reported that polysaccharides
have antitumor activities and that polysaccharides from different
herbs have different roles (7–10).
Normal cells in multicellular organisms constantly
signal to one another via molecules called growth factors and
cytokines. The signals conducted by growth factors and cytokines
can inform individual cells whether to divide or not (11). According to Hanahan and Weinberg
(12), the cell surface receptors
that transduce signals into the cell are targets that can lead to
dysregulation during tumor progression, resulting in
self-sufficiency in growth signaling, one of the major hallmarks of
cancer cells. Growth factor receptors are overexpressed in numerous
types of cancer, and may enable the cancer cells to become
hyper-responsive to ambient levels of growth factors and even
ligand-independent signaling (13).
This observation provides the rationale for research into the
development of anti-growth factor compounds. The present study
reviews the mechanisms that underlie the growth factor-mediated
growth, proliferation, angiogenesis and metastasis of HCC cells,
how they may be targeted by polysaccharides, and the current
research being conducted into the use of these polysaccharides for
the treatment of HCC.
Involvement of growth factors in HCC
Upon response to their specific ligands, growth
factor receptors mediate tumorigenic activity through a variety of
signaling pathways (14). A large
number of growth factors are produced in the human liver during the
foetal stage, including epidermal growth factor (EGF), insulin-like
growth factors (IGFs), hepatocyte growth factor (HGF), vascular
endothelial growth factor (VEGF), fibroblast growth factor (FGF),
platelet-derived growth factor (PDGF), transforming growth factor
(TGF)-α and TGF-β. However, the production of many of these growth
factors decreases or is absent in the normal adult liver (15–17). On
the other hand, following injury or damage, when liver regeneration
is required, adult hepatocytes are able to upregulate the
production of particular growth factors, including EGF, TGF-α, IGF
and VEGF (18). This normal,
transient upregulation is dysregulated in the chronically injured
liver, and such dysregulation of growth factor production and
growth factor receptor signaling in adult hepatocytes serves an
important role in hepatocarcinogenesis (19) (Fig.
1).
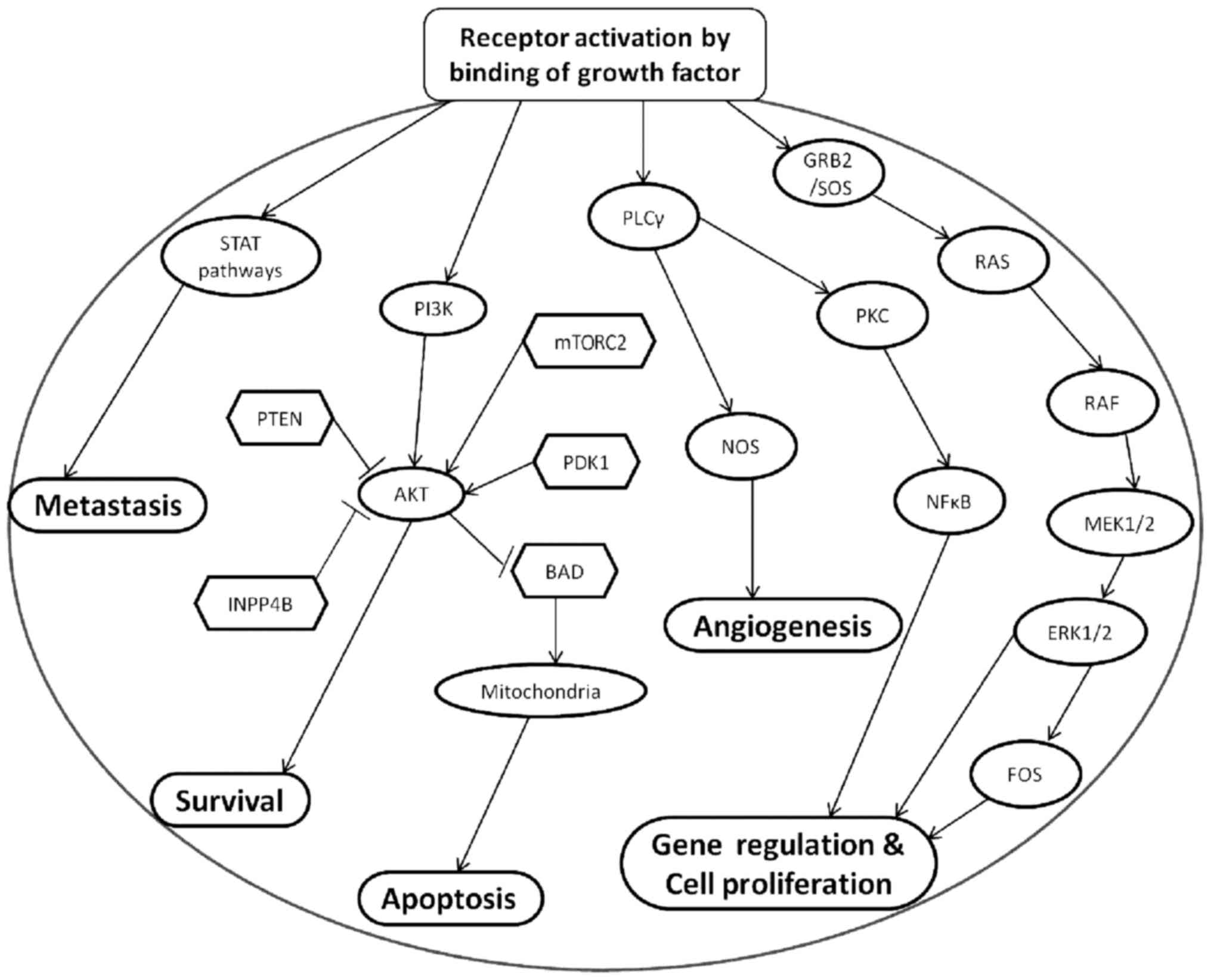 | Figure 1.Tumorigenesis, mediated by growth
factors. Growth factors activates their corresponding receptors,
leading to the subsequent activation of four pathways:
PI3K/AKT/mTOR pathway, Ras/Raf/MAPK pathway, NOS pathway and STAT
pathway. These four pathways can induce cell proliferation,
survival, angiogenesis and metastasis. PI3K, phosphoinositide
3-kinase; AKT, protein kinase B; mTOR, mammalian target of
rapamycin; MAPK, mitogen-activated protein kinase; NOS, nitric
oxide synthase; STAT, Signal Transducer and Activator of
Transcription; GRB2/SOS, growth factor receptor-bound protein 2/Son
of Sevenless; PLCγ, phospholipase Cγ; PKC, protein kinase C; PTEN,
phosphatase and tensin homolog; mTORC2, mTOR complex 2; PDK1,
pyruvate dehydrogenase kinase 1; BAD, Bcl2-associated agonist of
cell death; INPP4B, inositol polyphosphate-4-phosphatase type II B;
NFκB, nuclear factor κB; MEK1/2, MAPK kinase 1/2; ERK1/2,
extracellular signal-regulated kinase 1/2. |
VEGFs, prominent factors associated with aggressive
tumor behavior, belong to the PDGF supergene family (20,21).
VEGF-A, the major factor for angiogenesis, binds to two tyrosine
kinase receptors, namely VEGF receptor (VEGFR)-1 and VEGFR-2.
VEGFR-1 is a trans-membrane receptor and is responsible for
mediating angiogenesis and inflammatory responses. VEGFR-2 serves
important roles in physiological and pathological angiogenesis and
regulates the proliferation and migration of endothelial cells
(22,23). Previous studies have indicated that
high serum levels of VEGF have significant predictive ability for
the estimation of survival in HCC patients treated by hepatic
resection, radiofrequency ablation or trans-catheter arterial
chemoembolization (24,25). In HCC, VEGF expression is increased
through the expression of hypoxia-inducible factor-1α. In hypoxic
conditions, VEGF and VEGFR trigger the angiogenic cascade and
promote endothelial cell migration and proliferation. Thus, VEGF
blockade may be an effective target for HCC treatment (26,27).
Curcumin and Bevacizumab (VEGF blocker) can independently inhibit
HCC progression through the regulation of the VEGF/VEGFR/K-ras
pathway (28).
TGF-β1 is commonly recognized as a hallmark of HCC
and represents one of the most important pathways to be targeted
(29). TGF-β induces the
epithelial-mesenchymal transition (EMT), which is involved in
hepatocarcinogenesis and HCC metastasis (30). Stimulation of TGF-β1 leads to the
activation of phosphoinositide 3-kinase (PI3K)/protein kinase B
(Akt), whereas inhibition of PI3K/Akt activation represses EMT and
invasion of HCC cells (31).
EGF interacts with the EGF receptor (EGFR) to
stimulate cell growth, proliferation and differentiation, and EGFR
overexpression, which is known to be associated with tumorigenesis,
occurs in 40–70% of cases of human HCC (32). In addition, FGF and HGF, which are
heparin-binding growth factors, act as a potent mitogens for HCC
(33). In summary, all of the
aforementioned factors are associated with tumor growth and
development, and the control of the expression of these growth
factors can serve an important role in producing antitumor
effects.
Growth factor signaling as a therapeutic
target in HCC: Potential of polysaccharides
Clinical trials indicate that growth factor
receptors and their associated signaling pathways are important in
HCC cancer etiology and progression (34). Hence, growth factors, their receptors,
and signaling pathways mediated by these growth factors are
interesting targets for future therapeutic approaches. A number of
strategies, including inhibition of receptor expression using gene
therapy (antisense approach), antagonistic monoclonal antibodies
that prevent the binding of ligands to receptors, or
pharmacological (low-molecular-weight) receptor-selective tyrosine
kinase inhibitors have already been evaluated for their potency in
inhibiting the activity and downstream signaling cascades of these
receptors in HCC (34).
Initial clinical trials have also demonstrated that
multi-kinase inhibition is an effective novel treatment strategy in
HCC (35). In this respect,
sorafenib, an inhibitor of Raf, VEGF and PDGF signaling, is the
first multi-kinase inhibitor that has been approved by the Food
& Drug Administration for the treatment of advanced HCC
(35). Sorafenib administration is an
effective treatment for advanced HCC and can increase the survival
rate of these patients. Sorafenib inhibits the growth of hepatoma
cells by interfering with the secretion of IGF-1 (36). In addition, linifanib, a
multi-targeted receptor tyrosine kinase inhibitor, can inhibit
members of the VEGF and PDGF receptor families (37).
However these drugs have their own disadvantages
with regard to side effects, drug intolerance and resistance.
Although sorafenib has shown promising therapeutic effects, primary
and acquired resistance to the drug has been reported in numerous
studies (38,39). Furthermore, the overall survival time
of HCC patients who responded to sorafenib improved by only ~2
months (40,41). In this setting, polysaccharides may be
a good option. Many polysaccharides and polysaccharide-protein
complexes have been isolated from mushrooms, fungi, yeast, algae
and plants. Polysaccharides have a broad spectrum of biological
effects, such as antibiotic, antioxidant, anti-mutant,
anticoagulant, immunomodulating and anticancer activities (42–48). The
antitumor ability depends on a number of properties, such as
structure, dose, mechanism of action, and site of activity
(49). Several studies have
demonstrated that polysaccharides may have roles in the prevention
of tumorigenesis and induction of tumor cell apoptosis,
immunomodulation during chemotherapy, and inhibition of tumor
metastasis (50). Further research
into how polysaccharides may act via growth factors, cytokine
networks and signaling pathways, and their roles and mechanisms in
cancer regulation is required.
Sources of polysaccharides targeting
growth factors in HCC
Polysaccharides are the most abundant group of
biopolymers and, due to their biocompatibility, biodegradability
and non-toxicity, many studies have been conducted to evaluate
their therapeutic effects (51–53). A
large variety of polysaccharides can be isolated from plants
(dietary fibers, herbs and wood plants), algae, lichen,
microorganisms (fungi, yeasts and bacteria) and animals. Research
has been conducted to characterize the constituents of these
polysaccharides; however these kinds of studies are strikingly
limited.
A study by Lv et al (54) found that polysaccharides isolated from
tea were hetero-polysaccharides which consisted of mannose, ribose,
rhamnose, glucuronic acid, galacturonic acid, glucose, xylose,
galactose and arabinose with molar contents of 16.3, 10.3, 47.1,
5.6, 24.0, 128.4, 25.0, 101.4 and 71.1 µM, respectively (54). Another study demonstrating the
hepatoprotective effect of polysaccharides from Huangshan Maofeng
green tea also found that the main monosaccharide components of
this polysaccharide were galactose (mol.%, 35.0%), arabinose
(28.9%) and galacturonic acid (11.3%) (55). Capillary zone electrophoresis analysis
showed that polysaccharide isolated from Gynostemma
pentaphyllum Makino consisted of glucose (23.2%), galactose
(18.9%), arabinose (10.5%), rhamnose (7.7%), galacturonic acid
(4.7%), xylose (3.9%), mannose (3.1%), and glucuronic acid (1.2%)
(56). Characterization of
polysaccharides from two Pleurotus mushrooms, P.
eryngii and P. ostreatus, found that they were mainly
composed of mannose, along with other monosaccharides, including
glucose, galactose, xylose and rhamnose (57,58). The
monosaccharide constituents of Lentinus edodes
polysaccharide have also been characterized (59).
However, although many studies have found that,
grossly, these polysaccharides have potent antitumor activities in
HCC, it has not been determined which components of the
polysaccharides are responsible for such effects. Furthermore,
synthetic polysaccharides are often used in association with
chemotherapy (60–62). Traditional Chinese medicine (TCM) and
certain herbal medicines have been used in the treatment of cancer
for thousands of years in China, Japan and South Korea, as well as
some other Asian countries (63). As
adjunct anticancer agents, TCMs can have important anticancer roles
in inducing apoptosis and differentiation, improving the immune
system, inhibiting angiogenesis, and also in increasing the
sensitivity to and reducing the side effects of chemotherapeutics,
and improving patient survival time (64). For example, naturopathic therapy using
Cordyceps sinensis can prolong the survival time of patients
with HCC (65). Studies have
demonstrated that their roles in HCC are associated with the
regulation of growth factors and cytokines. The names, sources and
specific targets of polysaccharides are given in Table I.
 | Table I.Names of polysaccharides with their
sources and potential targets. |
Table I.
Names of polysaccharides with their
sources and potential targets.
| A, Polysaccharides
from natural sources |
|---|
|
|---|
| Source and
name | Targets |
|---|
| Traditional Chinese
medicine |
|
|
Aconitum koreanum
polysaccharides | IL-2, TNF-α,
IFN-γ |
|
Salvia miltiorrhiza
polysaccharides | TNF-α |
| Radix
Glycyrrhizae polysaccharide | IL-10, TGF-β, IL-2,
IL-12p70 |
|
Astragalus
polysaccharides | IL-1α, IL-2, IL-6,
TNF-α, IL-10 |
|
Exopolysaccharide fraction
from Cordyceps sinensis | TNF-α, IFN-γ |
| Plant-derived |
|
| Tea
carbohydrate polymers | IFN-γ, TNF-α,
VEGF |
|
Dihydromyricetin | TGF-β |
| Corn
silk polysaccharides | IL-2, IL-6,
TNF-α |
|
Gynostemma pentaphyllum
Makino polysaccharide | IL-2, TNF-α,
IFN-γ |
|
Artemisia apiacea
polysaccharide | IFN-γ, IL-4 |
| Dietary |
|
|
Pleurotus mushroom
polysaccharide | VEGF |
|
Tricholoma matsutake
polysaccharide | TNF-α, IFN-γ,
IL-2 |
|
Lentinus edodes
polysaccharide | TNF-α, IFN-γ,
IL-2 |
|
| B, Synthetic
polysaccharides |
|
| Name | Targets |
|
| Low molecular
weight chitosan | VEGF |
| Chitosan
nanoparticles | VEGFR-2 |
| Galactose modified
trimethyl chitosan-cysteine nanoparticles | VEGF |
|
| C, Others |
|
| Name | Targets |
|
| HS mimetic PI-88
series | FGF1, FGF2 |
| HS mimetic PG500
series | FGF1, FGF2,
VEGF |
Polysaccharides targeting a single
growth factor
Pleurotus mushroom polysaccharide-protein
complex (PP) has shown anticancer activities against liver cancer
cells in vitro and in vivo (66). PP inhibits proliferation by
inactivation of PI3K/Akt signaling (67). Studies have suggested that, in HCC, PP
reduces the expression of secretory VEGF, which in turn mediates
autocrine regulation of PI3K/Akt signaling in xenograft BALB/c nude
mice. PP can also enhance sensitivity to the chemotherapeutic drug
cisplatin (68).
Tea carbohydrate polymers are natural polymers with
antioxidant, hepatoprotective and antitumor activities (69). These have exhibited strong antitumor
activity in experimental HCC animals. It was found that
administration of tea carbohydrate for 40 days could significantly
inhibit tumour growth and that three different doses of
carbohydrate treatment (100, 200 and 300 mg/kg body weight/day)
significantly decreased microvessel density in a dose-dependent
manner. During this period, a significant enhancement in the serum
white blood cell count and interferon (IFN)-γ and tumor necrosis
factor α (TNF-α) levels, and a decrease in the expression of VEGF
and proliferating cell nuclear antigen were found in H22 tumor
tissues (70). Tea carbohydrates were
also found to augment the antitumor activity of doxorubicin
(71).
Radix Glycyrrhizae polysaccharide (GP) is a major
active compound extracted from Radix Glycyrrhizae, a commonly used
traditional herbal medicine. In a study of tumor-bearing mice, the
effect of GP on tumor growth inhibition was determined to be likely
caused by the to upregulation of the Th1/Th2 cytokine ratio in
serum by the decrease in the transcription factor Foxp3,
interleukin (IL)-10 and transforming growth factor (TGF)-β levels
(P<0.01), and the increase in IL-2 and IL-12p70 levels in serum
(P<0.01) (72).
Dihydromyricetin (DHM) is the active component in
extracts of Ampelopsis grossedentata. The principal
anticancer mechanism of DHM is via the induction of p53-dependent
apoptosis (73–75). A previous study demonstrated that DHM
enhances chemosensitivity to nedaplatin (a platinum-containing
chemotherapeutic drug) by activating the p53/B-cell lymphoma 2
(Bcl-2) signaling pathway, which resulted in mitochondrial
dysfunction and induced cell death and growth inhibition in HCC
cells (76). Furthermore, it has also
been reported that induction of cell apoptosis by DHM is regulated
via the TGF-β/Smad3 signaling pathway and that TGF-β expression is
decreased in the mouse HCC cell line Hepal-6 (77).
Polysaccharides targeting multiple
growth factors
Cell surface/extracellular matrix heparan sulfate
(HS) glycosaminoglycans are complex polysaccharides, and HS
mimetics function by blocking these interactions and inhibiting
processes crucial to tumor progression, and could therefore be
considered as a novel class of cancer therapeutics (78,79). The
HS mimetic PI-88 acts as an inhibitor of heparanase and
angiogenesis in HCC. A series of PI-88 analogs with augmented
chemical and biological properties are composed of single, defined
oligosaccharides with specific modifications and can inhibit
heparanase and FGF1, FGF2 and VEGF, and thus can act as potent
inhibitors of growth factor-induced endothelial cell proliferation
(80). The PG500 series of HS
mimetics have high affinity for growth factors; however, their
affinity is lower compared with the PI-88 series. A previous study
showed that PG500 compounds containing a larger lipophilic moiety
had reduced anticoagulant activity compared with PI-88 (81). However, the selected PG500 series
compounds all inhibited FGF1, FGF2 and VEGF-induced endothelial
cell proliferation. In a tube formation assay, it was found that
certain of the PG500 series compounds (PG536, PG537, PG545 and
PG562) inhibited tube formation by >90% at a concentration of 10
µM. Furthermore, in a rat aortic assay of angiogenesis, it was
found that PG536, PG545 and PG546 inhibited angiogenesis by >80%
following daily administration of 10 µM for 6–8 days. Treatment
with PG545, PG546 and PG547 also potently inhibited the development
of metastatic nodules when administered at 10 mg/kg. Finally, PG545
was found to significantly inhibit tumor development in a xenograft
model (HT29 colon adenocarcinoma cells) (81).
Polysaccharides targeting
pro-inflammatory cytokines
Cytokines are a subtype of growth factors that are
produced by hematopoietic and immune cell types, and include IFNs
and ILs. Cytokines normally function to stimulate a host response
to control cellular stress and minimize cellular damage. Cytokines
produced by CD4+ T-helper (Th) cells are categorized as Th1 or Th2;
Th1 cytokines (e.g., IL-1α, IL-1β, IL-2, IL-12p35, IL-12p40, IL-15,
TNF-α and IFN-γ) and Th2 cytokines (e.g., IL-4, IL-8, IL-10, and
IL-5) are generally referred to as proinflammatory and
anti-inflammatory cytokines, respectively (82). In the liver, cell damage and
regeneration mediated by viral hepatitis-induced immune responses
can cause dysregulated hepatocyte proliferation, which may
accelerate the development and progression of hepatic cancer
through transcription and activation of cytokines and growth
factors, oxidative DNA damage, DNA methylation, and hepatocyte
injury (83–86). Increasing evidence indicates the
involvement of cytokines in hepatocarcinogenesis [reviewed by
Bishayee (87)].
On the other hand, numerous studies have
demonstrated that cytokines have broad antitumor activity.
Accordingly, proinflammatory cytokines, including TNF-α, IL-6 and
IL-1β, and transcription factors that are required for signaling by
these cytokines, including NF-κB and STATs, are emerging as
potential targets for anticancer therapy (88). Serum cytokines play important roles in
suppressing tumor growth (89).
IL-1α, IL-2 and IL-6 are capable of inducing the proliferation of
responsive T-cells (90).
Wnt/β-catenin and EGFR signaling are the major cascades that are
activated through proinflammatory cytokines and involved in the
progression of epithelial tumors (91). IL-10 inhibits the synthesis of IL-2
and TNF-α (92).
Several in vivo experiments have demonstrated
that polysaccharides from various sources have the ability to
modulate tumor growth and immunity. Most affect tumor growth and
immune functions in hepatocarcinoma tumor-bearing mice by
increasing the expression levels of IL-2, IL-6 and TNF-α, and can
inhibit the growth of hepatoma and prolong the survival time of
these mice. These polysaccharides include those from Corn silk
(Zea mays L.) (93),
Aconitum koreanum and Salvia miltiorrhiza (94).
Polysaccharides from Astragalus membranaceus
(APS) have been widely studied for their anticancer potential. APS
was found to exert antitumor effects in H22 tumor-bearing mice and
also enhanced chemosensitivity to adriamycin (ADM). The antitumor
and synergistic activity of APS may be associated with its effect
of increasing the expression levels of IL-1α, IL-2, IL-6 and TNF-α
and decreasing IL-10 levels. APS was also found to downregulate
multidrug resistance 1 mRNA and P-glycoprotein expression levels,
which may be related to other anticancer effects (10,95).
Several studies aimed to assess the antitumor potentials of
polysaccharides from different sources and found that their
anticancer effects are mediated by the secretion of INF-γ. These
included polysaccharides obtained from G. pentaphyllum
Makino (96), a polysaccharide
isolated from Artemisia apiacea (97), and oral administration of A.
coreanum (98). Polysaccharides
obtained from cultured Cordyceps fungus have also been shown
to have pharmacological efficacy; specifically, the
exopolysaccharide fraction from Cordyceps sinensis was found
to significantly inhibit HCC tumor growth and lead to elevated
TNF-α and IFN-γ mRNA expression of splenic lymphocytes (99). Collectively, these data indicate the
increasing contribution to therapeutic effects of these
polysaccharides.
Combination of polysaccharides
5-fluorouracil (5-FU) is a chemotherapeutic drug
that is commonly used in HCC treatment. Chemotherapeutic drugs
combined with polysaccharide may act more effectively together;
thus, a combination of polysaccharides can be used with
chemotherapeutic drugs like 5-FU to enhance H22 cell growth
inhibition. A previous study found that polysaccharides from
Tricholoma matsutake (PTM) could activate splenic
lymphocytes, and another study demonstrated that polysaccharides
from Lentinus edodes (PL) had antitumor bioactivities at
concentrations ranging from 50 to 500 µg/ml, with maximum
inhibition occurring at a concentration of 200 µg/ml between 36 and
48 h (100,101). Notably, in vivo experiments
revealed significant increases in the cytotoxic T lymphocyte and
natural killer cell activities, the frequencies of CD4+ and CD8+ T
cells in the spleen, and the serum levels of TNF-α, IL-2 and IFN-γ
in mice treated with 5-FU+PTM+PL when compared with mice treated
with 5-FU, PTM or PL alone, 5-FU+PL, or 5-FU+PTM (102).
Helper polysaccharides
Polysaccharides have been studied for their roles in
facilitating successful drug delivery, and it was demonstrated that
certain polysaccharides are important in this regard.
In certain experimental tumor models, VEGF silencing
by RNA interference (RNAi) has been attempted and has achieved
positive results (103,104); however, due to the poor stability of
small interfering RNA (siRNA) molecules in vivo and the low
cellular uptake, a safe and efficient carrier is required for
therapeutic RNAi applications (105). Chitosan is a natural polysaccharide
composed of randomly distributed β-[1–4]-linked D-glucosamine and
N-acetyl-D-glucosamine. Low molecular weight chitosan (LMWC) can be
used as a carrier for RNAi drugs directed against VEGF (106). It was found that LMWC/VEGF shRNA
complexes could be efficiently transfected into murine
hepatocarcinoma Hepa1-6 cells and that this could inhibit VEGF
expression and suppress tumor angiogenesis (106). In another study, chitosan
nanoparticles (CNP) were shown to significantly inhibit tumor
growth and induce tumor necrosis in model mice xenografted with HCC
(BEL-7402) cells. The dose-dependent tumor suppression by CNP was
associated with the inhibition of tumor angiogenesis. Further
mechanistic evidence suggested that this inhibition of tumor
angiogenesis was linked to altered levels of VEGFR-2 (107).
In another study, tumor-bearing mice were orally
administered with VEGF siRNA and pDNA expressing shRNA specific for
survivin (a member of the inhibitors of apoptosis family) loaded
onto galactose-modified trimethyl chitosan-cysteine nanoparticles
with various degrees of galactosylation. The loaded nanoparticles
could effectively accumulate in the tumor tissues, resulting in the
downregulation of antiapoptotic survivin, which acted
synergistically with the siRNA-mediated inhibition of angiogenic
VEGF. This ultimately led to increased apoptosis and inhibition of
angiogenesis in hepatoma (108).
Conclusion and future perspectives
To date, a number of standard therapies have been
shown to result in modest improvements to the overall survival and
quality of life of HCC patients. However, they are associated with
significant toxicities. Furthermore, the ability of solid tumors to
develop multiple invasion and resistance pathways that allow them
to circumvent inhibition by a single signaling pathway is becoming
increasingly evident (109).
Expanding knowledge of the molecular signaling that underlies tumor
cell resistance and the poor survival of patients with HCC can
facilitate the development of new drugs with better safety
profiles. Furthermore, resistance is less likely to arise in
response to natural compounds (110).
As reviewed by Zong et al, the anticancer
efficacy of polysaccharides was first recognized in 1946 (44). Since then numerous studies have
suggested that polysaccharides can be utilized as key ingredients
for bio-based materials in life sciences, including
pharmaceuticals, and many polysaccharides have demonstrated
potential as anticancer agents (96–99). With
regard to drawbacks, such as low safety profiles and resistance of
current therapies, the development of polysaccharides as treatment
strategies for HCC is necessary.
Although there is potential for the use of these
kinds of polysaccharides against HCC, research into these
polysaccharides is limited. Regarding self-sufficiency in growth
signaling, a major hallmark of cancer, polysaccharides have been
shown to have good activity; as discussed in the present study,
polysaccharides from different sources have shown moderate
anticancer activity in HCC by targeting growth factors. However, an
active area of research should be dedicated to the development of
more efficient and economic approaches for the preparation and
modification of polysaccharides, and to elucidating the
structure-activity association. The active component of these
polysaccharides must be identified to better understand their
anticancer mechanism in HCC, and it is also important to pay
attention to the signaling pathways that are modified by the use of
polysaccharides. Furthermore it is essential that in vivo
studies are performed to confirm the results obtained in
vitro, and these polysaccharides must also be tested in a
clinical setting.
Considering the limited therapeutic options
available to treat HCC, studies investigating polysaccharides may
provide a rationale for the translation of these compounds into
potential therapeutics against HCC. Overall, recent discussion is
indicating a bright future for polysaccharides targeting growth
factors in HCC.
Acknowledgements
This study was funded by the Shanghai Natural
Science Foundation of China (grant no. 15ZR1438900), Shanghai
Construction Doctoral Research (grant no. B201404) and Shanghai
Municipal Health Bureau Scientific Research Program (grant no.
20134167).
References
|
1
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
AC Society, . Global cancer facts and
figures. 2008.http://www.cancer.org/acs/groups/content/@editorial/documents/document/acspc-044552.pdfAccessed:
December 16, 2016.
|
|
3
|
Lencioni R, Petruzzi P and Crocetti L:
Chemoembolization of hepatocellular carcinoma. Semin Intervent
Radiol. 30:3–11. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kim HY and Park JW: Clinical trials of
combined molecular targeted therapy and locoregional therapy in
hepatocellular carcinoma: Past, present and future. Liver Cancer.
3:9–17. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Levin B and Amos C: Therapy of
unresectable hepatocellular carcinoma. N Engl J Med. 332:1294–1296.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nocentini G: Ribonucleotide reductase
inhibitors: New strategies for cancer chemotherapy. Crit Rev Oncol
Hematol. 22:89–126. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Deng C, Fu H, Teng L, Hu Z, Xu X, Chen J
and Ren T: Anti-tumor activity of the regenerated triple-helical
polysaccharide from Dictyophora indusiata. Int J Biol Macromol.
61:453–458. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shen H, Tang G, Zeng G, Yang Y, Cai X, Li
D, Liu H and Zhou N: Purification and characterization of an
antitumor polysaccharide from Portulaca oleracea L. Carbohydr
Polym. 93:395–400. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yang Z, Xu J, Fu Q, Fu X, Shu T, Bi Y and
Song B: Antitumor activity of a polysaccharide from Pleurotus
eryngii on mice bearing renal cancer. Carbohydr Polym. 95:615–620.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang B, Xiao B and Sun T: Antitumor and
immunomodulatory activity of Astragalus membranaceus
polysaccharides in H22 tumor-bearing mice. Int J Biol Macromol.
62:287–290. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Odenthal J, Takes R and Friedl P:
Plasticity of tumor cell invasion: Governance by growth factors and
cytokines. Carcinogenesis. 37:1117–1128. 2016.PubMed/NCBI
|
|
12
|
Hanahan D and Weinberg RA: The hallmarks
of cancer. Cell. 100:57–70. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hanada K, Perry-Lalley DM, Ohnmacht GA,
Bettinotti MP and Yang JC: Identification of fibroblast growth
factor-5 as an overexpressed antigen in multiple human
adenocarcinomas. Cancer Res. 61:5511–5516. 2001.PubMed/NCBI
|
|
14
|
Habib SA, Aggour YA and Taha HA:
Downregulation of transforming growth factor- β (TGF-) and vascular
endothelial growth factor (VEGF) in ehrlich ascites
carcinoma-bearing mice using stearic acid-grafted carboxymethyl
chitosan (SA-CMC). Nat Sci. 4:808–818. 2012.
|
|
15
|
Wolf HK, Zarnegar R and Michalopoulos GK:
Localization of hepatocyte growth factor in human and rat tissues:
An immunohistochemical study. Hepatology. 14:488–494. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Awuah PK, Nejak-Bowen KN and Monga SP:
Role and regulation of PDGFRα signaling in liver development and
regeneration. Am J Pathol. 182:1648–1658. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Suzuki A, Iwama A, Miyashita H, Nakauchi H
and Taniguchi H: Role for growth factors and extracellular matrix
in controlling differentiation of prospectively isolated hepatic
stem cells. Development. 130:2513–2524. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Duncan SA: Mechanisms controlling early
development of the liver. Mech Dev. 120:19–33. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Breuhahn K, Longerich T and Schirmacher P:
Dysregulation of growth factor signaling in human hepatocellular
carcinoma. Oncogene. 25:3787–3800. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shibuya M: Vascular endothelial growth
factor and its receptor system: Physiological functions in
angiogenesis and pathological roles in various diseases. J Biochem.
153:13–39. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shibuya M and Claesson-Welsh L: Signal
transduction by VEGF receptors in regulation of angiogenesis and
lymphangiogenesis. Exp Cell Res. 312:549–560. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bhardwaj S, Roy H, Babu M, Shibuya M and
Yla-Herttuala S: Adventitial gene transfer of VEGFR-2 specific
VEGF-E chimera induces MCP-1 expression in vascular smooth muscle
cells and enhances neointimal formation. Atherosclerosis.
219:84–91. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Meyer JP, Edwards KJ, Kozlowski P, Backer
MV, Backer JM and Lewis JS: Selective imaging of VEGFR-1 and
VEGFR-2 using 89Zr-labeled single-chain VEGF mutants. J Nucl Med.
57:1811–1816. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kanematsu M, Semelka RC, Osada S and
Amaoka N: Magnetic resonance imaging and expression of vascular
endothelial growth factor in hepatocellular nodules in cirrhosis
and hepatocellular carcinomas. Top Magn Reson Imaging. 16:67–75.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Schoenleber SJ, Kurtz DM, Talwalkar JA,
Roberts LR and Gores GJ: Prognostic role of vascular endothelial
growth factor in hepatocellular carcinoma: Systematic review and
meta-analysis. Br J Cancer. 100:1385–1392. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Raskopf E, Dzienisowicz C, Hilbert T, Rabe
C, Leifeld L, Wernert N, Sauerbruch T, Prieto J, Qian C, Caselmann
WH and Schmitz V: Effective angiostatic treatment in a murine
metastatic and orthotopic hepatoma model. Hepatology. 41:1233–1240.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tan HY, Wang N, Tsao SW, Zhang Z and Feng
Y: Suppression of vascular endothelial growth factor via
inactivation of eukaryotic elongation factor 2 by alkaloids in
Coptidis rhizoma in hepatocellular carcinoma. Integr Cancer Ther.
13:425–434. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gao JZ, Du JL, Wang YL, Li J, Wei LX and
Guo MZ: Synergistic effects of curcumin and bevacizumab on cell
signaling pathways in hepatocellular carcinoma. Oncol Lett.
9:295–299. 2015.PubMed/NCBI
|
|
29
|
Hoshida Y, Nijman SM, Kobayashi M, Chan
JA, Brunet JP, Chiang DY, Villanueva A, Newell P, Ikeda K,
Hashimoto M, et al: Integrative transcriptome analysis reveals
common molecular subclasses of human hepatocellular carcinoma.
Cancer Res. 69:7385–7392. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ru NY, Wu J, Chen ZN and Bian H:
HAb18G/CD147 is involved in TGF-β-induced epithelial-mesenchymal
transition and hepatocellular carcinoma invasion. Cell Biol Int.
39:44–51. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yu W, Huang C, Wang Q, Huang T, Ding Y, Ma
C, Ma H and Chen W: MEF2 transcription factors promotes EMT and
invasiveness of hepatocellular carcinoma through TGF-β1
autoregulation circuitry. Tumour Biol. 35:10943–10951. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Buckley AF, Burgart LJ, Sahai V and Kakar
S: Epidermal growth factor receptor expression and gene copy number
in conventional hepatocellular carcinoma. Am J Clin Pathol.
129:245–251. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lai JP, Chien JR, Moser DR, Staub JK,
Aderca I, Montoya DP, Matthews TA, Nagorney DM, Cunningham JM,
Smith DI, et al: hSulf1 Sulfatase promotes apoptosis of
hepatocellular cancer cells by decreasing heparin-binding growth
factor signaling. Gastroenterology. 126:231–248. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Höpfner M, Schuppan D and Scherübl H:
Growth factor receptors and related signalling pathways as targets
for novel treatment strategies of hepatocellular cancer. World J
Gastroenterol. 14:1–14. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
35. Wilhelm SM, Adnane L, Newell P,
Villanueva A, Llovet JM and Lynch M: Preclinical overview of
sorafenib, a multikinase inhibitor that targets both Raf and VEGF
and PDGF receptor tyrosine kinase signaling. Mol Cancer Ther.
7:3129–3140. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sprinzl MF, Puschnik A, Schlitter AM,
Schad A, Ackermann K, Esposito I, Lang H, Galle PR, Weinmann A,
Heikenwälder M and Protzer U: Sorafenib inhibits macrophage-induced
growth of hepatoma cells by interference with insulin-like growth
factor-1 secretion. J Hepatol. 62:863–870. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Albert DH, Tapang P, Magoc TJ, Pease LJ,
Reuter DR, Wei RQ, Li J, Guo J, Bousquet PF, Ghoreishi-Haack NS, et
al: Preclinical activity of ABT-869, a multitargeted receptor
tyrosine kinase inhibitor. Mol Cancer Ther. 5:995–1006. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Blivet-Van Eggelpoël MJ, Chettouh H,
Fartoux L, Aoudjehane L, Barbu V, Rey C, Priam S, Housset C,
Rosmorduc O and Desbois-Mouthon C: Epidermal growth factor receptor
and HER-3 restrict cell response to sorafenib in hepatocellular
carcinoma cells. J Hepatol. 57:108–115. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhai B and Sun XY: Mechanisms of
resistance to sorafenib and the corresponding strategies in
hepatocellular carcinoma. World J Hepatol. 5:345–352. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Bruix J and Sherman M: American
Association for the Study of Liver Diseases: Management of
hepatocellular carcinoma: An update. Hepatology. 53:1020–1022.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S,
Kim JS, Luo R, Feng J, Ye S, Yang TS, et al: Efficacy and safety of
sorafenib in patients in the Asia-Pacific region with advanced
hepatocellular carcinoma: A phase III randomised, double-blind,
placebo-controlled trial. Lancet Oncol. 10:25–34. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ali BH, Ziada A and Blunden G: Biological
effects of gum arabic: A review of some recent research. Food Chem
Toxicol. 47:1–8. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yu Z, LiHua Y, Qian Y and Yan L: Effect of
Lentinus edodes polysaccharide on oxidative stress, immunity
activity and oral ulceration of rats stimulated by phenol.
Carbohydrate Polymers. 75:115–118. 2009. View Article : Google Scholar
|
|
44
|
Zong A, Cao H and Wang F: Anticancer
polysaccharides from natural resources: A review of recent
research. Carbohydr Polym. 90:1395–1410. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ajith TA and Janardhanan KK: Cytotoxic and
antitumor activities of a polypore macrofungus, Phellinus rimosus
(Berk) Pilat. J Ethnopharmacol. 84:157–162. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Santos-Neves JC, Pereira MI, Carbonero ER,
Gracher AHP, Alquini G, Gorin PAJ, Sassaki GL and Iacomini M: A
novel branched αβ-glucan isolated from the basidiocarps of the
edible mushroom Pleurotus florida. Carbohydrate Polymers.
73:309–314. 2008. View Article : Google Scholar
|
|
47
|
Wong SM, Wong KK, Chiu LCM and Cheung PCK:
Non-starch polysaccharides from different developmental stages of
Pleurotus tuber-regium inhibited the growth of human acute
promyelocytic leukemia HL-60 cells by cell-cycle arrest and/or
apoptotic induction. Carbohydrate Polymers. 68:206–217. 2007.
View Article : Google Scholar
|
|
48
|
Zhang M, Cheung PCK, Chiu LCM, Wong EYL
and Ooi VEC: Cell-cycle arrest and apoptosis induction in human
breast carcinoma MCF-7 cells by carboxymethylated β-glucan from the
mushroom sclerotia of Pleurotus tuber-regium. Carbohydrate
Polymers. 66:455–462. 2006. View Article : Google Scholar
|
|
49
|
Lavi I, Nimri L, Levinson D, Peri I, Hadar
Y and Schwartz B: Glucans from the edible mushroom Pleurotus
pulmonarius inhibit colitis-associated colon carcinogenesis in
mice. J Gastroenterol. 47:504–518. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Maehara Y, Tsujitani S, Saeki H, Oki E,
Yoshinaga K, Emi Y, Morita M, Kohnoe S, Kakeji Y, Yano T, et al:
Biological mechanism and clinical effect of protein-bound
polysaccharide K (KRESTIN(®)): Review of development and
future perspectives. Surg Today. 42:8–28. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Zeng Q, Zhou F, Lei L, Chen J, Lu J, Zhou
J, Cao K, Gao L, Xia F, Ding S, et al: Ganoderma lucidum
polysaccharides protect fibroblasts against UVB-induced photoaging.
Mol Med Rep. 2016.
|
|
52
|
Hu X, Zhang R, Xie Y, Wang H and Ge M: The
protective effects of polysaccharides from Agaricus blazei Murill
against cadmium-induced oxidant stress and inflammatory damage in
chicken livers. Biol Trace Elem Res. 2016. View Article : Google Scholar
|
|
53
|
Li S, Gao A, Dong S, Chen Y, Sun S, Lei Z
and Zhang Z: Purification, antitumor and immunomodulatory activity
of polysaccharides from soybean residue fermented with Morchella
esculenta. Int J Biol Macromo. 96:26–34. 2016. View Article : Google Scholar
|
|
54
|
Lv Y, Yang X, Zhao Y, Ruan Y, Yang Y and
Wang Z: Separation and quantification of component monosaccharides
of the tea polysaccharides from Gynostemma pentaphyllum by HPLC
with indirect UV detection. Food Chemistry. 112:742–746. 2009.
View Article : Google Scholar
|
|
55
|
Lu X, Zhao Y, Sun Y, Yang S and Yang X:
Characterisation of polysaccharides from green tea of Huangshan
Maofeng with antioxidant and hepatoprotective effects. Food Chem.
141:3415–3423. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Yang X, Zhao Y, Yang Y and Ruan Y:
Isolation and characterization of immunostimulatory polysaccharide
from an herb tea, Gynostemma pentaphyllum Makino. J Agric Food
Chem. 56:6905–6909. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Chen J, Yong Y, Xing M, Gu Y, Zhang Z,
Zhang S and Lu L: Characterization of polysaccharides with marked
inhibitory effect on lipid accumulation in Pleurotus eryngii.
Carbohydr Polym. 97:604–613. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Zhang Y, Dai L, Kong X and Chen L:
Characterization and in vitro antioxidant activities of
polysaccharides from Pleurotus ostreatus. Int J Biol Macromol.
51:259–265. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Li X, Zhang H and Xu H: Analysis of
chemical components of shiitake polysaccharides and its
anti-fatigue effect under vibration. Int J Biol Macromol.
45:377–380. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Wang M, Wang H, Tang Y, Kang D, Gao Y, Ke
M, Dou J, Xi T and Zhou C: Effective inhibition of a
Strongylocentrotus nudus eggs polysaccharide against hepatocellular
carcinoma is mediated via immunoregulation in vivo. Immunol Lett.
141:74–82. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Zhang CX and Huang KX: Mechanism of
apoptosis induced by a polysaccharide, from the loach Misgurnus
anguillicaudatus (MAP) in human hepatocellular carcinoma cells.
Toxicol Appl Pharmacol. 210:236–245. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Isoda N, Eguchi Y, Nukaya H, Hosho K, Suga
Y, Suga T, Nakazawa S and Sugano K: Clinical efficacy of superfine
dispersed lentinan (β-1,3-glucan) in patients with hepatocellular
carcinoma. Hepatogastroenterology. 56:437–441. 2009.PubMed/NCBI
|
|
63
|
Ruan WJ, Lai MD and Zhou JG: Anticancer
effects of Chinese herbal medicine, science or myth? J Zhejiang
Univ Sci B. 7:1006–1014. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Konkimalla VB and Efferth T:
Evidence-based Chinese medicine for cancer therapy. J
Ethnopharmacol. 116:207–210. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Niwa Y, Matsuura H, Murakami M, Sato J,
Hirai K and Sumi H: Evidence that naturopathic therapy including
Cordyceps sinensis prolongs survival of patients with
hepatocellular carcinoma. Integr Cancer Ther. 12:50–68. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Wang CR, Ng TB, Li L, Fang JC, Jiang Y,
Wen TY, Qiao WT, Li N and Liu F: Isolation of a polysaccharide with
antiproliferative, hypoglycemic, antioxidant and HIV-1 reverse
transcriptase inhibitory activities from the fruiting bodies of the
abalone mushroom Pleurotus abalonus. J Pharm Pharmacol. 63:825–832.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Xu WW, Li B, Lai ET, Chen L, Huang JJ,
Cheung AL and Cheung PC: Water extract from Pleurotus pulmonarius
with antioxidant activity exerts in vivo chemoprophylaxis and
chemosensitization for liver cancer. Nutr Cancer. 66:989–998. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Xu W, Huang JJ and Cheung PC: Extract of
Pleurotus pulmonarius suppresses liver cancer development and
progression through inhibition of VEGF-induced PI3K/AKT signaling
pathway. PLoS One. 7:e344062012. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Xu R, Ye H, Sun Y, Tu Y and Zeng X:
Preparation, preliminary characterization, antioxidant,
hepatoprotective and antitumor activities of polysaccharides from
the flower of tea plant (Camellia sinensis). Food Chem Toxicol.
50:2473–2480. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Chen B, Zhou W, Ning M, Wang Z, Zou L,
Zhang H and Wang Q: Evaluation of antitumour activity of tea
carbohydrate polymers in hepatocellular carcinoma animals. Int J
Biol Macromol. 50:1103–1108. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Liang G, Tang A, Lin X, Li L, Zhang S,
Huang Z, Tang H and Li QQ: Green tea catechins augment the
antitumor activity of doxorubicin in an in vivo mouse model for
chemoresistant liver cancer. Int J Oncol. 37:111–123.
2010.PubMed/NCBI
|
|
72
|
He X, Li X, Liu B, Xu L, Zhao H and Lu A:
Down-regulation of Treg cells and up-regulation of TH1/TH2 cytokine
ratio were induced by polysaccharide from Radix Glycyrrhizae in H22
hepatocarcinoma bearing mice. Molecules. 16:8343–8352. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Wu S, Liu B, Zhang Q, Liu J, Zhou W, Wang
C, Li M, Bao S and Zhu R: Dihydromyricetin reduced Bcl-2 expression
via p53 in human hepatoma HepG2 cells. PLoS One. 8:e768862013.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Liu J, Shu Y, Zhang Q, Liu B, Xia J, Qiu
M, Miao H, Li M and Zhu R: Dihydromyricetin induces apoptosis and
inhibits proliferation in hepatocellular carcinoma cells. Oncol
Lett. 8:1645–1651. 2014.PubMed/NCBI
|
|
75
|
Zhang Q, Liu J, Liu B, Xia J, Chen N, Chen
X, Cao Y, Zhang C, Lu C, Li M and Zhu R: Dihydromyricetin promotes
hepatocellular carcinoma regression via a p53 activation-dependent
mechanism. Sci Rep. 4:46282014.PubMed/NCBI
|
|
76
|
Jiang L, Zhang Q, Ren H, Ma S, Lu C, Liu
B, Liu J, Liang J, Li M and Zhu R: Dihydromyricetin enhances the
chemo-sensitivity of nedaplatin via regulation of the p53/Bcl-2
pathway in hepatocellular carcinoma cells. PLoS One.
10:e01249942015. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Liu B, Zhou W, Chen X, Xu F, Chen Y, Liu
J, Zhang Q, Bao S, Chen N, Li M and Zhu R: Dihydromyricetin induces
mouse hepatoma Hepal-6 cell apoptosis via the transforming growth
factor-β pathway. Mol Med Rep. 11:1609–1614. 2015.PubMed/NCBI
|
|
78
|
Lever R and Page C: Novel drug development
opportunities for heparin. Nat Rev Drug Discov. 1:140–148. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Presta M, Leali D, Stabile H, Ronca R,
Camozzi M, Coco L, Moroni E, Liekens S and Rusnati M: Heparin
derivatives as angiogenesis inhibitors. Curr Pharm Des. 9:553–566.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Ferro V, Dredge K, Liu L, Hammond E,
Bytheway I, Li C, Johnstone K, Karoli T, Davis K, Copeman E and
Gautam A: PI-88 and novel heparan sulfate mimetics inhibit
angiogenesis. Semin Thromb Hemost. 33:557–568. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Dredge K, Hammond E, Davis K, Li CP, Liu
L, Johnstone K, Handley P, Wimmer N, Gonda TJ, Gautam A, et al: The
PG500 series: Novel heparan sulfate mimetics as potent angiogenesis
and heparanase inhibitors for cancer therapy. Invest New Drugs.
28:276–283. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Budhu A and Wang XW: The role of cytokines
in hepatocellular carcinoma. J Leukoc Biol. 80:1197–1213. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Nakamoto Y, Guidotti LG, Kuhlen CV, Fowler
P and Chisari FV: Immune pathogenesis of hepatocellular carcinoma.
J Exp Med. 188:341–350. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Chuma M, Hige S, Nakanishi M, Ogawa K,
Natsuizaka M, Yamamoto Y and Asaka M: 8-Hydroxy-2′-deoxy-guanosine
is a risk factor for development of hepatocellular carcinoma in
patients with chronic hepatitis C virus infection. J Gastroenterol
Hepatol. 23:1431–1436. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Tanaka H, Fujita N, Sugimoto R, Urawa N,
Horiike S, Kobayashi Y, Iwasa M, Ma N, Kawanishi S, Watanabe S, et
al: Hepatic oxidative DNA damage is associated with increased risk
for hepatocellular carcinoma in chronic hepatitis C. Br J Cancer.
98:580–586. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Marra M, Sordelli IM, Lombardi A, Lamberti
M, Tarantino L, Giudice A, Stiuso P, Abbruzzese A, Sperlongano R,
Accardo M, et al: Molecular targets and oxidative stress biomarkers
in hepatocellular carcinoma: An overview. J Transl Med. 9:1712011.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Bishayee A: The role of inflammation and
liver cancer. Adv Exp Med Biol. 816:401–435. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Klampfer L: Cytokines, inflammation and
colon cancer. Curr Cancer Drug Targets. 11:451–464. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Chang HL, Lei LS, Yu CL, Zhu ZG, Chen NN
and Wu SG: Effect of Flammulina velutipes polysaccharides on
production of cytokines by murine immunocytes and serum levels of
cytokines in tumor-bearing mice. Zhong Yao Cai. 32:561–563.
2009.(In Chinese). PubMed/NCBI
|
|
90
|
Chen G, Xu J, Miao X, Huan Y, Liu X, Ju Y
and Han X: Characterization and antitumor activities of the
water-soluble polysaccharide from Rhizoma Arisaematis. Carbohydr
Polym. 90:67–72. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Wang LJ, Bai L, Su D, Zhang T and Mao ZY:
Proinflammatory conditions promote hepatocellular carcinoma onset
and progression via activation of Wnt and EGFR signaling pathways.
Mol Cell Biochem. 381:173–181. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Cassetta L, Cassol E and Poli G:
Macrophage polarization in health and disease.
ScientificWorldJournal. 11:2391–2402. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Yang J, Li X, Xue Y, Wang N and Liu W:
Anti-hepatoma activity and mechanism of corn silk polysaccharides
in H22 tumor-bearing mice. Int J Biol Macromol. 64:276–280. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Liu L, Jia J, Zeng G, Zhao Y, Qi X, He C,
Guo W, Fan D, Han G and Li Z: Studies on immunoregulatory and
anti-tumor activities of a polysaccharide from Salvia miltiorrhiza
Bunge. Carbohydr Polym. 92:479–483. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Tian QE, Li HD, Yan M, Cai HL, Tan QY and
Zhang WY: Astragalus polysaccharides can regulate cytokine and
P-glycoprotein expression in H22 tumor-bearing mice. World J
Gastroenterol. 18:7079–7086. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Schild L, Chen BH, Makarov P, Kattengell
K, Heinitz K and Keilhoff G: Selective induction of apoptosis in
glioma tumour cells by a Gynostemma pentaphyllum extract.
Phytomedicine. 17:589–597. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Chen J, Chen J, Wang X and Liu C:
Anti-tumour effects of polysaccharides isolated from Artemisia
annua L by inducing cell apoptosis and immunomodulatory
anti-hepatoma effects of polysaccharides. Afr J Tradit Complement
Altern Med. 11:15–22. 2013.PubMed/NCBI
|
|
98
|
Liang M, Li S, Shen B, Cai JP, Li C, Wang
ZY, Li XG, Gao J, Huang HY, Zhang XY and Li JY:
Anti-hepatocarcinoma effects of Aconitum coreanum polysaccharides.
Carbohydrate Polymers. 88:973–976. 2012. View Article : Google Scholar
|
|
99
|
Zhang W, Li J, Qiu S, Chen J and Zheng Y:
Effects of the exopolysaccharide fraction (EPSF) from a cultivated
Cordyceps sinensis on immunocytes of H22 tumor bearing mice.
Fitoterapia. 79:168–173. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Byeon SE, Lee J, Lee E, Lee SY, Hong EK,
Kim YE and Cho JY: Functional activation of macrophages, monocytes
and splenic lymphocytes by polysaccharide fraction from Tricholoma
matsutake. Arch Pharm Res. 32:1565–1572. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Kim JY, Byeon SE, Lee YG, Lee JY, Park J,
Hong EK and Cho JY: Immunostimulatory activities of polysaccharides
from liquid culture of pine-mushroom Tricholoma matsutake. J
Microbiol Biotechnol. 18:95–103. 2008.PubMed/NCBI
|
|
102
|
Ren M, Ye L, Hao X, Ren Z, Ren S, Xu K and
Li J: Polysaccharides from Tricholoma matsutake and Lentinus edodes
enhance 5-fluorouracil-mediated H22 cell growth inhibition. J
Tradit Chin Med. 34:309–316. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Kim DH and Rossi JJ: Strategies for
silencing human disease using RNA interference. Nat Rev Genet.
8:173–184. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Takeshita F and Ochiya T: Therapeutic
potential of RNA interference against cancer. Cancer Sci.
97:689–696. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Whitehead KA, Langer R and Anderson DG:
Knocking down barriers: Advances in siRNA delivery. Nat Rev Drug
Discov. 8:129–138. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Huang Z, Dong L, Chen J, Gao F, Zhang Z,
Chen J and Zhang J: Low-molecular weight chitosan/vascular
endothelial growth factor short hairpin RNA for the treatment of
hepatocellular carcinoma. Life Sci. 91:1207–1215. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Xu Y, Wen Z and Xu Z: Chitosan
nanoparticles inhibit the growth of human hepatocellular carcinoma
xenografts through an antiangiogenic mechanism. Anticancer Res.
29:5103–5109. 2009.PubMed/NCBI
|
|
108
|
Han L, Tang C and Yin C: Oral delivery of
shRNA and siRNA via multifunctional polymeric nanoparticles for
synergistic cancer therapy. Biomaterials. 35:4589–4600. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Roy M, Luo YH, Ye M and Liu J: Nonsmall
cell lung cancer therapy: Insight into multitargeted small-molecule
growth factor receptor inhibitors. Biomed Res Int. 2013:9647432013.
View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Wang E, Chen X, Wang K, Wang J, Chen D,
Geng Y, Lai W and Wei X: Plant polysaccharides used as
immunostimulants enhance innate immune response and disease
resistance against Aeromonas hydrophila infection in fish. Fish
Shellfish Immunol. 59:196–202. 2016. View Article : Google Scholar : PubMed/NCBI
|















